Abstract
Background:
Botulinum toxin A (BTxA) has gained popularity as a nonsurgical aesthetic treatment for skin rejuvenation. However, previous studies on intradermal BTxA have shown inconsistent results. This systematic review and meta-analysis with trial sequential analysis aimed to assess the efficacy and safety of intradermal BTxA for facial rejuvenation.
Methods:
Following PRISMA guidelines, a comprehensive search was conducted in various databases from January 2008 to March 2023. Outcome measures included sebum production, pore size, skin hydration, skin texture, erythema index, facial wrinkles, and facelift. Eligible studies included human-based clinical trials and prospective cohort studies published in English, focusing on healthy populations requiring facial rejuvenation. Two authors independently screened the titles and abstracts, followed by a full-text review to determine study eligibility. Data extraction and quality assessment were performed by two authors using predefined criteria.
Results:
Ten studies met the inclusion criteria, including five randomized controlled trials and five prospective cohort studies with 153 participants. Studies revealed positive effects of intradermal BTxA on various outcome measures related to facial rejuvenation. These effects included improvements in sebum production, pore size, erythema index, facial wrinkles, skin texture and elasticity, and overall facelift but not skin hydration. All failed to reach the required information size in the trial sequential analysis.
Conclusions:
Findings suggest positive outcomes in multiple attributes of skin quality and facial rejuvenation. However, more high-quality research is needed to establish definitive conclusions. These findings contribute to the evidence base for nonsurgical aesthetic treatments, emphasizing the importance of ongoing research in this field.
Takeaways
Question: What is the evidence for the effectiveness of intradermal injection of botulinum toxin A (BTxA) in improving skin quality attributes and facial rejuvenation?
Findings: Analysis of 10 studies (five randomized trials, five cohort studies) with 153 participants showed that intradermal BTxA improves several rejuvenation markers (sebum production, pore size, erythema, wrinkles, skin texture, and elasticity), except hydration. None met the sample size for trial sequential analysis.
Meaning: Preliminary results suggest BTxA benefits various skin aspects, highlighting the need for more extensive research for definitive conclusions.
INTRODUCTION
The pursuit of youthful skin and aesthetic appeal continues to drive nonsurgical aesthetic treatments. Among these, botulinum toxin A (BTxA) is considered a significant treatment modality in the field of facial rejuvenation.1,2
Botulinum toxin A has also demonstrated a wide range of therapeutic potentials such as hyperhidrosis and dystonia, spasticity, overactive bladder, and migraines, providing relief and improving quality of life for patients.3,4
A wealth of clinical studies and patient experiences have highlighted its effectiveness in reducing wrinkles and fine lines.5 Recently, intradermal (often referred to as “microbotox,” “mesobotox,” “babybotox,” and “microdroplets”) injection of BTxA for skin rejuvenation; skin texture, tone, and elasticity; and the delicate erasure of time’s footprints beckons aesthetic practitioners’ interest again to what was pioneered by Woffles Wu.6–11 Despite widespread use and acceptance of this treatment in facial aesthetics, much of the supporting evidence is anecdotal or derived from studies of lower methodological quality.
This systematic review and meta-analysis thus aimed to assess the effectiveness of BTxA on the enhancement of skin quality attributes and facial rejuvenation. Furthermore, traditional meta-analyses may face the risk of reaching premature conclusions due to random errors and potential bias. By incorporating trial sequential analysis (TSA), these risks can be mitigated to enhance the quality and reliability of the findings.12,13
MATERIALS AND METHODS
This systematic review with meta-analyses was conducted in accordance with the Systematic Reviews and Meta-Analysis (PRISMA) guidelines.14,15 The review was based on recommendations outlined in the Cochrane Handbook of Systematic Reviews of Interventions.16 A concise description of the study protocol was registered to the International Prospective Register of Systematic Reviews (PROSPERO) (www.crd.york.ac.UK/prospero, record ID: CRD42023388601).
Data Sources and Search Strategy
The search began in October 2022, updated in March 2023, across databases like PubMed/MEDLINE, Embase, Cochrane CENTRAL, CBM, Web of Science, CNKI, VIP, and Wanfang, covering literature from January 2008 to March 2023. Online registries and grey literature sources, including ClinicalTrials.gov and OpenGrey, were also reviewed for unpublished trials. Search terms related to BTxA and its effects on facial rejuvenation were used, combining free text and index terms with Boolean operators for efficiency. The detailed search strategy is presented in Supplemental Digital Content 1, and references of retrieved articles were manually checked for more studies. (See table, Supplemental Digital Content 1, which displays the search strategy. http://links.lww.com/PRSGO/D440.)
Study Selection Criteria
Studies were included if they met the eligibility criteria described as PICOS: P (patients): general healthy population requiring facial rejuvenation; I (intervention): intradermal (and the associated terms) BTxA; C (control): none; O (outcome): assessing the overall effect of BTxA on facial rejuvenation, namely skin quality, sebum production, skin hydration, pore size, erythema index, facial wrinkle, skin texture and elasticity and facelift; S (study design): human-based clinical trials and prospective cohort studies published in English only. Articles exclusively focused on one area of the face were also excluded.
Two authors (E.R. and P.R.) independently screened the titles and abstracts and removed duplications. Studies searched were exported to the EndNote Reference Library software version 20.0.1 (Clarivate Analytics).
Full texts of potentially useful articles were reviewed in their entirety, and any discrepancies and disagreements were addressed and resolved by a third author (J.C.).
Outcomes of Interest and Outcome Measure
The outcomes of interest were sebum production, pore size, skin hydration, skin texture, erythema index, wrinkles and overall facelift. The detailed outcomes of interest and outcome measures are presented in Table 1.
Table 1.
Outcomes of Interest and Outcome Measures
| Outcomes | Outcome of Interest | Outcome Measures |
|---|---|---|
| Sebum production | Reduction in excessive sebum production | Sebumeter measurements to quantify sebum levels on the skin surface, commonly expressed as sebum units (µg/cm2) |
| Skin hydration | Enhancement of skin hydration levels | Corneometer measurements to assess skin hydration by measuring the skin’s capacitance, presented as arbitrary units (AU) |
| Pore size | Reduction in visible pore size | Quantitative analysis of pore size using imaging techniques or microtopography-based measurements expressed in millimetres or as a percentage change |
| Erythema index | Reduction in skin redness or inflammation | Chromameter or spectrophotometer measurements of erythema index, providing quantitative values based on colorimetry |
| Facial wrinkle | Decrease in the severity and depth of facial wrinkles | Objective evaluation using three-dimensional imaging systems or subjective assessment using validated scales like the Wrinkle Severity Rating Scale (WSRS) |
| Skin texture and elasticity | Improvement in skin smoothness and elasticity | Quantitative assessment using imaging techniques, such as fringe projection or cutometer measurements, providing parameters like roughness, elasticity, or firmness |
| Facelift | Enhancement in facial contour and lifting effect | Visual assessment by trained evaluators, objective measurements using validated grading scales (eg, Lemperle scale), or three-dimensional imaging techniques to analyze volumetric changes and facial symmetry |
Data Extraction
Two authors (E.R. and P.R.) independently extracted data with a predesigned data extraction form, in which study characteristics (first author, publication year and country), participant characteristics, (including the number of participants in the intervention and control group), intervention characteristics (the total dose/dilution of BTxA injection), and outcomes of interest were included.
Quality Assessment of Studies
Two authors (E.R. and P.R.) evaluated each study’s methodological quality using the Cochrane risk of bias assessment.17,18 To ensure consensus, a third author (J.C.) was consulted to resolve any disagreements. The risk of bias was assessed based on criteria such as random sequence generation, allocation concealment, participant and personnel blinding, outcome assessment blinding, incomplete outcome data, selective outcome reporting, and other biases. Inter-rater reliability was calculated using kappa values, indicating quality from very good to poor. For test-retest reliability, authors reassessed half their articles one month later to reduce recall bias.19
The Newcastle-Ottawa Scale (NOS) was used to assess the quality of the cohort studies. NOS score 1–5 was considered a high risk for bias, 6–7 was moderate, and a score of more than 7 was considered a low risk of bias.20
Grading the Quality of Evidence
The assessment of each outcome measure was conducted by two authors (P.R. and E.R.) using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach, as outlined in the GRADEpro Guideline Development Tool.21 The inter-rater agreement for myelination and sulcation was measured using a free-marginal kappa statistic, with a value of к more than 0.7 indicating high reliability.21
Publication Bias
Funnel plots were generated, and bias assessment was conducted using Egger bias statistics.22 If evidence of publication bias was present, the trim and fill method was used to address this.23
Statistical Analysis
Review manager (“Revman”) for Mac (version 5.4.1, Cochrane Collaboration, Oxford, United Kingdom) was used for all statistical analyses. The data from studies were pooled using a random-effects model. Results were analyzed by analyzing standard mean differences (SMDs) with their respective 95% confidence intervals (CIs). Random-effects meta-analysis (DerSimonian and Laird) and fixed-effects meta-analysis (Mantel-Haenszel) were used to calculate the effect, and a more conservative point estimate of the two was used for the final reporting.24–26 Sensitivity analysis was done to see if any individual study was driving the results and to implore reasons for high heterogeneity. As per Higgins et al, the scale for heterogeneity was considered.27,28 Finally, TSA was conducted to determine the required information size (RIS) and the cumulative Z-curve’s breach of important trial sequential monitoring boundaries.12,13,29
Patient and Public Involvement
There was no patient or public involvement in the design or reviewing process.
Deviation from the Protocol
There was no deviation from the protocol.
RESULTS
Study Selection Process
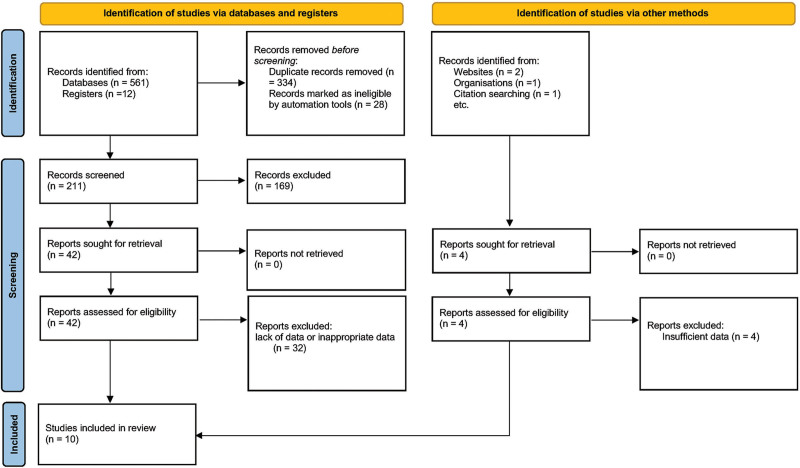
The search strategy yielded a total of 577 findings. After initial screening, 211 unique articles remained. Among these, 169 articles were excluded due to their study design, which included narrative reviews, meta-analyses, and case studies. Of the remaining 42 studies, 32 were excluded due to lack of data or inappropriate data. Finally, five randomized controlled trials (RCTs)30–34 and five prospective studies35–39 were deemed suitable for the quantitative analysis (n = 10; Fig. 1).
Fig. 1.
PRISMA flow diagram.
The PRISMA checklist has also been included as Supplemental Digital Content 2 to ensure adherence to reporting guidelines.40 (See table, Supplemental Digital Content 2, which displays the PRISMA checklist. http://links.lww.com/PRSGO/D441.)
Characteristics of the Included Studies and Subjects
A total of 153 participants were included in these studies. Eight studies were split face design, and at least one arm was treated with BTxA. Mean age of the participants was 41 (30–54) years; 92.1% of all participants were women. OnabotulinumtoxinA [(ONA); Botox; Allergan Aesthetics, an AbbVie Company, Irvine, Calif.] was utilized in three studies,30,33,35 whereas abobotulinumtoxinA [(ABO); Dysport; Medicis Aesthetics, Scottsdale, Arizona; Azzalure, Galderma Laboratories, Lausanne, Switzerland (outside of the United States)] was used in three studies.33,37,38 IncobotulinumtoxinA [(INCO); Xeomin, Merz, Frankfurt am Main, Germany],34 prabotulinumtoxinA [(PRABO); Jeuveau, Evolus, Inc. Newport Beach, Calif.],31letibotulinumtoxinA [(LETIBO) Botulax, Hugel America, Inc],36 Botulinumtoxin A [(REFI) Refinex; KC Pharmaceuticals, Pomona, California],32 and lanbotulinumtoxin A [(LANBO) Hengli, Prosigne, Lantox, Lazox, Redux, Liftox, HBTX-A and CBTX-A, Lanzhou Institute of Biological Products, Lanzhou, China]39 each appeared in one study (Table 2).
Table 2.
Characteristics of the Included Studies
| No. | Author | Year | Type of Study | Botulinum Toxin A Type | Dose/Dilution | Outcome (s) of Interest | Outcome Measure(s) | Total No. Patients Recruited |
|
|---|---|---|---|---|---|---|---|---|---|
| Intervention | Control | ||||||||
| 1 | Chang et al32 | 2008 | Prospective | ONA | 100 U in 10 mL NS (0.02 mL per spot); 20–25 U into one half of the face. | Facelift, skin tightness and wrinkles. | Photograph numeric scale; Haematoxylin and eosin (H&E) stain, Masson trichrome stain, elastin (Verhoeff-van Gieson) stain and type I procollagen and immunohistochemical stains. |
9 hemiface |
9 hemiface |
| 2 | Kapoor et al27 | 2010 | Interventional, comparative, split face clinical trial. | ONA | 2 U/0.1mL; 30 facial injections on half of the face, each 0.1mL. | Face rejuvenation: skin texture, skin tightness, pore size, sebum production. |
Blinded observers’ clinical assessment and pre and post photographic comparison. | 10 hemiface |
10 hemiface |
| 3 | Jun et al33 | 2018 | Double-blinded, split-face, pilot study (intradermal versus intramuscular) | Botulax | 0.05 mL and 2 U per spot; total 5 spots per side of the forehead. |
Forehead wrinkle reduction. | Photographic review, wrinkle score was graded on a five-point scale. |
3 hemiface |
3 hemiface |
| 4 | Kim et al28 | 2019 | Prospective, randomized, double-blind, split face |
PRABO | 1 U per 0.1 mL. a total of 15 U of was injected intradermally into one cheek. | Facial erythema, skin hydration, trans epidermal water loss, melanin content, erythema index, elasticity, and sebum production. |
Clinician Erythema Assessment score, Global Aesthetic Improvement Scale score, corneometer/ mexameter/ reviscometer/sebumeter. | 23 subjects | 23 subjects |
| 5 | Sayed et al29 | 2019 | Split face-controlled pilot study | Botulinum toxin A (Refinex) | The 100 unit reconstituted with 5 mL NS to achieve a 2 U/0.1 mL; total dose injected per patient was 10 U. | Pore size, sebum production. | Digital Photography, dermoscopic evaluation, optic coherence topography evaluation. | 20 hemiface | 20 hemiface |
| 6 | Sapra et al30 | 2017 | Single-blind, split-face, randomized, pilot study |
ONA/ ABO | 100 units of ONA reconstituted with 5cc of NS/ 300 units of ABO was reconstituted with 6cc of NS. Total ONA units(u) in one half of the face: 76.5; total ABO units(u) in one half of the face: 189.5. |
Severity of wrinkles, pore size, skin texture, skin tightness, degree of lift or droop, and sebum production. |
Blinded evaluator using baseline and posttreatment photographs using Visia Complexion Analysis System and Vectra3D (Canfield Scientific, Inc, Fairfield, N.J.); self-reported satisfaction questionnaire. |
10 hemiface |
10 hemiface |
| 7 | Shin et al31 | 2022 | Prospective, double-blind, randomized, split face. |
INCO | A single injection volume of 0.025 mL (0.5 U per spot) injected intradermally, no more than 20 U. |
Severity of wrinkles, pore size, skin texture and sebum production. |
Pre and post digital photography comparison; sebumeter; Lemperle wrinkle score. | 18 hemiface |
18 hemiface |
| 8 | Sirithanabadeekul et al34 | 2018 | Single-center, prospective, randomized, double-blind, pilot, split face study |
ABO | Dilution of 15 mL to give 3.33 units per 0.1 mL/dilution of 7.5 mL to give 6.67 units per 0.1 mL; volume of injection was 1.5 mL/side (total of 100 units on the common concentration side and 50 units on the double diluted side). |
Facelift | Photographic images using Visia (Canfield Scientific, Inc., Fairfield, N.J.). | 10 hemiface |
10 hemiface |
| 9 | Wanitphakdeedecha et al35 | 2020 | Prospective observational study | ABO | Dilution of 1 vial:7 mL (500 U in 7 mL of NS); 0.04 mL, was injected per point. | Facelift | Photographic documentation was obtained using a two-, and three-dimensional imaging system, Vectra H1, (Canfield Scientific, Inc, Fairfield, N.J.). |
30 Hemiface |
30 Hemiface |
| 10 | Zhu et al36 | 2017 | Prospective observational study | BoNTA powder (HENGLI) |
30 U BoNTA (diluted in 2.6 mL saline solution, concentration at 12.5 U/mL); 2 µL in every point. |
Erythema, melanin, trans epidermal water loss, elasticity, skin surface roughness, hydration. | Subjective satisfaction scale, corneometer/mexameter/ reviscometer/sebumeter. | 20 Subjects | 20 Subjects |
Outcomes of Interest
Four articles explored the effect on sebum production,30–32,34 four on pore size,30,32–34 two on skin hydration,31,39 five on skin texture,30,31,33,34,39 two on erythema index,31,39 four on wrinkles,33–36 and four on overall facelift33,35,37,38 following intradermal BTxA injection.
Dose and Dilution
Studies showed varied dilutions and doses across different BTxA products. For instance, Chang et al administered 20–25 U on half of the face using a 100 U in 10 mL normal saline (NS) solution, equating to 0.02 mL per spot. Kapoor et al and Jun et al used 2 U per 0.1 mL and 0.05 mL (2 U) per spot, respectively, across different facial areas. Kim et al injected 15 U intradermally into one cheek, whereas Sayed et al used 10 U per patient with a 2 U per 0.1 mL concentration. Sapra et al compared 76.5 units of ONA and 189.5 units of ABO on different face halves with specific dilutions. Shin et al limited their dose to 20 U total, Sirithanabadeekul et al offered two dilution options for their injections, and Wanitphakdeedecha et al used a dilution resulting in 500 U in 7 mL of NS. Zhu et al applied 30 U of BTxA with a concentration of 12.5 U per mL. This variability underscores the lack of standardization in BTxA application techniques (Table 2).
Quality of the Evidence
Risk of Bias Assessment
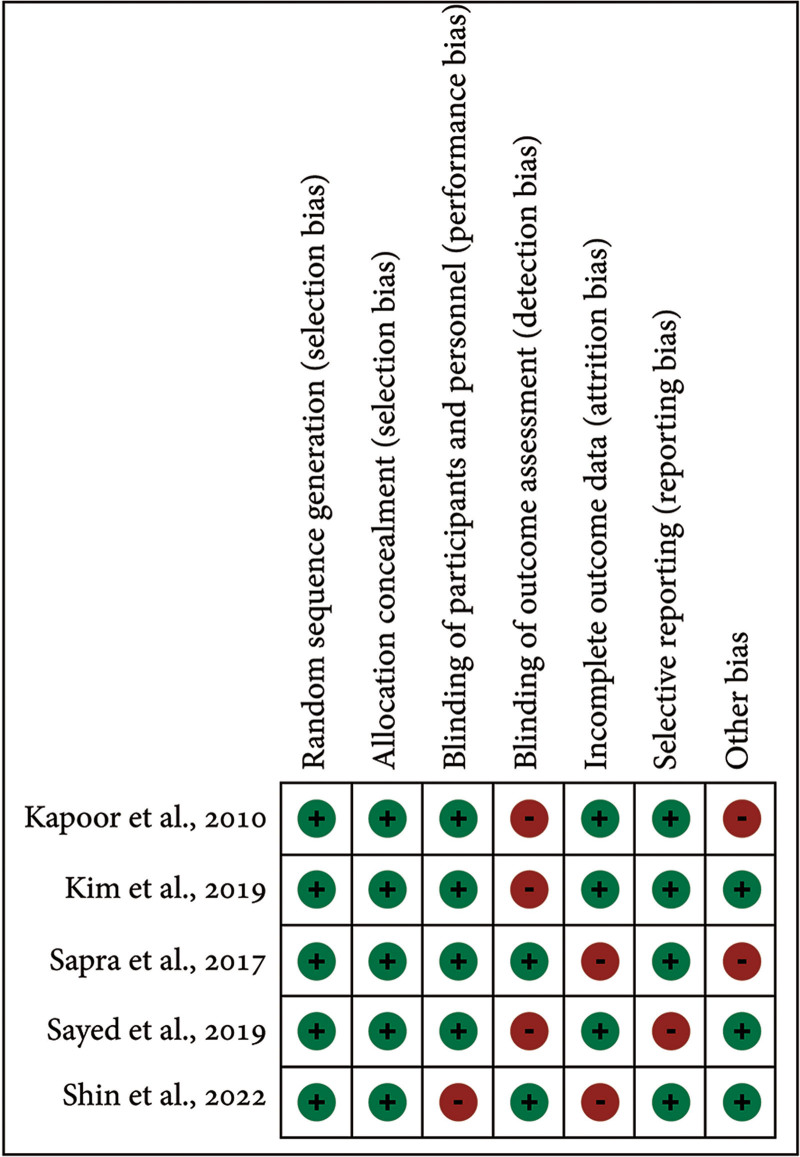
The included RCTs were classified as moderate risk. One study exhibited bias in the blinding of the participants and personnel,34 three in the blinding of outcome assessment,30–32 two in incomplete outcome data,33,34 one in selective reporting,32 and two in other domains30,33 (Figs. 2 and 3).
Fig. 2.
Risk of bias summary: review of authors’ judgements about each risk of bias item for included RCTs.
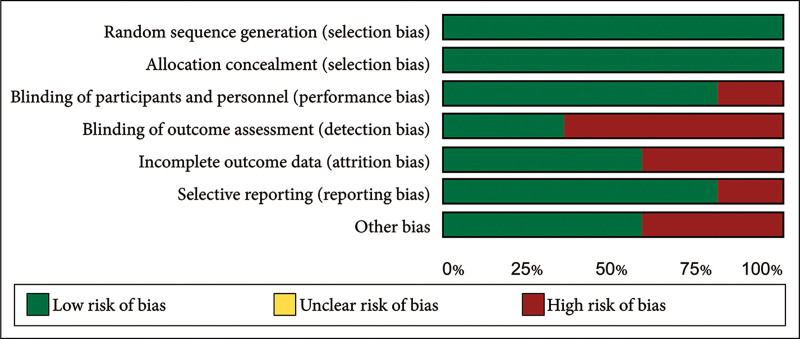
Fig. 3.
Overall risk of bias graph presented as percentages across all included RCTs.
All studies showed a strong methodological approach with a low risk of bias in random sequence generation and allocation concealment. Blinding of participants and personnel had a low risk of bias (20%), whereas blinding of outcome assessment showed a high risk (60%). About 60% of studies had a low risk, and 40% had a high risk of attrition bias. Selective reporting bias was low overall (20%), with comprehensive outcome reporting. Other biases were low in 60% of studies and high in 40%. The NOS assessed the quality of five other studies. Four studies were of good quality based on patient selection, three had excellent comparability, and four showed good quality in outcome/exposure assessment (Table 3).
Table 3.
Quality Reporting and Commercial Sponsorship Disclosures
| No. | Author | Year | Botulinum Toxin A Type | ROB | Newcastle-Ottawa Score | GREDE Evidence Profile | Commercial Sponsor |
|---|---|---|---|---|---|---|---|
| 1 | Chang et al32 | 2008 | ONA | — | Low | Low | Allergan, Taiwan |
| 2 | Kapoor et al27 | 2010 | ONA | Moderate | — | Low | None |
| 3 | Jun et al33 | 2018 | Botulax | — | Low | Low | None |
| 4 | Kim et al28 | 2019 | PRABO | Moderate | — | Low | Daewoong Pharmaceutical |
| 5 | Sayed et al29 | 2019 | Botulinum toxin A (Refinex) | Moderate | — | Low | None |
| 6 | Sapra et al30 | 2017 | ONA/ ABO | Moderate | — | Moderate | None |
| 7 | Shin et al31 | 2022 | INCO | Moderate | — | Moderate | Medytox, Korea |
| 8 | Sirithanabadeekul et al.34 | 2018 | ABO | — | Low | Low | None |
| 9 | Wanitphakdeedecha et al35 | 2020 | ABO | — | Moderate | Low | None |
| 10 | Zhu et al36 | 2017 | BoNTA powder (HENGLI) |
— | Moderate | Low | None |
GRADE Assessment and Reliability Statistics
For GRADE, all κs were very good (0.88). Test-retest reliability following a 1-month interval between assessments showed the same result (Table 3).
Sponsorship Disclosure
Out of the 10 included trials, only three were commercially sponsored. The study conducted by Chang et al32 was supported by Allergan Pharmaceuticals, Taiwan; Kim et al28 was sponsored by Daewoong Pharmaceutical, Korea; and lastly, Shin et al31 was sponsored by Medytox, Korea (Table 3).
Publication Bias
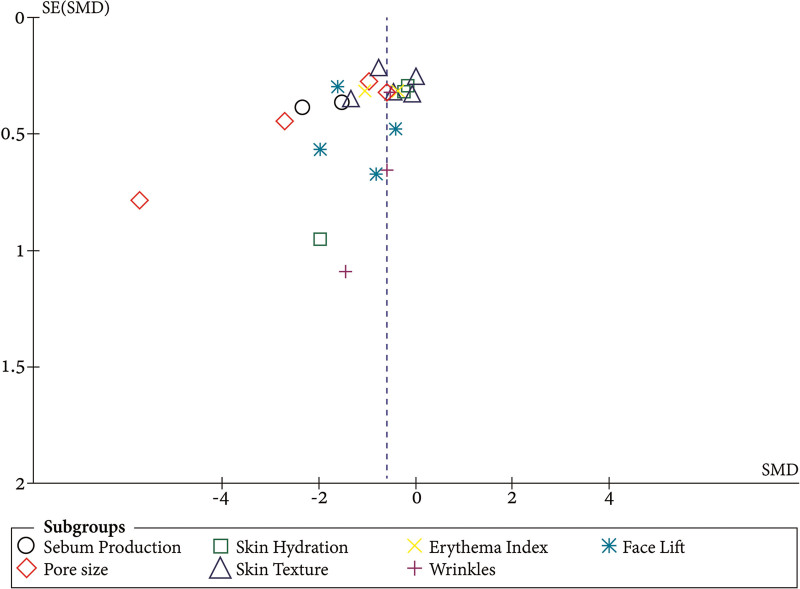
There was no evidence of publication bias despite the small number of studies included. The funnel plots of studies included in the pore size showed a symmetrical shape with an outlier, which indicates true heterogeneity rather than publication bias (Fig. 4).
Fig. 4.
Funnel plot for the publication bias of the included studies. SE: Standard error.
Results of Meta-analysis
On the Skin Quality Attributes
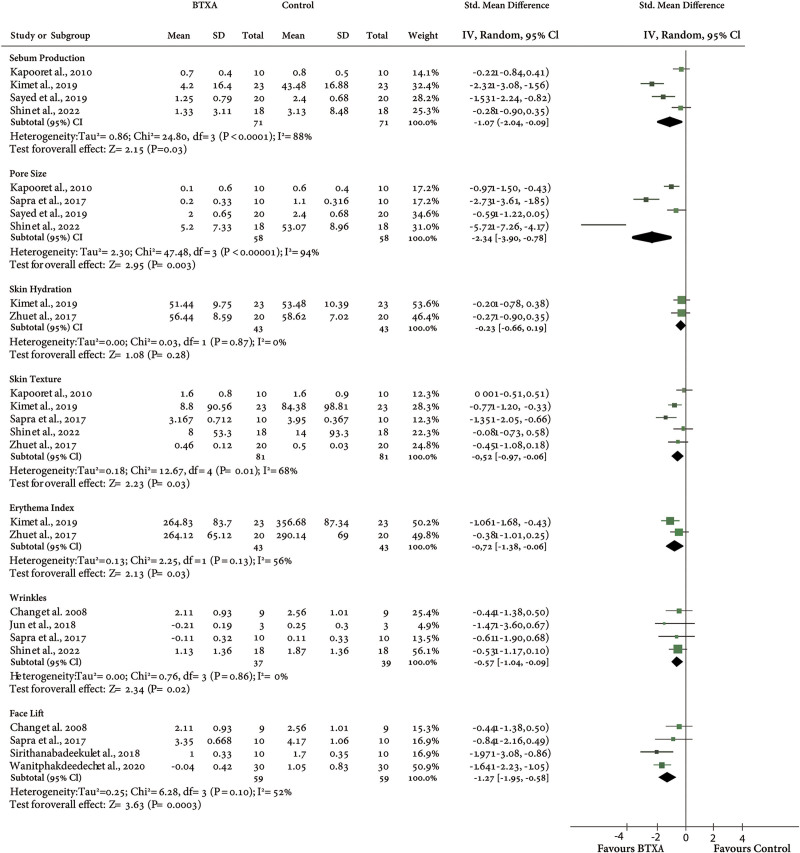
The meta-analysis of the sebum secretion shows a significant reduction in sebum production compared with the saline injection side [SMD = − 1.07, 95% CI; (− 2.04 to − 0.09), P = 0.03 and z score = 2.15]. The heterogeneity among the included studies was significant and moderately high [I2 = 88%, P < 0.0000].
There was a significant improvement for the pore size subgroup [SMD = − 2.34, 95% CI −3.90 to 0.78, P = 0.003]. A moderately high and significant heterogeneity was found [I2 = 94%, P < 0.00001].
For skin hydration and skin texture, the injection of BTxA does not improve skin hydration compared with the control side [SMD = − 0.23, 95% CI; − 0.66, 0.19, P = 0.26], but significantly improved skin texture [SMD = −0.52, 95% CI; −0.97, −0.06, P = 0.03] with moderate heterogeneity percentage (I2 = 68%].
For the meta-analysis of erythema index, wrinkles and facelift were all significantly improved after the injection of BTxA in comparison with the control (erythema index; SMD = − 0.72, 95% CI; − 1.38, − 0.06, P = 0.03), (wrinkles; SDM = -0.57, 95% CI; -1.04, -0.09, P = 0.02), (facelift; SMD = − 1.27, 95% CI; − 1.95, − 0.58, P = 0.0003) (Fig. 5).
Fig. 5.
Comparison of BTxA with control for the skin attribute changes. IV, inverse variance.
On the Outcome with Different BTxA Products
ONA, PRABO, REFI, and INCO explored the effect on sebum production,30–32,34; ONA, REFI, and INCO on pore size30,32–34; ONA, ABO, INCO, and LANBO on skin texture30,31,33,34,39; LANBO and PRABO on erythema index31,39; ONA, ABO, LETIBO, and INCO on wrinkles33–36; and finally, ONA and ABO reported overall facelift.33,35,37,38 Further analysis revealed no statistical difference in the outcome neither for the product (SMD = − 0.29, 95% CI; − 0.72, 0.32, P = 0.42), nor dilution (SMD = − 0.28, 95% CI; − 0.76, 0.34, P = 0.34), with high heterogeneity (I2 = 86%, P < 0.0001).
Sensitivity Analysis
A sensitivity analysis was conducted by excluding one study at a time from the three sponsored trials (Chang et al32; Kim et al28; Shin et al31), followed by the generation of pooled SMD for the rest of the studies. No significant changes were observed, suggesting the results were robust.
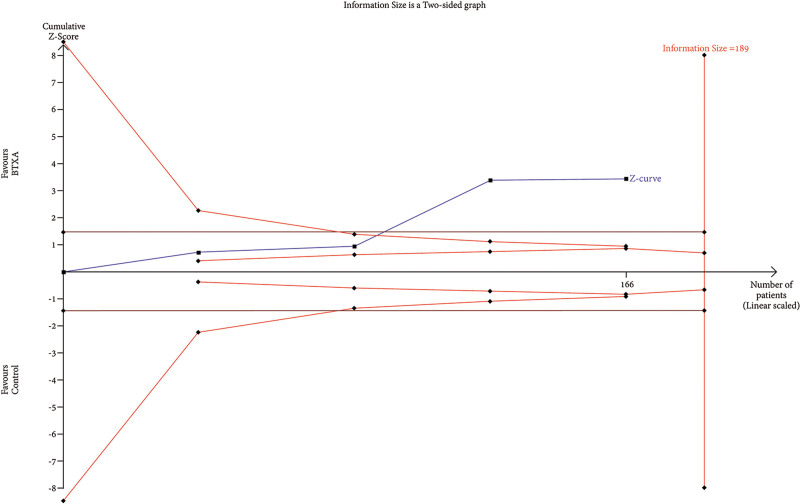
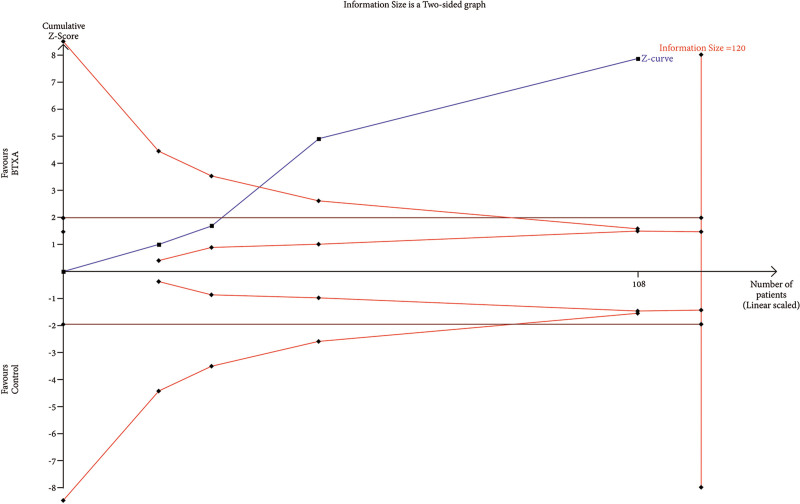
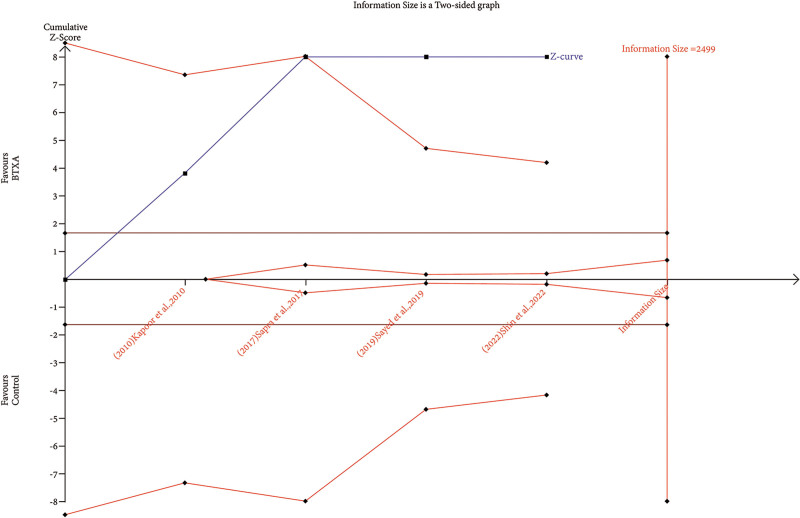
Trial Sequential Analysis
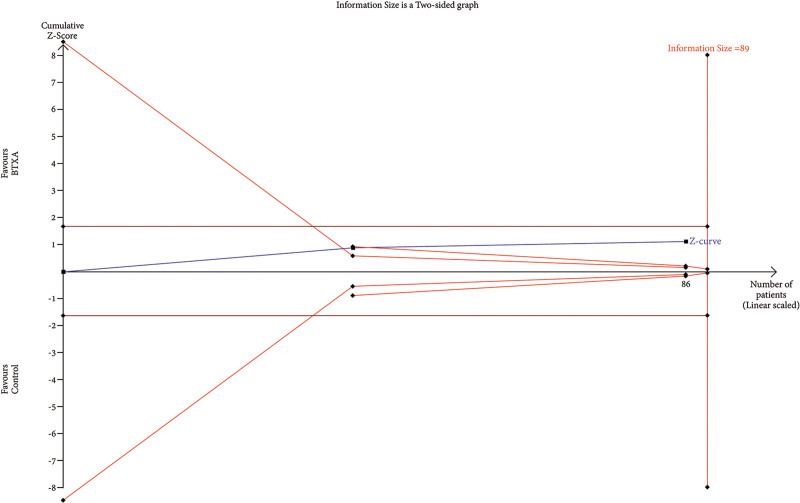
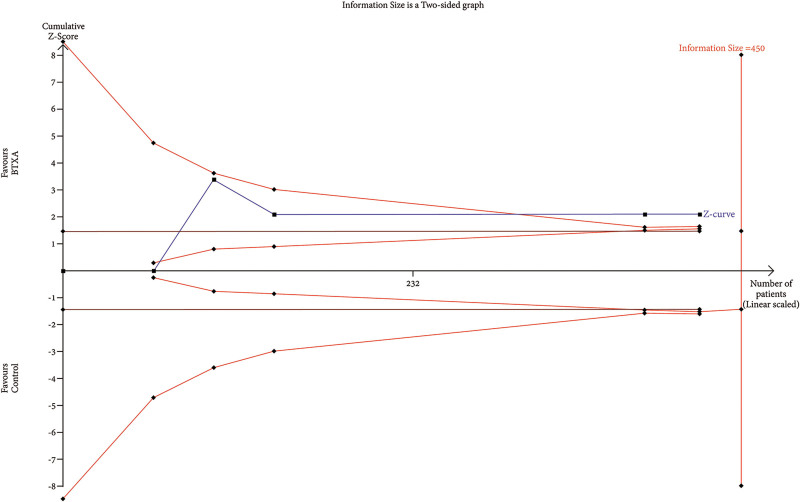
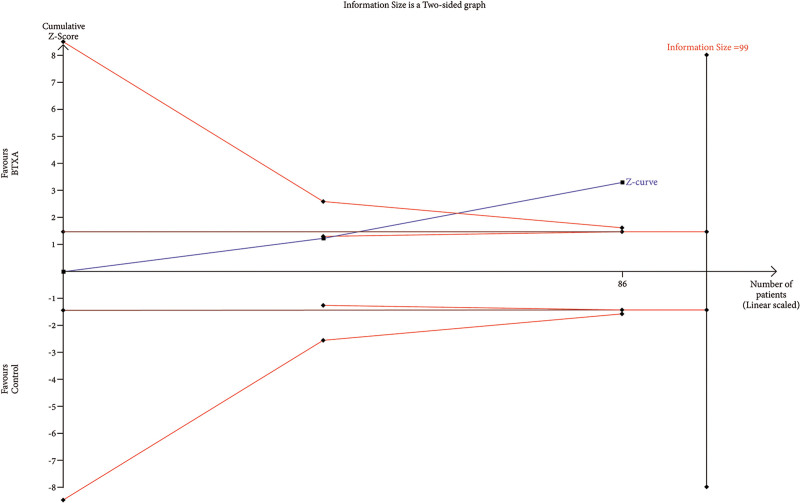
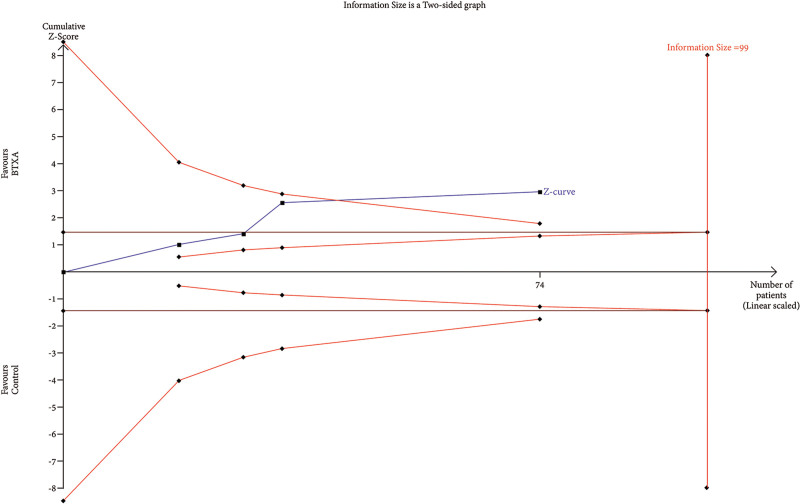
RIS data were estimated using a variance-based model with a significance level (α) of 0.05 and a power (β) of 0.20. The cumulative Z-curve for each parameter, as depicted in Figures 6–12, reveals that it crossed the trial sequential monitoring boundary for beneficial effects. However, it did not reach the required level of RIS, indicating that the available evidence is currently insufficient to draw definitive conclusions (Figs. 6–12).
Fig. 6.
TSA of trials explored the effectiveness of BTxA in the reduction of sebum production. The cumulative Z-curve has not crossed the RIS (189) but crossed conventional benefit boundary.
Fig. 12.
TSA of trials explored the effectiveness of BTxA in facelift. The cumulative Z-curve has not crossed the RIS (120) but crossed conventional benefit boundary.
Fig. 7.
TSA of trials explored the effectiveness of BTxA in the reduction of pore size. The cumulative Z-curve has not crossed the RIS (2499) but crossed conventional benefit boundary.
Fig. 8.
TSA of trials explored the effectiveness of BTxA in improvement of skin hydration. The cumulative Z-curve has not crossed the RIS (89) but crossed conventional benefit boundary and touched area of futility, indicating that any future meta-analysis is unlikely to show a significant difference, even though more clinical trials may be conducted until the RIS is achieved.
Fig. 9.
TSA of trials explored the effectiveness of BTxA in improvement of skin texture. The cumulative Z-curve has not crossed the RIS (450) but crossed conventional benefit boundary.
Fig. 10.
TSA of trials explored the effectiveness of BTxA in improvement of erythema index. The cumulative Z-curve has not crossed the RIS (99) but crossed conventional benefit boundary.
Fig. 11.
TSA of trials explored the effectiveness of BTxA in improvement of wrinkles. The cumulative Z-curve has not crossed the RIS (99) but crossed conventional benefit boundary.
DISCUSSION
The analysis revealed a significant reduction in sebum production, facial pores, erythema, wrinkle improvement, and facelift effects, but results for skin hydration were inconclusive across various BTxA products and dilutions. Despite these findings, none met the required RIS boundary for conclusive evidence, indicating the need for more RCTs to confirm these outcomes.
The observed variability in BTxA doses and dilutions across studies highlights a lack of standardized treatment protocols for facial rejuvenation, complicating direct comparisons due to differing efficacy, result longevity, and side effects. This inconsistency impacts clinical and economic aspects, influencing treatment frequency, costs, and challenges in achieving consistent outcomes. The absence of standardization also obscures the treatment’s ideal efficacy-safety balance.
Numerous in vivo and in vitro studies have posited elaborate mechanisms elucidating the enhancement of various skin quality attributes, especially sebum production, after the intradermal administration of BTxA.41–53
Within the sebaceous glands, two primary receptor types, muscarinic acetylcholine (mAChR) and nicotinic acetylcholine (nAChR), are involved; m2AChR is expressed in the suprabasal sebocytes, whereas the α7nAChR is highly immunoreactive in the ductal cells of the sebaceous glands.46,54,55 ACh, interacting with α7nAChR, contributes to lipid synthesis in sebocytes by activating ERK signaling. This interaction potentially promotes sebocyte differentiation, as mature sebocytes exhibit high levels of α7nAChR expression.41,52,54
BTxA can influence the release of vascular endothelial growth factor, which affects vasodilation, and it can inhibit the release of cathelicidin and other inflammatory mediators. Additionally, it has been observed that BTxA may inhibit mast cell degranulation, thereby promoting an antiinflammatory effect. Substance P and calcitonin gene-related peptides, which are involved in the regulation of inflammation, are also thought to contribute to the alleviation of facial flushing.54,56
The exact way BTxA reduces pore size is not fully understood, but it may involve decreasing sweat and sebaceous gland activity and relaxing muscles, which indirectly might shrink pores. Its antiinflammatory effects could also reduce inflammation around pores, and BTxA may help remodel the skin’s structure, leading to smaller pores by lessening skin stretch.
Despite focusing on prospective trials, noteworthy retrospective studies exist. Park et al assessed intradermal incobotulinumtoxin A’s impact on sebum secretion, face laxity, and facial pores. They observed significant reductions within 1 week, sustaining through 12 weeks, with peak improvements at week 4.57 A study conducted by Rose et al showed significant improvement in sebum production of the forehead in patients with oily skin.58 A retrospective clinical analysis by Shah et al with 20 patients demonstrated that intradermal injection of BTxA significantly reduces (17 of 20 patients) sebum production and pore size within 4 weeks of treatment.59
Our study found that BTxA did not significantly improve skin hydration, lacking a direct mechanism and solid clinical proof for this effect. Research has since explored using hyaluronic acid alongside or after BTxA to address hydration. Applying hyaluronic acid before BTxA may improve treatment distribution and longevity, aiding in skin restoration and potentially enhancing BTxA benefits.60
This is the most recent systematic review and meta-analysis, including TSA on the efficacy of intradermal BTxA on skin quality, to our knowledge. Key strengths include PROSPERO registration for transparency, strict inclusion and exclusion criteria for precise study selection, and thorough statistical analysis using TSA and multilevel sensitivity analysis for strong findings. Additionally, comparing meta-analysis results with real-world evidence provides a complete view for healthcare professionals.61,62
However, statistical significance does not always mean noticeable benefits in real life. Clinical outcomes of BTxA vary due to individual factors, dosage, and dilution, requiring personalized treatment plans. This variability can affect results outside controlled studies. Therefore, some patients may see minor improvements, whereas others expect more significant changes. Clinicians should set realistic expectations.
Limitations in our systematic review and meta-analyses should be acknowledged. Small sample sizes for most outcomes hinder broader applicability. Variability in outcome definitions introduces potential reporting bias. Heterogeneity in studies, attributed to diverse BTxA types, dilution protocols, study populations, and follow-up durations, complicates the analysis. Despite these challenges, we conducted sensitivity, subgroup, and trial sequential analyses to validate our meta-analysis results.
Future studies should prioritize larger sample sizes for enhanced statistical power and generalizability. Standardizing outcome definitions is crucial, and researchers must address heterogeneity through standardized treatment protocols, including dose, dilution, extending follow-up, and uniform inclusion criteria.
CONCLUSIONS
Despite promising results following intradermal BTxA for facial rejuvenation and the popularity of practice, this study emphasizes the need for more RCTs to validate these findings. Such measures will contribute to illuminating the path forward, marking the journey from the era of eminence to the dawn of evidence-based practice in aesthetic medicine.
DISCLOSURES
Dr. Rahman worked as a consultant and speaker for Allergan Aesthetics (Irvine, Calif.); Dr. Rao, Dr. Garcia, Dr. Ioannidis, Dr. Kefalas, Dr. Kajaija, Dr. Friederich, and Dr. Parikh are speakers for Allergan aesthetics an Abbvie Company (Irvine, Calif.); Dr. Philipp-Dormston reports being a clinical trial investigator, scientific advisor, and/or speaker for Allergan Aesthetics an Abbvie Company (Irvine, Calif.), Galderma (Lausanne, Switzerland), and Merz Pharmaceuticals GmbH; Dr. Almeida has been a consultant to Allergan Inc. and Merz, and participated in clinical trials for Allergan and Galderma. Dr. J. Carruthers is a consultant and investigator for Allergan Aesthetics, an Abbvie Company, Merz Pharmaceuticals GmbH, Solstice Neurosciences, and Revance, Inc. Dr. A. Carruthers is a consultant for and has received research grants from Allergan Aesthetics an Abbvie Company (Irvine, Calif.), Merz Pharmaceuticals GmbH, and Revance, Inc; Dr. Mosahebi served a consultant and speaker for Allergan Aesthetics an Abbvie Company (Irvine, Calif.), Merz Pharmaceuticals GmbH; Dr. Wu is a speaker and consultant for Allergan Aesthetics an Abbvie Company, Merz Pharmaceuticals GmbH; Dr. Goodman is a speaker, investigator, and consultant for Allergan Aesthetics an Abbvie Company. (Irvine, Calif.) and Galderma (Lausanne, Switzerland). All the other authors have no financial interest to declare in relation to the content of this article.
Supplementary Material
Footnotes
Published online 23 August 2024.
Disclosure statements are at the end of this article, following the correspondence information.
Related Digital Media are available in the full-text version of the article on www.PRSGlobalOpen.com.
Drs. Rahman and Rao contributed equally to this work.
REFERENCES
- 1.França K, Kumar A, Fioranelli M, et al. The history of botulinum toxin: from poison to beauty. Wien Med Wochenschr. 2017;167:46–48. [DOI] [PubMed] [Google Scholar]
- 2.Erbguth FJ. From poison to remedy: the chequered history of botulinum toxin. J Neural Transm. 2008;115:559–565. [DOI] [PubMed] [Google Scholar]
- 3.Simpson L. The life history of a botulinum toxin molecule. Toxicon. 2013;68:40–59. [DOI] [PubMed] [Google Scholar]
- 4.Setler PE, Riley T. Therapeutic use of botulinum toxins: background and history. Clin J Pain. 2002;18:S119-S124. [DOI] [PubMed] [Google Scholar]
- 5.Rahman E, Mosahebi A, Carruthers JDA, et al. The efficacy and duration of onabotulinum toxin A in improving upper facial expression lines with 64-unit dose optimization: a systematic review and meta-analysis with trial sequential analysis of the randomized controlled trials. Aesthet Surg J. 2023;43:215–229. [DOI] [PubMed] [Google Scholar]
- 6.Wu WTL. Skin resurfacing with microbotox and the treatment of keloids. In: Benedetto AV, ed. Botulinum Toxins in Clinical Aesthetic Practice. 2nd ed. London: Informa Healthcare; 2011:190–205. [Google Scholar]
- 7.Wu WTL. Microbotox of the lower face and neck: evolution of a personal technique and its clinical effects. Plast Reconstr Surg. 2015;136:92S–100S. [DOI] [PubMed] [Google Scholar]
- 8.Fabi SG, Park JY, Goldie K, et al. Microtoxin for improving pore size, skin laxity, sebum control, and scars: a roundtable on integrating intradermal botulinum toxin type A microdoses into clinical practice. Aesthet Surg J. 2023;43:1015–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu WTL. Reply: microbotox of the lower face and neck: evolution of a personal technique and its clinical effects. Plast Reconstr Surg. 2016;138:1073e–1074e. [DOI] [PubMed] [Google Scholar]
- 10.Wu WTL. Nonsurgical facial rejuvenation with the 4R principle: innovative uses of BOTOX and facelifting with the woffles lift, a barbed suture Sling. In: Aesthetic Surgery of the Facial Mosaic. Berlin: Springer; 2007. [Google Scholar]
- 11.Tonnard PL, et al. Part II: the place of toxins and fillers in the modern plastic surgery practice: chapter 11 The Microbotox Technique. (n.d.). In: Centrofacial Rejuvenation. [Google Scholar]
- 12.Kulinskaya E, Wood J. Trial sequential methods for meta-analysis. Res Synth Methods. 2014;5:212–220. [DOI] [PubMed] [Google Scholar]
- 13.Wetterslev J, Jakobsen JC, Gluud C. Trial sequential analysis in systematic reviews with meta-analysis. BMC Med Res Methodol. 2017;17:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hutton B, Salanti G, Caldwell DM, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162:777–784. [DOI] [PubMed] [Google Scholar]
- 15.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. J Clin Epidemiol. 2021;134:178–189. [DOI] [PubMed] [Google Scholar]
- 16.Higgins JPT, Thomas J, Chandler J, et al. Cochrane Handbook for Systematic Reviews of Interventions, version 6.2.; 2021. Updated February 2021. UK: John Wiley & Sons Ltd. [Google Scholar]
- 17.Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. [DOI] [PubMed] [Google Scholar]
- 18.Cumpston M, Li T, Page MJ, et al. Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev. 2019;10:ED000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cohen J. A coefficient of agreement for nominal scales. Educ Psychol Meas. 1960;20:37–46. [Google Scholar]
- 20.Oremus M, Oremus C, Hall GBC, et al. Inter-rater and test-retest reliability of quality assessments by novice student raters using the Jadad and Newcastle-Ottawa scales. BMJ Open. 2012;2:e001368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Egger M, Smith GD, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. Br Med J. 1997;315:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455–463. [DOI] [PubMed] [Google Scholar]
- 24.DerSimonian R, Laird N. Meta-analysis in clinical trials revisited. Contemp Clin Trials. 2015;45:139–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. [DOI] [PubMed] [Google Scholar]
- 26.Demets DL. Methods for combining randomized clinical trials: strengths and limitations. Stat Med. 1987;6:753–760. [DOI] [PubMed] [Google Scholar]
- 27.Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. [DOI] [PubMed] [Google Scholar]
- 28.Higgins JPT, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. Br Med J. 2003;327:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thorlund K, Devereaux PJ, Wetterslev J, et al. Can trial sequential monitoring boundaries reduce spurious inferences from meta-analyses? Int J Epidemiol. 2009;38:276–286. [DOI] [PubMed] [Google Scholar]
- 30.Kapoor R, Shome D, Jain V, et al. Facial rejuvenation after intradermal botulinum toxin: is it really the botulinum toxin or is it the pricks? Dermatol Surg. 2010;36:2098–2105. [DOI] [PubMed] [Google Scholar]
- 31.Kim MJ, Kim JH, Cheon HI, et al. Assessment of skin physiology change and safety after intradermal injections with botulinum toxin: a randomized, double-blind, placebo-controlled, split- face pilot study in rosacea patients with facial erythema. Dermatol Surg. 2019;45:1155–1162. [DOI] [PubMed] [Google Scholar]
- 32.Sayed KS, Hegazy R, Gawdat HI, et al. The efficacy of intradermal injections of botulinum toxin in the management of enlarged facial pores and seborrhea: a split face-controlled study. J Dermatolog Treat. 2021;32:771–777. [DOI] [PubMed] [Google Scholar]
- 33.Sapra P, Demay S, Sapra S, et al. A single-blind, split-face, randomized, pilot study comparing the effects of intradermal and intramuscular injection of two commercially available botulinum toxin a formulas to reduce signs of facial aging. J Clin Aesthet Dermatol. 2017;10:34–44. [PMC free article] [PubMed] [Google Scholar]
- 34.Shin DM, Lee J, Noh H, et al. A double-blind, split-face, randomized study on the effects and safety of intradermal injection of botulinum toxin A (incobotulinum toxin A) in the cheek. Ann Dermatol. 2022;34:442–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chang SP, Tsai HH, Chen WY, et al. The wrinkles soothing effect on the middle and lower face by intradermal injection of botulinum toxin type A. Int J Dermatol. 2008;47:1287–1294. [DOI] [PubMed] [Google Scholar]
- 36.Jun JY, Park JH, Youn CS, et al. Intradermal injection of botulinum toxin: a safer treatment modality for forehead wrinkles. Ann Dermatol. 2018;30:458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sirithanabadeekul P, Lapsomboonsiri S, Rungjang A, et al. Split face comparison between common concentration vs double dilution of intradermal abobotulinum toxin type A (dysport) injection for facial lifting in Asians. J Cosmet Dermatol. 2018;17:355–360. [DOI] [PubMed] [Google Scholar]
- 38.Wanitphakdeedecha R, Yan C, Apinuntham C, et al. Intradermal micro-dosing of abobotulinumtoxinA for face-lifting: how long does it last? Dermatol Ther (Heidelb). 2020;10:779–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhu J, ji X, Xu Y, et al. The efficacy of intradermal injection of type A botulinum toxin for facial rejuvenation. Dermatol Ther. 2017;30:e12433. [DOI] [PubMed] [Google Scholar]
- 40.Rethlefsen ML, Kirtley S, Waffenschmidt S, et al. PRISMA-S: an extension to the PRISMA statement for reporting literature searches in systematic reviews. Syst Rev. 2021;10:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kurzen H, Berger H, Jäger C, et al. Phenotypical and molecular profiling of the extraneuronal cholinergic system of the skin. J Investig Dermatol. 2004;123:937–949. [DOI] [PubMed] [Google Scholar]
- 42.Kurzen H, Wessler I, Kirkpatrick CJ, et al. The non-neuronal cholinergic system of human skin. Horm Metab Res. 2007;39:125–135. [DOI] [PubMed] [Google Scholar]
- 43.Haberberger RV, Pfeil U, Lips KS, et al. Expression of the high-affinity choline transporter, CHT1, in the neuronal and non-neuronal cholinergic system of human and rat skin. J Investig Dermatol. 2002;119:943–948. [DOI] [PubMed] [Google Scholar]
- 44.Grando SA, Pittelkow MR, Schallreuter KU. Adrenergic and cholinergic control in the biology of epidermis: physiological and clinical significance. J Investig Dermatol. 2006;126:1948–1965. [DOI] [PubMed] [Google Scholar]
- 45.Zouboulis CC, Yoshida GJ, Wu Y, et al. Sebaceous gland: milestones of 30-year modelling research dedicated to the “brain of the skin.” Exp Dermatol. 2020;29:1069–1079. [DOI] [PubMed] [Google Scholar]
- 46.Zouboulis CC. The brain of the skin: sebaceous gland. In: Pappas A, ed. Lipids and Skin Health.; New York: Springer; 2015;109–126. [Google Scholar]
- 47.Zouboulis CC, Baron JM, Böhm M, et al. Frontiers in sebaceous gland biology and pathology. Exp Dermatol. 2008;17:542–551. [DOI] [PubMed] [Google Scholar]
- 48.Makrantonaki E, Adjaye J, Herwig R, et al. Age-specific hormonal decline is accompanied by transcriptional changes in human sebocytes in vitro. Aging Cell. 2006;5:331–344. [DOI] [PubMed] [Google Scholar]
- 49.Ramot Y, Böhm M, Paus R. Translational neuroendocrinology of human skin: concepts and perspectives. Trends Mol Med. 2021;27:60–74. [DOI] [PubMed] [Google Scholar]
- 50.Stegemann A, Böhm M. Targeting the α7 nicotinic acetylcholine receptor—a novel road towards the future treatment of skin diseases. Exp Dermatol. 2020;29:924–931. [DOI] [PubMed] [Google Scholar]
- 51.Li ZJ, Park SB, Sohn KC, et al. Regulation of lipid production by acetylcholine signalling in human sebaceous glands. J Dermatol Sci. 2013;72:116–122. [DOI] [PubMed] [Google Scholar]
- 52.Kurzen H, Schallreuter KU. Novel aspects in cutaneous biology of acetylcholine synthesis and acetylcholine receptors. Exp Dermatol. 2004;13:27–30. [DOI] [PubMed] [Google Scholar]
- 53.Thomas SE, Conway J, Ebling FJG, et al. Measurement of sebum excretion rate and skin temperature above and below the neurological lesion in paraplegic patients. Br J Dermatol. 1985;112:569–573. [DOI] [PubMed] [Google Scholar]
- 54.Rho NK, Gil YC. Botulinum neurotoxin type a in the treatment of facial seborrhea and acne: evidence and a proposed mechanism. Toxins (Basel). 2021;13:817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Makrantonaki E, Brink TC, Zampeli V, et al. Identification of biomarkers of human skin ageing in both genders. Wnt signalling - a label of skin ageing? PLoS One. 2012;7:e50393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Steinhoff M, Schmelz M, Schauber J. Facial erythema of rosacea—aetiology, different pathophysiologies and treatment options. Acta Derm Venereol. 2016;96:579–586. [DOI] [PubMed] [Google Scholar]
- 57.Park JY, Cho SI, Hur K, et al. Intradermal microdroplet injection of diluted incobotulinumtoxin-A for sebum control, face lifting, and pore size improvement. J Drugs Dermatol. 2021;20:49–54. [DOI] [PubMed] [Google Scholar]
- 58.Rose AE, Goldberg DJ. Safety and efficacy of intradermal injection of botulinum toxin for the treatment of oily skin. Dermatol Surg. 2013;39:443–448. [DOI] [PubMed] [Google Scholar]
- 59.Shah AR. Use of intradermal botulinum toxin to reduce sebum production and facial pore size. J Drugs Dermatol. 2008;7:847–850. [PubMed] [Google Scholar]
- 60.Kim JS. Fine wrinkle treatment and hydration on the facial dermis using HydroToxin mixture of MicroBotox and MicroHyaluronic acid. Aesthet Surg J. 2021;41:NP538–NP549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Franklin JM, Patorno E, Desai RJ, et al. Emulating randomized clinical trials with nonrandomized real-world evidence studies first results from the RCT DUPLICATE initiative. Circulation. 2021;143:1002–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.LoCasale RJ, Pashos CL, Gutierrez B, et al. Bridging the gap between RCTs and RWE through endpoint selection. Ther Innov Regul Sci. 2021;55:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.