Abstract
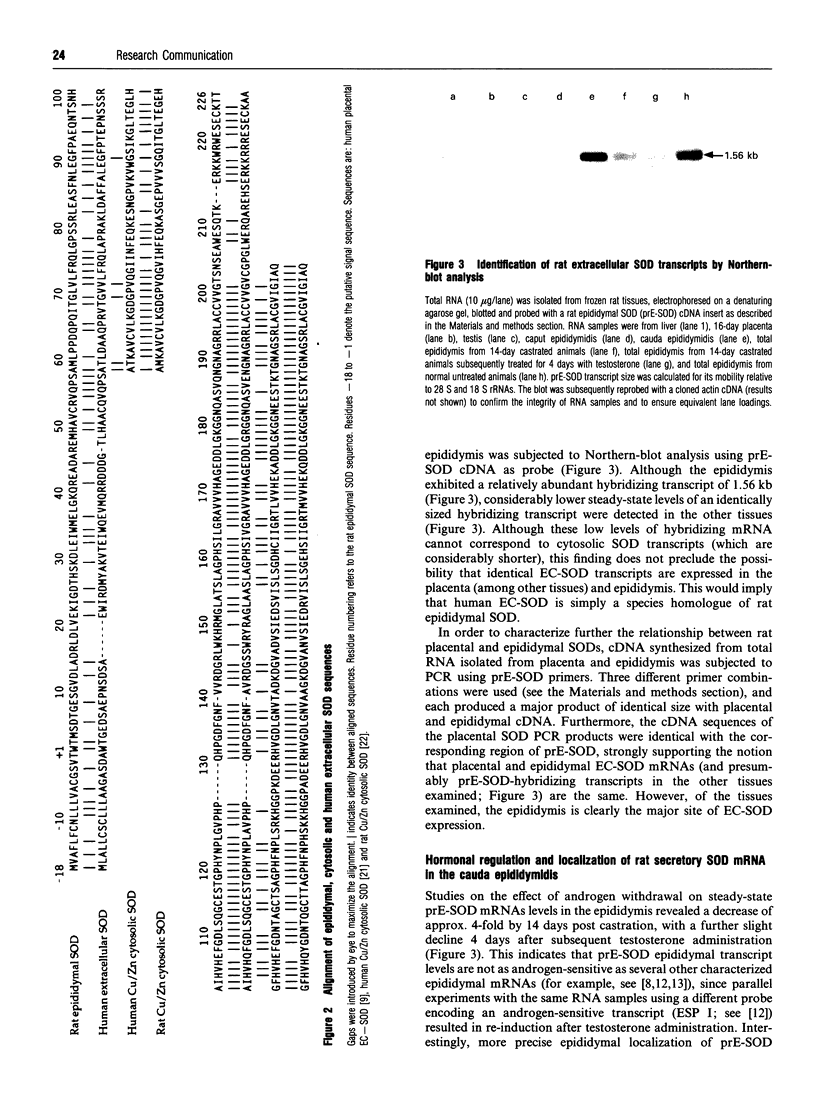
Superoxide dismutase (SOD) plays a key role in combating loss of fertility of spermatozoa due to lipid peroxidation. Here we report the sequence of a cDNA encoding a secreted form of SOD isolated from a rat epididymal library. Northern-blot analysis indicates that the corresponding transcript is expressed principally in the cauda region of the epididymis, consistent with the high levels of SOD enzyme activity found in cauda-epididymidal plasma. Much lower levels of an identically sized transcript exist in all tissues examined, including placenta. PCR and subsequent sequence analysis of rat placental SOD strongly suggest that it is identical in sequence with epididymal SOD.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aitken R. J., Clarkson J. S. Cellular basis of defective sperm function and its association with the genesis of reactive oxygen species by human spermatozoa. J Reprod Fertil. 1987 Nov;81(2):459–469. doi: 10.1530/jrf.0.0810459. [DOI] [PubMed] [Google Scholar]
- Alvarez J. G., Storey B. T. Role of glutathione peroxidase in protecting mammalian spermatozoa from loss of motility caused by spontaneous lipid peroxidation. Gamete Res. 1989 May;23(1):77–90. doi: 10.1002/mrd.1120230108. [DOI] [PubMed] [Google Scholar]
- Barratt C. L., Bolton A. E., Cooke I. D. Functional significance of white blood cells in the male and female reproductive tract. Hum Reprod. 1990 Aug;5(6):639–648. doi: 10.1093/oxfordjournals.humrep.a137162. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Cutler R. G. Human longevity and aging: possible role of reactive oxygen species. Ann N Y Acad Sci. 1991;621:1–28. doi: 10.1111/j.1749-6632.1991.tb16965.x. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Getzoff E. D., Cabelli D. E., Fisher C. L., Parge H. E., Viezzoli M. S., Banci L., Hallewell R. A. Faster superoxide dismutase mutants designed by enhancing electrostatic guidance. Nature. 1992 Jul 23;358(6384):347–351. doi: 10.1038/358347a0. [DOI] [PubMed] [Google Scholar]
- Getzoff E. D., Tainer J. A., Weiner P. K., Kollman P. A., Richardson J. S., Richardson D. C. Electrostatic recognition between superoxide and copper, zinc superoxide dismutase. Nature. 1983 Nov 17;306(5940):287–290. doi: 10.1038/306287a0. [DOI] [PubMed] [Google Scholar]
- Girotti M., Jones R., Emery D. C., Chia W., Hall L. Structure and expression of the rat epididymal secretory protein I gene. An androgen-regulated member of the lipocalin superfamily with a rare splice donor site. Biochem J. 1992 Jan 1;281(Pt 1):203–210. doi: 10.1042/bj2810203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallewell R. A., Masiarz F. R., Najarian R. C., Puma J. P., Quiroga M. R., Randolph A., Sanchez-Pescador R., Scandella C. J., Smith B., Steimer K. S. Human Cu/Zn superoxide dismutase cDNA: isolation of clones synthesising high levels of active or inactive enzyme from an expression library. Nucleic Acids Res. 1985 Mar 25;13(6):2017–2034. doi: 10.1093/nar/13.6.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hass M. A., Iqbal J., Clerch L. B., Frank L., Massaro D. Rat lung Cu,Zn superoxide dismutase. Isolation and sequence of a full-length cDNA and studies of enzyme induction. J Clin Invest. 1989 Apr;83(4):1241–1246. doi: 10.1172/JCI114007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjalmarsson K., Marklund S. L., Engström A., Edlund T. Isolation and sequence of complementary DNA encoding human extracellular superoxide dismutase. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6340–6344. doi: 10.1073/pnas.84.18.6340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries S. E., Whittall R., Minty A., Buckingham M., Williamson R. There are approximately 20 actin gene in the human genome. Nucleic Acids Res. 1981 Oct 10;9(19):4895–4908. doi: 10.1093/nar/9.19.4895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki A., Gagnon C. Formation of reactive oxygen species in spermatozoa of infertile patients. Fertil Steril. 1992 Feb;57(2):409–416. doi: 10.1016/s0015-0282(16)54855-9. [DOI] [PubMed] [Google Scholar]
- Jones R., Mann T. Toxicity of exogenous fatty acid peroxides towards spermatozoa. J Reprod Fertil. 1977 Jul;50(2):255–260. doi: 10.1530/jrf.0.0500255. [DOI] [PubMed] [Google Scholar]
- Kirkland J. L. The biochemistry of mammalian senescence. Clin Biochem. 1992 Apr;25(2):61–75. doi: 10.1016/0009-9120(92)80047-k. [DOI] [PubMed] [Google Scholar]
- Mennella M. R., Jones R. Properties of spermatozoal superoxide dismutase and lack of involvement of superoxides in metal-ion-catalysed lipid-peroxidation and reactions in semen. Biochem J. 1980 Nov 1;191(2):289–297. doi: 10.1042/bj1910289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paoletti F., Mocali A. Determination of superoxide dismutase activity by purely chemical system based on NAD(P)H oxidation. Methods Enzymol. 1990;186:209–220. doi: 10.1016/0076-6879(90)86110-h. [DOI] [PubMed] [Google Scholar]
- Parge H. E., Hallewell R. A., Tainer J. A. Atomic structures of wild-type and thermostable mutant recombinant human Cu,Zn superoxide dismutase. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):6109–6113. doi: 10.1073/pnas.89.13.6109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry A. C., Jones R., Niang L. S., Jackson R. M., Hall L. Genetic evidence for an androgen-regulated epididymal secretory glutathione peroxidase whose transcript does not contain a selenocysteine codon. Biochem J. 1992 Aug 1;285(Pt 3):863–870. doi: 10.1042/bj2850863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker J. E., Jones R., Moore A., Hamilton D. W., Hall L. Analysis of major androgen-regulated cDNA clones from the rat epididymis. Mol Cell Endocrinol. 1990 Nov 12;74(1):61–68. doi: 10.1016/0303-7207(90)90205-m. [DOI] [PubMed] [Google Scholar]



