Abstract
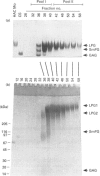
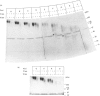
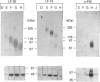
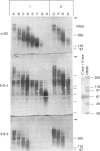
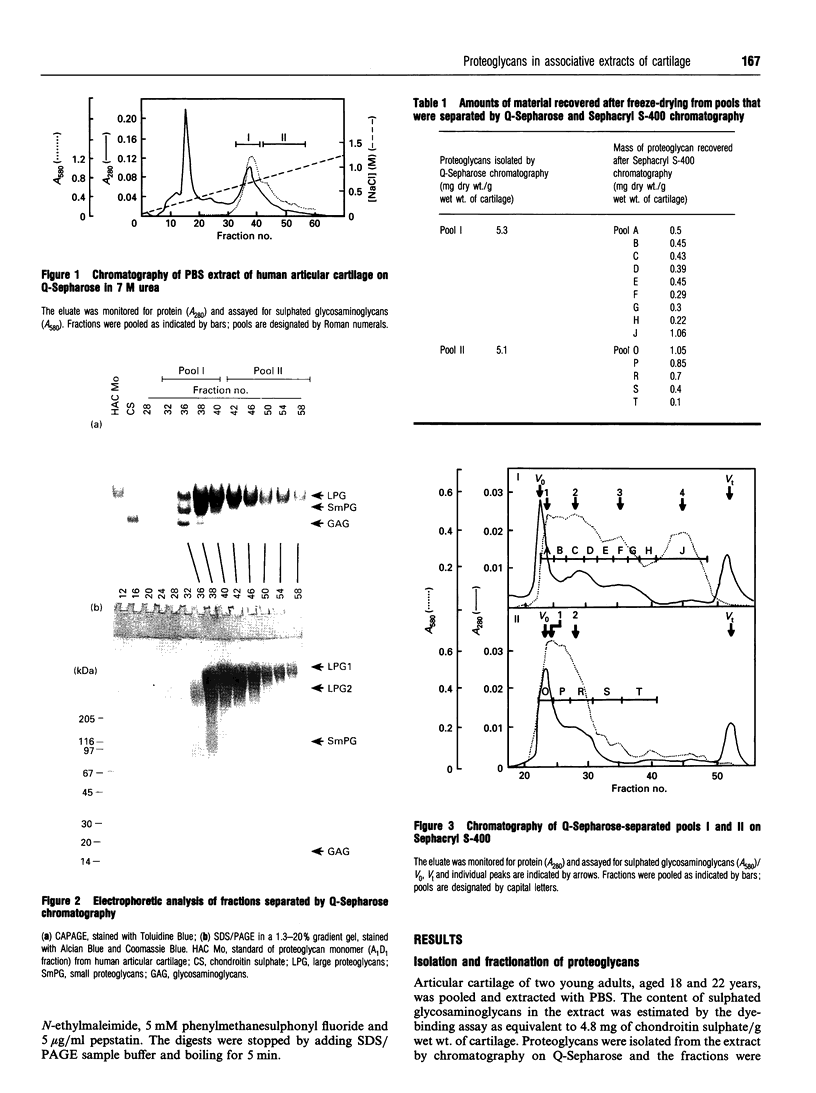
Approx. 10% of the total proteoglycan content of normal young human articular cartilage was extracted under associative conditions with Dulbecco's PBS. Proteoglycans isolated from the extract by Q-Sepharose chromatography were separated by gel chromatography and characterized by gradient gel SDS/PAGE and immunoblotting. Three species of small proteoglycans, two main populations of aggrecan and a population of its smaller fragments were identified. The major populations of aggrecan contained chondroitin sulphate chains, all or part of the N-terminal G1 and G2 domains and, therefore, intact keratan sulphate domains. The larger population was estimated by gradient SDS/PAGE to have a molecular mass of approx. 600 kDa or greater. The second population had an apparent molecular mass of approx. 300-600 kDa. Core proteins derived from these populations of proteoglycans separated on SDS/PAGE into several clusters of bands in the range from 120 to approx. 360 kDa. The extract further contained smaller fragments which lacked chondroitin sulphate but reacted with antibodies against keratan sulphate, and against epitopes present in the G2 domain of aggrecan. The presence of the G2 domain in a broad range of populations of decreasing size indicated extensive cleavage of the aggrecan core protein within its chondroitin sulphate domain. These findings suggest that fragmentation of aggrecan probably occurs in vivo in normal articular cartilage of young individuals. Associative extracts also contained decorin, biglycan and fibromodulin. These were resolved from aggrecan by gel chromatography and identified by immunodetection.
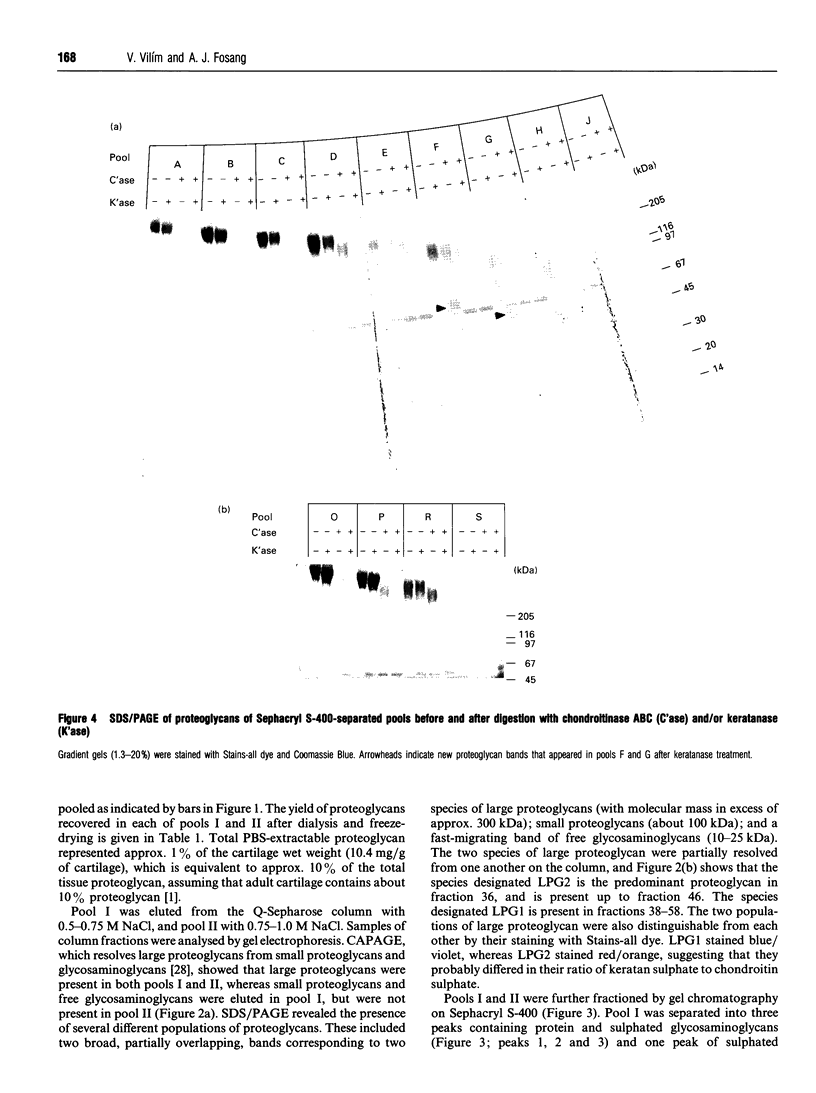
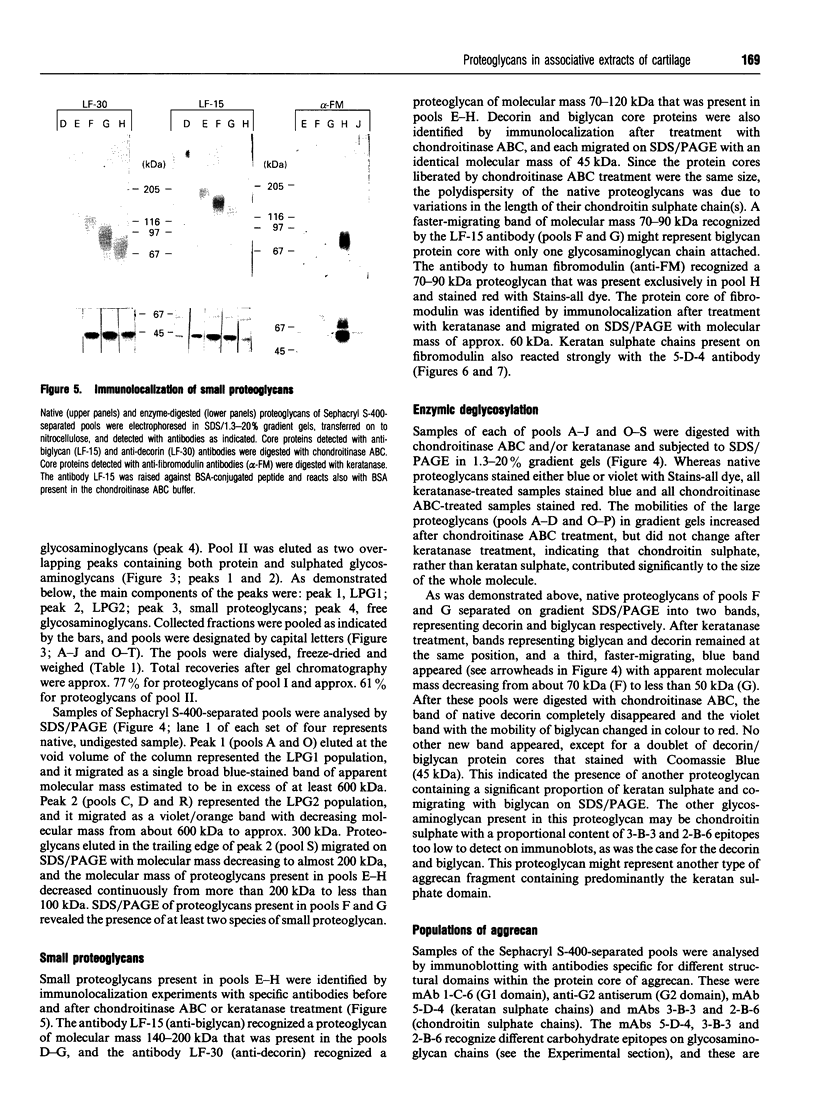
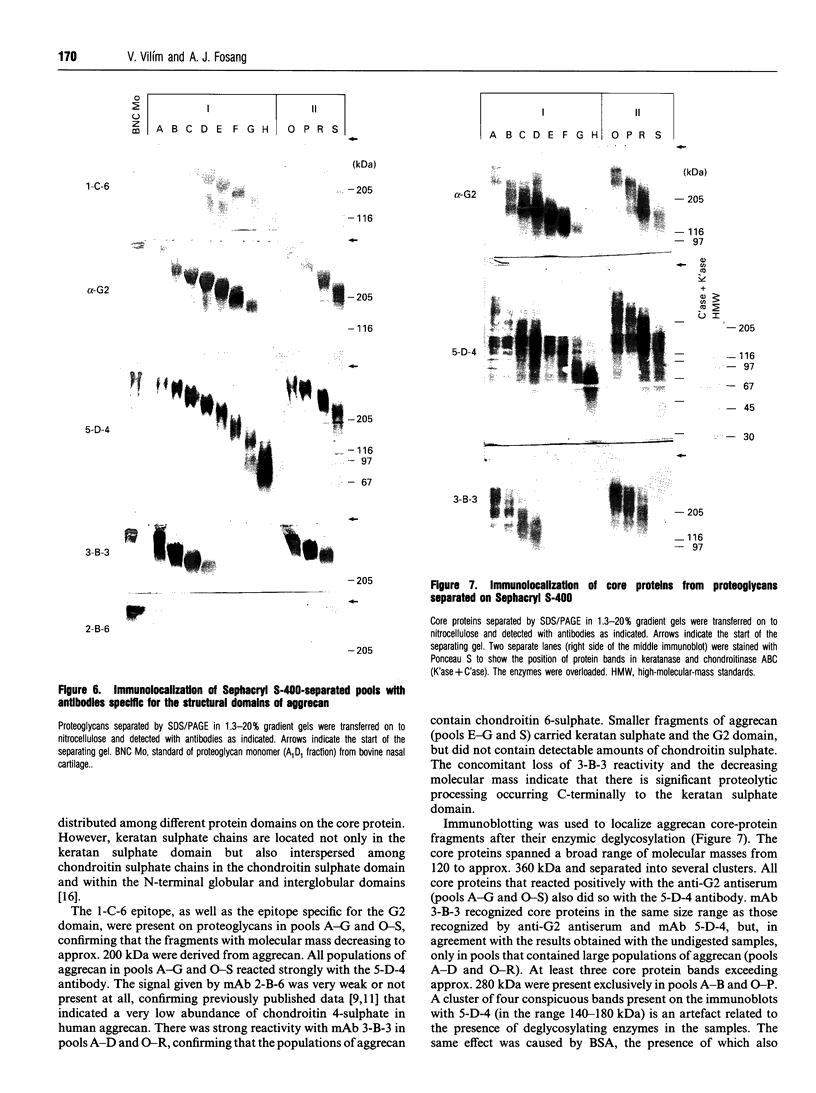
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bayliss M. T., Ridgway G. D., Ali S. Y. Delayed aggregation of proteoglycans in adult human articular cartilage. Biosci Rep. 1984 Oct;4(10):827–833. doi: 10.1007/BF01138164. [DOI] [PubMed] [Google Scholar]
- Bayliss M. T., Venn M., Maroudas A., Ali S. Y. Structure of proteoglycans from different layers of human articular cartilage. Biochem J. 1983 Feb 1;209(2):387–400. doi: 10.1042/bj2090387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell M. A., Handley C. J., D'Souza S. E. Turnover of proteoglycans in articular-cartilage cultures. Characterization of proteoglycans released into the medium. Biochem J. 1989 Apr 1;259(1):21–25. doi: 10.1042/bj2590021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carney S. L., Bayliss M. T., Collier J. M., Muir H. Electrophoresis of 35S-labeled proteoglycans on polyacrylamide-agarose composite gels and their visualization by fluorography. Anal Biochem. 1986 Jul;156(1):38–44. doi: 10.1016/0003-2697(86)90150-8. [DOI] [PubMed] [Google Scholar]
- Caterson B., Christner J. E., Baker J. R. Identification of a monoclonal antibody that specifically recognizes corneal and skeletal keratan sulfate. Monoclonal antibodies to cartilage proteoglycan. J Biol Chem. 1983 Jul 25;258(14):8848–8854. [PubMed] [Google Scholar]
- Caterson B., Griffin J., Mahmoodian F., Sorrell J. M. Monoclonal antibodies against chondroitin sulphate isomers: their use as probes for investigating proteoglycan metabolism. Biochem Soc Trans. 1990 Oct;18(5):820–823. doi: 10.1042/bst0180820. [DOI] [PubMed] [Google Scholar]
- Doege K. J., Sasaki M., Kimura T., Yamada Y. Complete coding sequence and deduced primary structure of the human cartilage large aggregating proteoglycan, aggrecan. Human-specific repeats, and additional alternatively spliced forms. J Biol Chem. 1991 Jan 15;266(2):894–902. [PubMed] [Google Scholar]
- Farndale R. W., Sayers C. A., Barrett A. J. A direct spectrophotometric microassay for sulfated glycosaminoglycans in cartilage cultures. Connect Tissue Res. 1982;9(4):247–248. doi: 10.3109/03008208209160269. [DOI] [PubMed] [Google Scholar]
- Fisher L. W., Termine J. D., Young M. F. Deduced protein sequence of bone small proteoglycan I (biglycan) shows homology with proteoglycan II (decorin) and several nonconnective tissue proteins in a variety of species. J Biol Chem. 1989 Mar 15;264(8):4571–4576. [PubMed] [Google Scholar]
- Flannery C. R., Lark M. W., Sandy J. D. Identification of a stromelysin cleavage site within the interglobular domain of human aggrecan. Evidence for proteolysis at this site in vivo in human articular cartilage. J Biol Chem. 1992 Jan 15;267(2):1008–1014. [PubMed] [Google Scholar]
- Fosang A. J., Hardingham T. E. 1-C-6 epitope in cartilage proteoglycan G2 domain is masked by keratan sulphate. Biochem J. 1991 Jan 15;273(Pt 2):369–373. doi: 10.1042/bj2730369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fosang A. J., Neame P. J., Hardingham T. E., Murphy G., Hamilton J. A. Cleavage of cartilage proteoglycan between G1 and G2 domains by stromelysins. J Biol Chem. 1991 Aug 25;266(24):15579–15582. [PubMed] [Google Scholar]
- Fosang A. J., Neame P. J., Last K., Hardingham T. E., Murphy G., Hamilton J. A. The interglobular domain of cartilage aggrecan is cleaved by PUMP, gelatinases, and cathepsin B. J Biol Chem. 1992 Sep 25;267(27):19470–19474. [PubMed] [Google Scholar]
- Galloway W. A., Murphy G., Sandy J. D., Gavrilovic J., Cawston T. E., Reynolds J. J. Purification and characterization of a rabbit bone metalloproteinase that degrades proteoglycan and other connective-tissue components. Biochem J. 1983 Mar 1;209(3):741–752. doi: 10.1042/bj2090741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg H. A., Domenicucci C., Pringle G. A., Sodek J. Mineral-binding proteoglycans of fetal porcine calvarial bone. J Biol Chem. 1988 Aug 25;263(24):12092–12101. [PubMed] [Google Scholar]
- Hardingham T. E., Fosang A. J. Proteoglycans: many forms and many functions. FASEB J. 1992 Feb 1;6(3):861–870. [PubMed] [Google Scholar]
- Hedbom E., Heinegård D. Interaction of a 59-kDa connective tissue matrix protein with collagen I and collagen II. J Biol Chem. 1989 Apr 25;264(12):6898–6905. [PubMed] [Google Scholar]
- Heimer R. Proteoglycan profiles obtained by electrophoresis and triple immunoblotting. Anal Biochem. 1989 Aug 1;180(2):211–215. doi: 10.1016/0003-2697(89)90117-6. [DOI] [PubMed] [Google Scholar]
- Heinegård D., Björne-Persson A., Cöster L., Franzén A., Gardell S., Malmström A., Paulsson M., Sandfalk R., Vogel K. The core proteins of large and small interstitial proteoglycans from various connective tissues form distinct subgroups. Biochem J. 1985 Aug 15;230(1):181–194. doi: 10.1042/bj2300181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinegård D., Oldberg A. Structure and biology of cartilage and bone matrix noncollagenous macromolecules. FASEB J. 1989 Jul;3(9):2042–2051. doi: 10.1096/fasebj.3.9.2663581. [DOI] [PubMed] [Google Scholar]
- Heinegård D., Sommarin Y., Hedbom E., Wieslander J., Larsson B. Assay of proteoglycan populations using agarose-polyacrylamide gel electrophoresis. Anal Biochem. 1985 Nov 15;151(1):41–48. doi: 10.1016/0003-2697(85)90050-8. [DOI] [PubMed] [Google Scholar]
- Heinegård D., Wieslander J., Sheehan J., Paulsson M., Sommarin Y. Separation and characterization of two populations of aggregating proteoglycans from cartilage. Biochem J. 1985 Jan 1;225(1):95–106. doi: 10.1042/bj2250095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hronowski L. J., Anastassiades T. P. Nonspecific interaction of proteoglycans with chromatography media and surfaces: effect of this interaction on the isolation efficiencies. Anal Biochem. 1990 Nov 15;191(1):50–57. doi: 10.1016/0003-2697(90)90386-n. [DOI] [PubMed] [Google Scholar]
- Hughes C., Murphy G., Hardingham T. E. Metalloproteinase digestion of cartilage proteoglycan. Pattern of cleavage by stromelysin and susceptibility to collagenase. Biochem J. 1991 Nov 1;279(Pt 3):733–739. doi: 10.1042/bj2790733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilic M. Z., Handley C. J., Robinson H. C., Mok M. T. Mechanism of catabolism of aggrecan by articular cartilage. Arch Biochem Biophys. 1992 Apr;294(1):115–122. doi: 10.1016/0003-9861(92)90144-l. [DOI] [PubMed] [Google Scholar]
- Mehmet H., Scudder P., Tang P. W., Hounsell E. F., Caterson B., Feizi T. The antigenic determinants recognized by three monoclonal antibodies to keratan sulphate involve sulphated hepta- or larger oligosaccharides of the poly(N-acetyllactosamine) series. Eur J Biochem. 1986 Jun 2;157(2):385–391. doi: 10.1111/j.1432-1033.1986.tb09680.x. [DOI] [PubMed] [Google Scholar]
- Melching L. I., Roughley P. J. The synthesis of dermatan sulphate proteoglycans by fetal and adult human articular cartilage. Biochem J. 1989 Jul 15;261(2):501–508. doi: 10.1042/bj2610501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mok M. T., Ilic M. Z., Handley C. J., Robinson H. C. Cleavage of proteoglycan aggregate by leucocyte elastase. Arch Biochem Biophys. 1992 Feb 1;292(2):442–447. doi: 10.1016/0003-9861(92)90014-n. [DOI] [PubMed] [Google Scholar]
- Ratcliffe A., Doherty M., Maini R. N., Hardingham T. E. Increased concentrations of proteoglycan components in the synovial fluids of patients with acute but not chronic joint disease. Ann Rheum Dis. 1988 Oct;47(10):826–832. doi: 10.1136/ard.47.10.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratcliffe A., Tyler J. A., Hardingham T. E. Articular cartilage cultured with interleukin 1. Increased release of link protein, hyaluronate-binding region and other proteoglycan fragments. Biochem J. 1986 Sep 1;238(2):571–580. doi: 10.1042/bj2380571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roughley P. J., White R. J. Dermatan sulphate proteoglycans of human articular cartilage. The properties of dermatan sulphate proteoglycans I and II. Biochem J. 1989 Sep 15;262(3):823–827. doi: 10.1042/bj2620823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roughley P. J., White R. J., Poole A. R. Identification of a hyaluronic acid-binding protein that interferes with the preparation of high-buoyant-density proteoglycan aggregates from adult human articular cartilage. Biochem J. 1985 Oct 1;231(1):129–138. doi: 10.1042/bj2310129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampaio L. de O., Bayliss M. T., Hardingham T. E., Muir H. Dermatan sulphate proteoglycan from human articular cartilage. Variation in its content with age and its structural comparison with a small chondroitin sulphate proteoglycan from pig laryngeal cartilage. Biochem J. 1988 Sep 15;254(3):757–764. doi: 10.1042/bj2540757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandy J. D., Boynton R. E., Flannery C. R. Analysis of the catabolism of aggrecan in cartilage explants by quantitation of peptides from the three globular domains. J Biol Chem. 1991 May 5;266(13):8198–8205. [PubMed] [Google Scholar]
- Sandy J. D., Flannery C. R., Neame P. J., Lohmander L. S. The structure of aggrecan fragments in human synovial fluid. Evidence for the involvement in osteoarthritis of a novel proteinase which cleaves the Glu 373-Ala 374 bond of the interglobular domain. J Clin Invest. 1992 May;89(5):1512–1516. doi: 10.1172/JCI115742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandy J. D., Neame P. J., Boynton R. E., Flannery C. R. Catabolism of aggrecan in cartilage explants. Identification of a major cleavage site within the interglobular domain. J Biol Chem. 1991 May 15;266(14):8683–8685. [PubMed] [Google Scholar]
- Saxne T., Heinegård D. Synovial fluid analysis of two groups of proteoglycan epitopes distinguishes early and late cartilage lesions. Arthritis Rheum. 1992 Apr;35(4):385–390. doi: 10.1002/art.1780350404. [DOI] [PubMed] [Google Scholar]
- Saxne T., Heinegård D., Wollheim F. A., Pettersson H. Difference in cartilage proteoglycan level in synovial fluid in early rheumatoid arthritis and reactive arthritis. Lancet. 1985 Jul 20;2(8447):127–128. doi: 10.1016/s0140-6736(85)90229-6. [DOI] [PubMed] [Google Scholar]
- Stevens J. W., Oike Y., Handley C., Hascall V. C., Hampton A., Caterson B. Characteristics of the core protein of the aggregating proteoglycan from the Swarm rat chondrosarcoma. J Cell Biochem. 1984;26(4):247–259. doi: 10.1002/jcb.240260405. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triphaus G. F., Schmidt A., Buddecke E. Age-related changes in the incorporation of [35S]sulfate into two proteoglycan populations from human cartilage. Hoppe Seylers Z Physiol Chem. 1980 Dec;361(12):1773–1779. doi: 10.1515/bchm2.1980.361.2.1773. [DOI] [PubMed] [Google Scholar]
- Vilim V., Krajickova J. Electrophoretic separation of large proteoglycans in large-pore polyacrylamide gradient gels (1.32-10.0% T) and a one-step procedure for simultaneous staining of proteins and proteoglycans. Anal Biochem. 1991 Aug 15;197(1):34–39. doi: 10.1016/0003-2697(91)90351-s. [DOI] [PubMed] [Google Scholar]
- Vilim V., Krajickova J. Proteoglycans of human articular cartilage. Identification of several populations of large and small proteoglycans and of hyaluronic acid-binding proteins in successive cartilage extracts. Biochem J. 1991 Feb 1;273(Pt 3):579–585. doi: 10.1042/bj2730579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel K. G., Paulsson M., Heinegård D. Specific inhibition of type I and type II collagen fibrillogenesis by the small proteoglycan of tendon. Biochem J. 1984 Nov 1;223(3):587–597. doi: 10.1042/bj2230587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webber C., Glant T. T., Roughley P. J., Poole A. R. The identification and characterization of two populations of aggregating proteoglycans of high buoyant density isolated from post-natal human articular cartilages of different ages. Biochem J. 1987 Dec 15;248(3):735–740. doi: 10.1042/bj2480735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witter J., Roughley P. J., Webber C., Roberts N., Keystone E., Poole A. R. The immunologic detection and characterization of cartilage proteoglycan degradation products in synovial fluids of patients with arthritis. Arthritis Rheum. 1987 May;30(5):519–529. doi: 10.1002/art.1780300506. [DOI] [PubMed] [Google Scholar]