Abstract
Objectives
For patients with brain disorders, regaining the ability to drive is crucial to their reintegration into society. Despite the existence of numerous assessment methods for determining the ability to resume driving, the most effective approach remains unclear. This study evaluated patients with brain disorders who had received support for driving resumption. We examined the factors influencing the acquisition of driving ability in this specific population.
Methods
This retrospective observational study was conducted from July 2019 to March 2022. Initially, a desk-based assessment was conducted using neuropsychological tests. Successful candidates subsequently underwent an on-road assessment at an affiliated driving school. Patients who passed both assessments were granted permission to resume driving. The participants were categorized into pass and fail groups based on their assessments, and a comparative analysis was conducted. Age, sex, type of brain disorder, functional independence measures (FIMs), assessments of higher cognitive skills, and physical function test results were evaluated.
Results
Forty-five patients (average age: 62±13 years) underwent evaluation. Logistic regression analysis for the desk-based assessment identified the Rey-Osterrieth complex figure test (ROCFT) (three-minute delayed recall) as the most influential factor (cutoff value: 21.5 points; sensitivity: 65%; specificity, 72.7%). In the on-road assessment, the 10-m walking test was significantly faster in the passing group than in the failing group (p<0.005).
Conclusions
We demonstrated that the ROCFT (three-minute delayed recall) was the most effective neuropsychological assessment tool for evaluating driving resumption. The assessment of walking speed may also be able to predict the resumption of driving in patients with brain disorders.
Keywords: rehabilitation, driving resumption assessment, neuropsychological assessment, brain disorder, driving ability
Introduction
Automobile accidents involving patients with brain disorders occur frequently, and there is ongoing debate regarding the adequacy of tests determining their driving abilities. For patients with brain disorders, driving a car is an important means of reintegrating into society [1,2]. For them, the ability to drive is highly beneficial for maintaining activities of daily living (ADL) and quality of life (QOL). Therefore, in recent years, support for the resumption of driving has gained attention in rehabilitation medicine [3-6]. In the United States, higher cognitive function screening measures are recommended to assess driving ability [7]. However, the best method of assessing the driving abilities of patients with brain disorders has yet to be determined [6,8]. In Japan, there are no unified or clear criteria, and effective assessments have not yet been established. Individual medical institutions in Japan, including our hospital, develop their own criteria. Doctors make decisions regarding the resumption of driving using different assessments at each hospital. As the Japanese population ages, the number of patients with brain disorders is increasing. Therefore, it is important to assess the driving ability of these patients accurately. In this study, we evaluated patients for whom we provided support for driving resumption and examined the factors influencing the acquisition of driving abilities. The purpose of this study is to examine the higher cognitive skills and physical functions required for patients with brain disorders to resume driving. We hypothesized that attention function, visual-spatial cognition, and physical functions such as walking ability are important for resuming driving.
Materials and methods
Ethics
This study complied with the principles of the Declaration of Helsinki for Human Research. The ethics committee of our hospital approved this study (no. 2022-1), and informed consent was obtained from the patients or their legal guardians.
Study design and patients
This retrospective observational study was conducted between July 2019 and March 2022. Patients with brain disorders who were hospitalized at our hospital and underwent assessments for the resumption of driving were recruited for this study. This study focused on patients with non-traumatic brain disorders. We excluded the patients exhibiting clinically evident severe paralysis or higher brain dysfunction.
Desk-based assessment and on-road assessment
At our hospital, when patients express a desire to resume driving, a desk-based assessment is conducted, involving several assessments of higher cognitive skills, such as the Mini-Mental State Examination (MMSE), Kohs block-design test (Kohs), Trail Making Test (TMT) Parts A and B, Clinical Assessment for Attention Test (CAT), Rey-Osterrieth Complex Figure Test (ROCFT), Behavioural Assessment of the Dysexecutive Syndrome (BADS), Wechsler Adult Intelligence Scale - Fourth Edition (WAIS-IV), and Cognitive-Related Behavioral Assessment (CBA). The attending physician comprehensively evaluates the results of these assessments to determine the overall outcome of the desk-based assessment. If the desk-based assessment is successful, a practical on-road driving evaluation is then conducted at an affiliated driving school. The criteria for passing the on-road assessments were based on the responses from driving instructors at the driving school. Finally, patients who pass both the desk-based and on-road assessments are granted permission by the attending physician to resume driving.
Evaluated factors
We evaluated factors including age, sex, type of brain disorder, functional independence measures (FIM), several assessments of higher-order cognitive skills (MMSE, Kohs, TMT, CAT, ROCFT, BADS, WAIS-IV, CBA), and physical function tests, including the Fugl-Meyer Assessment (FMA), Brunnstrom Stage (BRS), Action Research Arm Test (ARAT), Functional Ambulation Categories (FAC), 10-m walking test, Postural Assessment Scale for Stroke (PASS), and the Scale for the Assessment and Rating of Ataxia (SARA). We divided the participants into two groups, the pass and fail groups, based on their performance in the desk-based and on-road assessments, and conducted between-group comparisons.
Statistical analysis
First, the desk-based assessment results were used to classify patients into pass/fail groups, and the results of each test were compared using the Mann-Whitney U test. Logistic regression analysis with the forward-backward stepwise selection method was performed to examine the passing or failing of the desk-based assessment by age, sex, hemisphere of damage (left or right), and factors that showed significant differences as independent factors. The on-road assessment results were subsequently used to classify patients who passed the desk-based assessment into pass/fail groups, and the results of each test were also compared using the Mann-Whitney U test after confirming non-normal distribution using the Shapiro-Wilk test. All statistical analyses were performed using Statistical Product and Service Solutions (SPSS, version 26.0; IBM SPSS Statistics for Windows, Armonk, NY).
Results
The 45 patients who were included in the study consisted of 35 men and 10 women. The average age is 62.4±1.95 years, with an average duration from onset to examination of 23.4±2.93 days. The average upper limb BRS is 5.27±0.20, hand BRS is 5.24±0.21, and lower limb BRS is 5.27±0.19 (Table 1).
Table 1. Characteristics of the patients.
BRS: Brunnstrom Stage
| Average | Patients (n=45) |
| Age | 62.4±1.95 (years) |
| Duration from onset to examination | 23.4±2.93 (days) |
| Upper limb BRS | 5.27±0.20 |
| Hand BRS | 5.24±0.21 |
| Lower limb BRS | 5.27±0.19 |
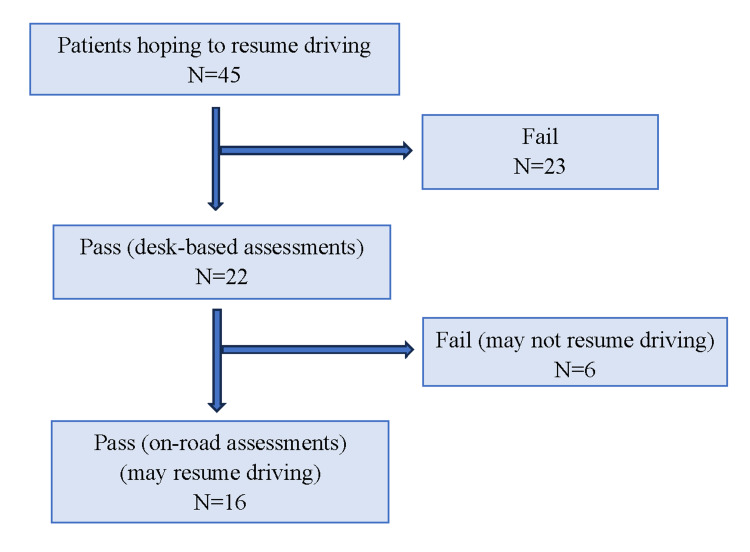
The underlying causes of brain disorders were cerebral infarction in 30 patients, cerebral hemorrhage in 11, and other causes in four (meningioma, brain abscess, hypoxic brain injury, and normal pressure hydrocephalus). As shown in Figure 1, desk-based assessments were conducted for the 45 individuals who expressed a desire to resume driving, and on-road assessments were conducted for the 22 individuals who passed the desk-based assessment. Sixteen patients passed the on-road assessment and were permitted to resume driving, while six failed.
Figure 1. The examination results for both the desk-based and on-road assessments.
Assessment outcomes
Desk-Based Assessment
In the comparison between the pass and fail groups in the desk-based assessment, significant differences were observed in the Kohs (p=0.046), TMT Part A (p=0.008), and among the items "△" time (p=0.026), "mark" time (p=0.001), "3" accuracy rate (p=0.043), and "ka" correct response rate (p=0.019) within the visual cancellation task in the CAT, as well as in the ROCFT (three-minute delayed recall) (p=0.018) (Table 2).
Table 2. Comparison of the pass and fail groups in the desk-based assessment.
| Pass group (n=22) | Fail group (n=23) | p-value | |
| Kohs block-design test | 97.0±19.2 | 84.0±23.4 | 0.046 |
| Trail Making Test-Part A | 42.5±14.5 | 61.6±28.3 | 0.008 |
| Visual cancellation task ”△” time | 52.6±12.2 | 85.0±96.2 | 0.026 |
| Visual cancellation task ”mark” time | 59.4±11.0 | 87.1±44.3 | 0.001 |
| Visual cancellation task ”3” accuracy rate | 100±0 | 99.7±0.8 | 0.043 |
| Visual cancellation task ”ka” correct response rate | 98.4±2.0 | 94.8±5.8 | 0.019 |
| Rey–Osterrieth Complex Figure Test (3-min delayed recall) | 23.5±7.6 | 17.2±8.5 | 0.018 |
However, no significant differences were observed in the age or physical function assessments (Table 3).
Table 3. Comparison of the pass and fail groups in the desk-based assessment.
| Pass group (n=22) | Fail group (n=23) | p-value | |
| Age | 62.1±12.8 | 62.7±13.8 | 0.847 |
| Functional Independence Measures (motor) | 87.4±4.5 | 82.5±10.6 | 0.062 |
| Functional Independence Measures (cognition) | 32.6±3.1 | 32.6±2.9 | 0.913 |
| Fugl–Meyer Assessment (upper) | 61.6±5.4 | 60.6±6.1 | 0.612 |
| Fugl–Meyer Assessment (lower) | 33.4±1.1 | 32.3±2.1 | 0.094 |
| Action Research Arm Test | 54.5±7.4 | 50.6±9.3 | 0.432 |
| Functional Ambulation Categories | 4.5±0.8 | 3.9±1.1 | 0.274 |
| 10-meter walking (time) | 8.1±3.9 | 12.0±10.0 | 0.156 |
| Postural Assessment Scale for Stroke | 33.7±5.9 | 32.9±3.9 | 0.314 |
| Scale for the Assessment and Rating of Ataxia | 4.8±4.8 | 6.3±5.6 | 0.914 |
Logistic regression analysis examining the presence or absence of passing the desk-based assessment showed a significant difference in the ROCFT (three-minute delayed recall) (Table 4).
Table 4. Logistic regression analysis examining the presence or absence of passing the desk-based assessment showed a significant difference in the Rey–Osterrieth complex figure test (three-minute delayed recall).
Dependent variable: passing the desk-based assessment; independent variables: age, sex, hemisphere of damage (left or right), and factors that showed significant differences
| Odds ratio | p-value | 95%CI | ||
| Lower | Upper | |||
| Rey–Osterrieth Complex Figure Test (3-min delayed recall) | 1.153 | 0.022 | 1.021 | 1.301 |
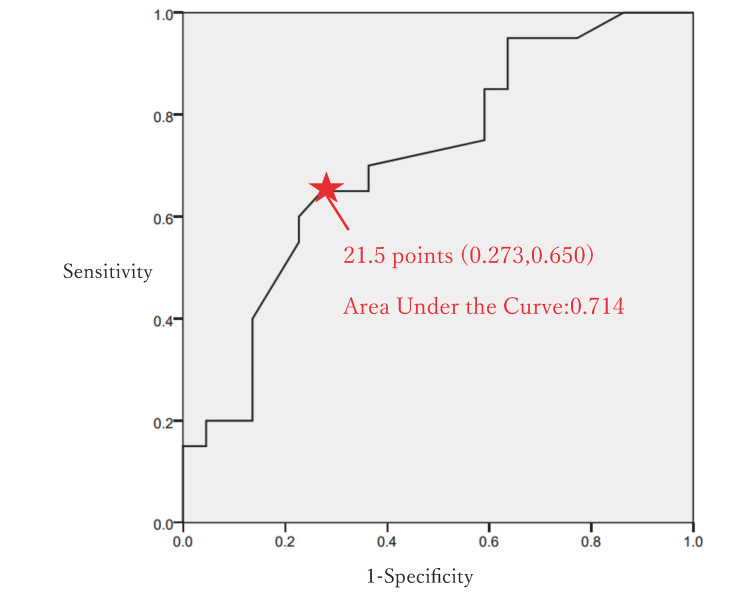
Furthermore, receiver operating characteristic (ROC) curve analysis was conducted to determine the cutoff value of the ROCFT (three-minute delayed recall) for the pass group. The cutoff value was determined to be 21.5/36 points (AUC: 0.714; sensitivity: 65%; specificity: 72.7%) (Figure 2).
Figure 2. Cutoff value determination for the Rey–Osterrieth complex figure test in the desk-based assessment.
A receiver operating characteristic curve analysis was conducted to determine the cutoff value of the Rey–Osterrieth complex figure test (three-minute delayed recall) for the pass group in the desk-based assessment. The cutoff value was determined to be 21.5/36 points (area under the curve: 0.714, sensitivity: 65%, specificity: 72.7%).
On-Road Assessment
In the comparison between the pass (driving resumption group) and fail groups (driving non-resumption group) in the on-road assessment, no significant differences were observed in the neuropsychological assessments (Table 5).
Table 5. Comparison of the pass and fail groups in the on-road assessment.
| Pass group (n=16) | Fail group (n=6) | p-value | |
| Mini Mental State Examination-J | 29.4±1.1 | 29.3±0.8 | 0.693 |
| Kohs block-design test | 100.9±16.1 | 86.5±24.4 | 0.154 |
| Trail Making Test-Part A | 39.3±12.9 | 51.0±16.0 | 0.115 |
| Trail Making Test-Part B | 69.3±29.0 | 91.8±44.3 | 0.231 |
| Visual cancellation task “△” time | 52.0±13.0 | 54.2±10.3 | 0.747 |
| Visual cancellation task “△” accuracy rate | 99.9±0.5 | 100 | 0.858 |
| Visual cancellation task “mark” time | 59.44±11.9 | 59.2±8.9 | 0.971 |
| Visual cancellation task “mark” correct response rate | 99.9±0.5 | 99±1.7 | 0.329 |
| Visual cancellation task “mark” accuracy rate | 99.9±0.5 | 100 | 0.858 |
| Visual cancellation task “3” time | 101.9±23.2 | 115.5±28.1 | 0.407 |
| Visual cancellation task “3” correct response rate | 99.3±1.5 | 98.8±0.8 | 0.7 |
| Visual cancellation task “3” accuracy rate | 100 | 100 | 1 |
| Visual cancellation task “ka” time | 127.0±29.8 | 134.5±25.0 | 0.329 |
| Visual cancellation task “ka” correct response rate | 98.6±2.0 | 97.8±2.0 | 0.449 |
| Visual cancellation task “ka” accuracy rate | 99.3±2.8 | 100 | 0.858 |
| Rey–Osterrieth Complex Figure Test (copy) | 34.3±1.5 | 34.8±2.2 | 0.445 |
| Rey–Osterrieth Complex Figure Test (3-min delayed recall) | 23.9±7.4 | 22.2±8.9 | 0.612 |
| Behavioural Assessment of the Dysexecutive Syndrome (zoo map) | 2.6±1.5 | 2.8±1.6 | 0.905 |
| Wechsler Adult Intelligence Scale - Fourth Edition (puzzle task) | 11.1±4.1 | 10.6±3.4 | 0.842 |
| Cognitive-Related Behavioral Assessment | 28.4±1.5 | 27.8±1.0 | 0.327 |
However, a significant difference was observed in the 10-m walking test in the age and physical function assessments (Table 6).
Table 6. Comparison of the pass and fail groups in the on-road assessment.
| Pass group(n=16) | Fail group(n=6) | p-value | |
| Age | 60.2±13.7 | 67.3±9.4 | 0.294 |
| Functional Independence Measures (motor) | 88.2±3.9 | 84.8±5.9 | 0.398 |
| Functional Independence Measures (cognition) | 33.0±2.9 | 31.2±3.6 | 0.398 |
| Fugl–Meyer Assessment (upper) | 61.4±5.5 | 65 | 0.75 |
| Fugl–Meyer Assessment (lower) | 33.4±1.2 | 34±0 | 0.5 |
| Action Research Arm Test | 54.4±7.8 | 56 | 0.5 |
| Functional Ambulation Categories | 4.7±0.5 | 3 | 0.25 |
| 10-meter walking (time) | 6.6±2.0 | 13.6±4.7 | 0.005 |
| Postural Assessment Scale for Stroke | 35.6±1.1 | 29.3±10.7 | 0.267 |
| Scale for the Assessment and Rating of Ataxia | 6.0±5.0 | 1 | 0.5 |
Discussion
In this study, we examined the test items that affect driving resumption in individuals with brain disorders. Neuropsychological assessments in the desk-based assessment, such as the Kohs, TMT Part A, visual cancellation task in CAT, and ROCFT, may have potential usefulness, with particular emphasis on the ROCFT (three-minute delayed recall). The factor influencing the resumption of driving in patients who passed the desk assessment was the 10-m walking time, suggesting the importance of lower limb function and gait ability as predictive factors for driving resumption.
Driving is a complex activity that requires various high-level cognitive skills. According to the United States guidelines, the cognitive skills used in driving are short- and long-term memory, visuospatial cognition function (including visual perception and visual processing and visual search and visuospatial skills), selective and divided attention, executive skills, language, and vigilance [7]. In driving, attention and visual-spatial cognition are essential abilities. Tests such as the TMT, CAT, and ROCFT are considered important assessments that can evaluate these necessary skills for driving. However, there is currently no established neuropsychological assessment that is effective in predicting the ability to resume driving, and there are no unified criteria between facilities. The TMT and ROCFT have previously been reported to be useful screening tools for evaluating driving ability [4]. Combining assessments that focus on attention and visuospatial cognitive function could be important. In this study, we also found that the TMT and ROCFT were useful for measuring attention and visuospatial cognitive functions, respectively.
When driving a car, attention functions such as the ability to "maintain a safe following distance," "pay attention to signals and road signs," "be aware of pedestrians and oncoming vehicles," and "switch attention in response to changing situations" are essential. TMT is reportedly both convenient and useful in the assessment of attention function [3,9,10]. Furthermore, in stroke patients, driving suitability can be predicted with 86% accuracy using three assessments: the visual cancellation task test, ROCFT, and on-road assessment [11]. The reference values for the TMT show significant variations among facilities, and there is no consensus on whether Part A or B is more useful. However, in our study, both TMT Part A and the CAT visual cancellation task were found to be useful in the desk-based assessment. These tests are considered valuable due to their simplicity and relatively short administration times.
When driving a car, visuospatial cognitive functions such as the ability to "drive within lanes accurately," "maintain a safe following distance," and "assess the speed and direction of other vehicles" are essential. The ROCFT is useful for assessing visuospatial cognitive functions [4,9,12]. Combined neuropsychological assessments, including the ROCFT and WAIS digit symbol task, classify the resumption of driving with an accuracy rate of 94.4 [13], and one report indicates that ROCFT scores correlate with the passing of the on-road assessment [6]. In addition, logistic regression analysis has identified the ROCFT as an independent predictor of on-road assessments of patients with brain disorders [14]. In our study, logistic regression analysis demonstrated the usefulness of the ROCFT. Furthermore, ROCFT scores are less affected by aphasia. Like the TMT, the ROCFT is simple and suitable for screening assessments. However, there is currently no established cutoff value for the ROCFT in predicting driving ability. In this study, the ROCFT was considered more crucial as a neuropsychological assessment for driving resumption, and a cutoff value of 21.5 points was identified.
Regarding the relationship between physical function and driving, in patients with brain disorders, individuals with higher scores on the motor items of the FIM can resume driving [15]. In patients who passed the desk-based assessment in this study, the time taken for the 10-m walking test was identified as a factor influencing the resumption of driving. This suggests that not only higher cognitive function but also motor functions, such as lower limb function and walking ability could be predictive factors for driving resumption.
This study had several limitations. First, this was a retrospective study. We are constrained by the tests that were already available at the time of data collection. This limitation can affect the comprehensiveness and relevance of the neuropsychological assessments used in our study. Further prospective studies are necessary to confirm the cutoff values in the future. Second, the sample size was small, leading to potential bias in patient backgrounds, including the causes of brain disorders. Larger-scale clinical studies are warranted in the future. Third, since our hospital did not have a driving simulator, it was not available for use in driving resumption assessments. Finally, it has not been confirmed whether patients who resumed driving could continue driving without encountering problems. Therefore, long-term follow-up is required.
Conclusions
In this study, we examined the factors influencing the resumption of driving in patients with brain disorders. Among the neuropsychological assessments for evaluating the resumption of driving, the ROCFT score may have significant potential. As a passing criterion for the desk-based assessment, the cutoff value for the ROCFT (three-minute delayed recall) was determined to be 21.5 points. Furthermore, in addition to higher cognitive function, motor functions such as lower limb function and walking ability could emerge as potential predictive factors for driving resumption. Large-scale clinical studies with long-term follow-up are warranted in the future.
Disclosures
Human subjects: Consent was obtained or waived by all participants in this study. The Institutional Review Board of Ishikawa Hospital issued approval No. 2022-1. The Ethics Committee of our hospital approved this study.
Animal subjects: All authors have confirmed that this study did not involve animal subjects or tissue.
Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following:
Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work.
Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work.
Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
Author Contributions
Concept and design: Ryo Yoshikawa, Yuzo Tsuda, Yoshimichi Kobayashi, Risa Harada, Yoshitada Sakai, Yoshiaki Saji
Drafting of the manuscript: Ryo Yoshikawa, Yuzo Tsuda
Critical review of the manuscript for important intellectual content: Ryo Yoshikawa, Atsuko Matsuda, Yasumitsu Fujii, Yoshimichi Kobayashi, Risa Harada, Yoshitada Sakai, Yoshiaki Saji
Supervision: Ryo Yoshikawa, Yoshitada Sakai
Acquisition, analysis, or interpretation of data: Yuzo Tsuda, Atsuko Matsuda, Yasumitsu Fujii
References
- 1.Role of premorbid factors in predicting safe return to driving after severe TBI. Pietrapiana P, Tamietto M, Torrini G, Mezzanato T, Rago R, Perino C. Brain Inj. 2005;19:197–211. doi: 10.1080/02699050400017197. [DOI] [PubMed] [Google Scholar]
- 2.Identifying safe versus unsafe drivers following brain impairment: the Coorabel Programme. Fox GK, Bashford GM, Caust SL. Disabil Rehabil. 1992;14:140–145. doi: 10.3109/09638289209165850. [DOI] [PubMed] [Google Scholar]
- 3.A systematic review and meta-analysis on the association between driving ability and neuropsychological test performances after moderate to severe traumatic brain injury. Egeto P, Badovinac SD, Hutchison MG, Ornstein TJ, Schweizer TA. J Int Neuropsychol Soc. 2019;25:868–877. doi: 10.1017/S1355617719000456. [DOI] [PubMed] [Google Scholar]
- 4.Predictors of driving ability following stroke: a systematic review. Marshall SC, Molnar F, Man-Son-Hing M, et al. Top Stroke Rehabil. 2007;14:98–114. doi: 10.1310/tsr1401-98. [DOI] [PubMed] [Google Scholar]
- 5.Prediction of driving after stroke: a prospective study. Akinwuntan AE, Feys H, De Weerdt W, Baten G, Arno P, Kiekens C. Neurorehabil Neural Repair. 2006;20:417–423. doi: 10.1177/1545968306287157. [DOI] [PubMed] [Google Scholar]
- 6.Predicting driving performance after stroke. Nouri FM, Lincoln NB. BMJ. 1993;307:482–483. doi: 10.1136/bmj.307.6902.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carr DB, Schwartzberg JG, Manning L, Sempek J. Physician’s Guide to Assessing and Counseling Older Drivers, 2nd Edition. Washington DC: NHTSA; 2010. Physician’s Guide to Assessing and Counseling Older Drivers, 2nd Edition. [Google Scholar]
- 8.Return to driving after traumatic brain injury: a British perspective. Brooks N, Hawley CA. Brain Inj. 2005;19:165–175. doi: 10.1080/02699050410001720004. [DOI] [PubMed] [Google Scholar]
- 9.Cognitive, on-road, and simulator-based driving assessment after stroke. Hird MA, Vetivelu A, Saposnik G, Schweizer TA. J Stroke Cerebrovasc Dis. 2014;23:2654–2670. doi: 10.1016/j.jstrokecerebrovasdis.2014.06.010. [DOI] [PubMed] [Google Scholar]
- 10.Screening for fitness to drive after stroke: a systematic review and meta-analysis. Devos H, Akinwuntan AE, Nieuwboer A, Truijen S, Tant M, De Weerdt W. Neurology. 2011;76:747–756. doi: 10.1212/WNL.0b013e31820d6300. [DOI] [PubMed] [Google Scholar]
- 11.Confirmation of the accuracy of a short battery to predict fitness-to-drive of stroke survivors without severe deficits. Akinwuntan AE, Devos H, Feys H, Verheyden G, Baten G, Kiekens C, De Weerdt W. J Rehabil Med. 2007;39:698–702. doi: 10.2340/16501977-0113. [DOI] [PubMed] [Google Scholar]
- 12.Malingering and mild brain injury: how low is too low. Meyers JE, Galinsky AM, Volbrecht M. Appl Neuropsychol. 1999;6:208–216. doi: 10.1207/s15324826an0604_3. [DOI] [PubMed] [Google Scholar]
- 13.Prediction of driving capacity after traumatic brain injury: a systematic review. Ortoleva C, Brugger C, Van der Linden M, Walder B. J Head Trauma Rehabil. 2012;27:302–313. doi: 10.1097/HTR.0b013e3182236299. [DOI] [PubMed] [Google Scholar]
- 14.Neuropsychological assessment of fitness to drive following acquired cognitive impairment. Bliokas VV, Taylor JE, Leung J, Deane FP. Brain Inj. 2011;25:471–487. doi: 10.3109/02699052.2011.559609. [DOI] [PubMed] [Google Scholar]
- 15.Driving with cognitive deficits: neurorehabilitation and legal measures are needed for driving again after severe traumatic brain injury. Leon-Carrion J, Dominguez-Morales MR, Martin JM. Brain Inj. 2005;19:213–219. doi: 10.1080/02699050400017205. [DOI] [PubMed] [Google Scholar]