Abstract
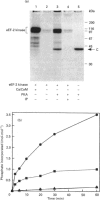
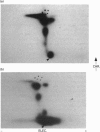
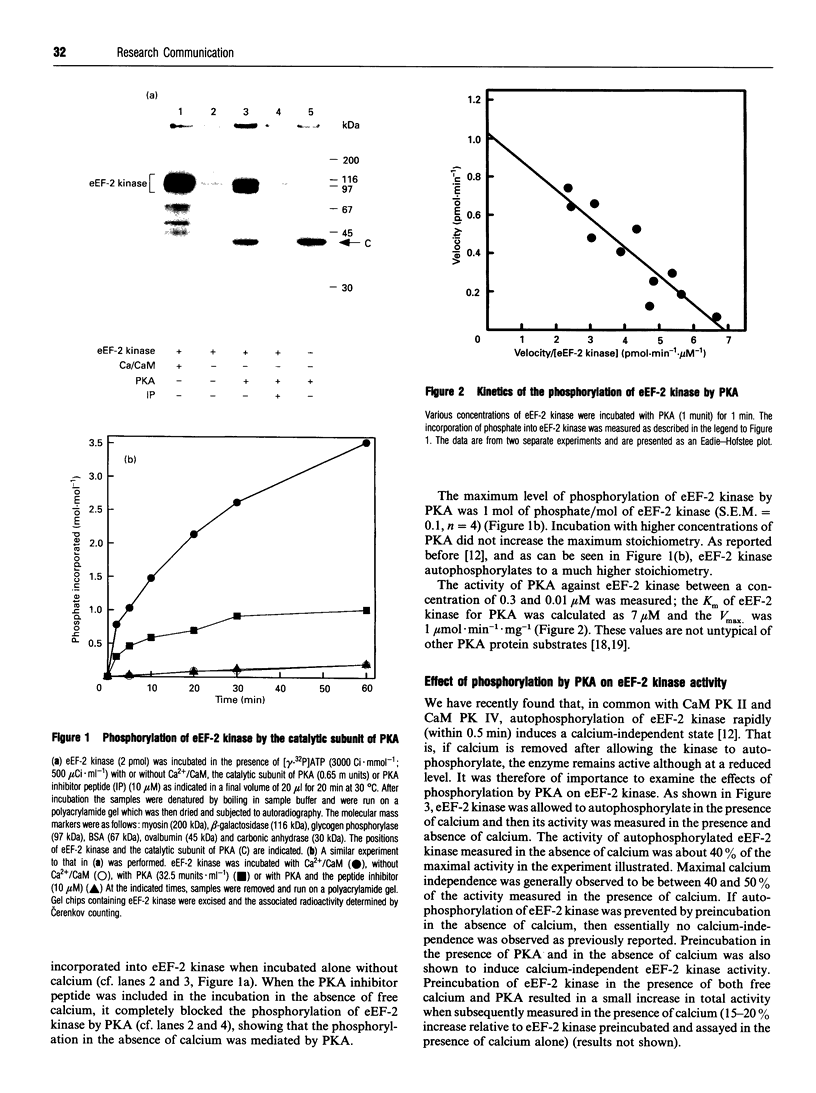
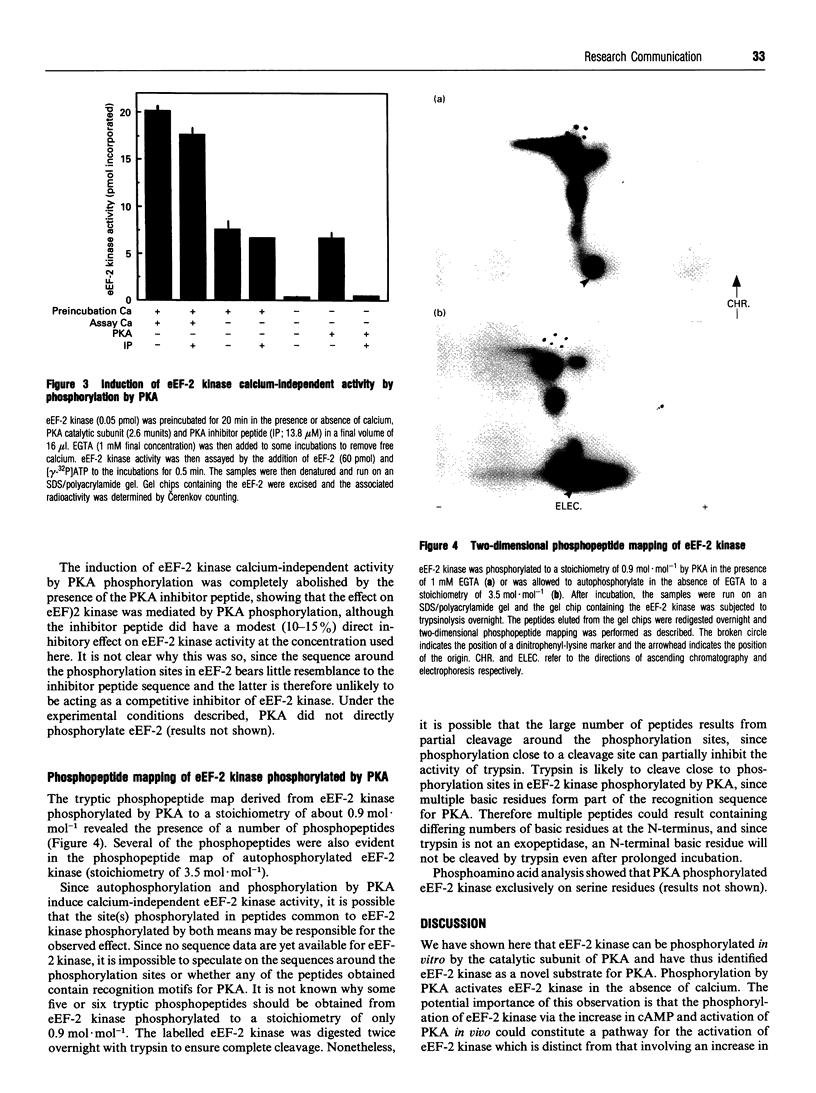
The catalytic subunit of cyclic AMP-dependent protein kinase (PKA) phosphorylated purified calcium/calmodulin-dependent eukaryotic elongation factor-2 (eEF-2) kinase, isolated from rabbit reticulocyte lysates. It maximally incorporated about 1 mol of phosphate/mol of eEF-2 kinase. The Km of eEF-2 kinase for PKA was calculated to be 7 microM. Phosphorylation of eEF-2 kinase by PKA induced calcium-independent activity which amounted to 40-50% of the total activity measured in the presence of calcium. Furthermore, the level of calcium-independent activity induced by phosphorylation by PKA was similar to that induced by the calcium-stimulated autophosphorylation of eEF-2 kinase. Phosphopeptide mapping of eEF-2 kinase labelled by autophosphorylation and by PKA revealed a number of common phosphopeptides. This suggests that PKA may phosphorylate the same site(s) which are phosphorylated autocatalytically and which are responsible for the induction of calcium-independent activity. The possible implications these findings have for the control of translation are discussed.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ayuso-Parrilla M. S., Martín-Requero A., Pérez-Días J., Parrilla R. Role of glucagon on the control of hepatic protein synthesis and degradation in the rat in vivo. J Biol Chem. 1976 Dec 25;251(24):7785–7790. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Bylund D. B., Krebs E. G. Effect of denaturation on the susceptibility of proteins to enzymic phosphorylation. J Biol Chem. 1975 Aug 25;250(16):6355–6361. [PubMed] [Google Scholar]
- Cohen P. The role of calcium ions, calmodulin and troponin in the regulation of phosphorylase kinase from rabbit skeletal muscle. Eur J Biochem. 1980 Oct;111(2):563–574. doi: 10.1111/j.1432-1033.1980.tb04972.x. [DOI] [PubMed] [Google Scholar]
- Conti M. A., Adelstein R. S. The relationship between calmodulin binding and phosphorylation of smooth muscle myosin kinase by the catalytic subunit of 3':5' cAMP-dependent protein kinase. J Biol Chem. 1981 Apr 10;256(7):3178–3181. [PubMed] [Google Scholar]
- Frangakis M. V., Ohmstede C. A., Sahyoun N. A brain-specific Ca2+/calmodulin-dependent protein kinase (CaM kinase-Gr) is regulated by autophosphorylation. Relevance to neuronal Ca2+ signaling. J Biol Chem. 1991 Jun 15;266(17):11309–11316. [PubMed] [Google Scholar]
- Hincke M. T., Nairn A. C. Phosphorylation of elongation factor 2 during Ca(2+)-mediated secretion from rat parotid acini. Biochem J. 1992 Mar 15;282(Pt 3):877–882. doi: 10.1042/bj2820877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kameshita I., Fujisawa H. Phosphorylation and functional modification of calmodulin-dependent protein kinase IV by cAMP-dependent protein kinase. Biochem Biophys Res Commun. 1991 Oct 15;180(1):191–196. doi: 10.1016/s0006-291x(05)81275-6. [DOI] [PubMed] [Google Scholar]
- Mackie K. P., Nairn A. C., Hampel G., Lam G., Jaffe E. A. Thrombin and histamine stimulate the phosphorylation of elongation factor 2 in human umbilical vein endothelial cells. J Biol Chem. 1989 Jan 25;264(3):1748–1753. [PubMed] [Google Scholar]
- Menaya J., Parrilla R., Ayuso M. S. Action of phenylephrine on protein synthesis in liver cells. Biochem J. 1987 Dec 15;248(3):903–909. doi: 10.1042/bj2480903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller S. G., Kennedy M. B. Regulation of brain type II Ca2+/calmodulin-dependent protein kinase by autophosphorylation: a Ca2+-triggered molecular switch. Cell. 1986 Mar 28;44(6):861–870. doi: 10.1016/0092-8674(86)90008-5. [DOI] [PubMed] [Google Scholar]
- Moazed D., Noller H. F. Intermediate states in the movement of transfer RNA in the ribosome. Nature. 1989 Nov 9;342(6246):142–148. doi: 10.1038/342142a0. [DOI] [PubMed] [Google Scholar]
- Moldave K. Eukaryotic protein synthesis. Annu Rev Biochem. 1985;54:1109–1149. doi: 10.1146/annurev.bi.54.070185.005333. [DOI] [PubMed] [Google Scholar]
- Munday M. R., Hardie D. G. Isolation of three cyclic-AMP-independent acetyl-CoA carboxylase kinases from lactating rat mammary gland and characterization of their effects on enzyme activity. Eur J Biochem. 1984 Jun 15;141(3):617–627. doi: 10.1111/j.1432-1033.1984.tb08237.x. [DOI] [PubMed] [Google Scholar]
- Nairn A. C., Palfrey H. C. Identification of the major Mr 100,000 substrate for calmodulin-dependent protein kinase III in mammalian cells as elongation factor-2. J Biol Chem. 1987 Dec 25;262(36):17299–17303. [PubMed] [Google Scholar]
- Nishikawa M., de Lanerolle P., Lincoln T. M., Adelstein R. S. Phosphorylation of mammalian myosin light chain kinases by the catalytic subunit of cyclic AMP-dependent protein kinase and by cyclic GMP-dependent protein kinase. J Biol Chem. 1984 Jul 10;259(13):8429–8436. [PubMed] [Google Scholar]
- Palfrey H. C., Nairn A. C., Muldoon L. L., Villereal M. L. Rapid activation of calmodulin-dependent protein kinase III in mitogen-stimulated human fibroblasts. Correlation with intracellular Ca2+ transients. J Biol Chem. 1987 Jul 15;262(20):9785–9792. [PubMed] [Google Scholar]
- Palfrey H. C. Presence in many mammalian tissues of an identical major cytosolic substrate (Mr 100 000) for calmodulin-dependent protein kinase. FEBS Lett. 1983 Jun 27;157(1):183–190. doi: 10.1016/0014-5793(83)81142-9. [DOI] [PubMed] [Google Scholar]
- Proud C. G. Protein phosphorylation in translational control. Curr Top Cell Regul. 1992;32:243–369. doi: 10.1016/b978-0-12-152832-4.50008-2. [DOI] [PubMed] [Google Scholar]
- Redpath N. T., Price N. T., Severinov K. V., Proud C. G. Regulation of elongation factor-2 by multisite phosphorylation. Eur J Biochem. 1993 Apr 15;213(2):689–699. doi: 10.1111/j.1432-1033.1993.tb17809.x. [DOI] [PubMed] [Google Scholar]
- Redpath N. T., Proud C. G. Purification and phosphorylation of elongation factor-2 kinase from rabbit reticulocytes. Eur J Biochem. 1993 Mar 1;212(2):511–520. doi: 10.1111/j.1432-1033.1993.tb17688.x. [DOI] [PubMed] [Google Scholar]
- Redpath N. T., Proud C. G. The tumour promoter okadaic acid inhibits reticulocyte-lysate protein synthesis by increasing the net phosphorylation of elongation factor 2. Biochem J. 1989 Aug 15;262(1):69–75. doi: 10.1042/bj2620069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimann E. M., Beham R. A. Catalytic subunit of cAMP-dependent protein kinase. Methods Enzymol. 1983;99:51–55. doi: 10.1016/0076-6879(83)99039-0. [DOI] [PubMed] [Google Scholar]
- Requero A. M., Díaz J. P., Ayuso-Parrilla M. S., Parrilla R. On the mechanism of the glucagon-induced inhibition of hepatic protein synthesis. Arch Biochem Biophys. 1979 Jun;195(1):223–234. doi: 10.1016/0003-9861(79)90344-8. [DOI] [PubMed] [Google Scholar]
- Ryazanov A. G., Natapov P. G., Shestakova E. A., Severin F. F., Spirin A. S. Phosphorylation of the elongation factor 2: the fifth Ca2+/calmodulin-dependent system of protein phosphorylation. Biochimie. 1988 May;70(5):619–626. doi: 10.1016/0300-9084(88)90245-3. [DOI] [PubMed] [Google Scholar]
- Ryazanov A. G., Shestakova E. A., Natapov P. G. Phosphorylation of elongation factor 2 by EF-2 kinase affects rate of translation. Nature. 1988 Jul 14;334(6178):170–173. doi: 10.1038/334170a0. [DOI] [PubMed] [Google Scholar]