Abstract
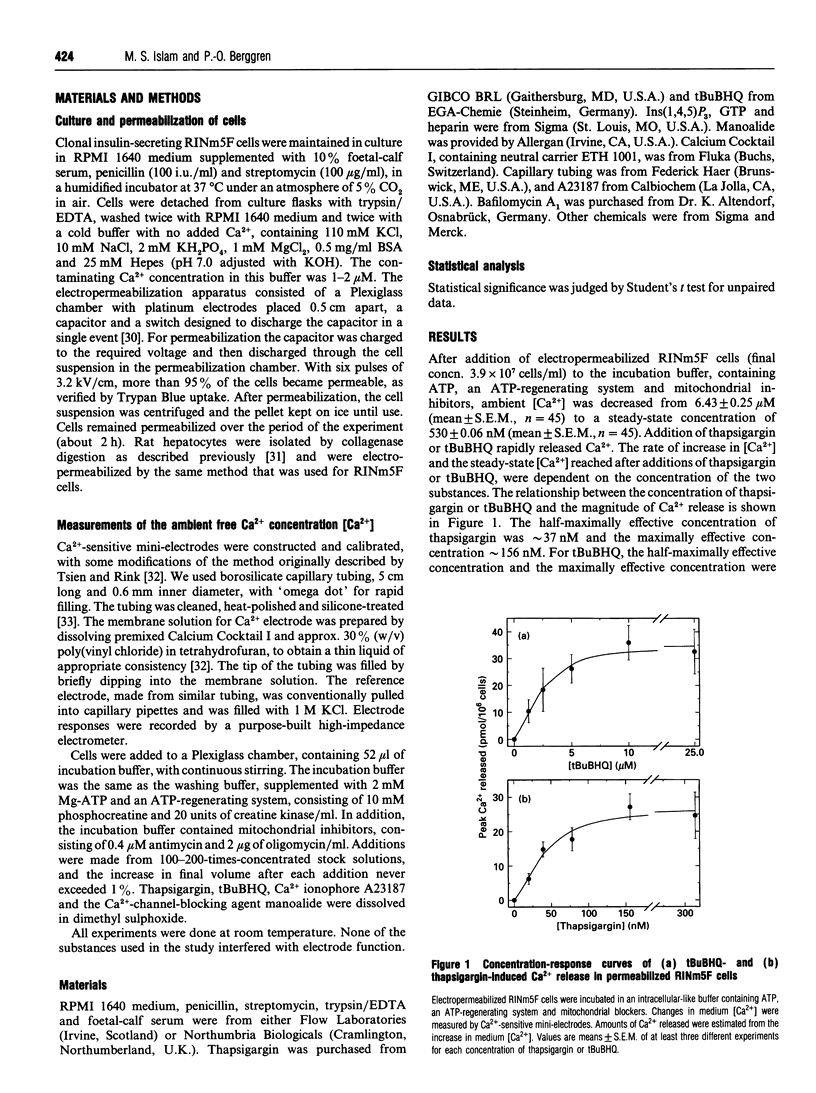
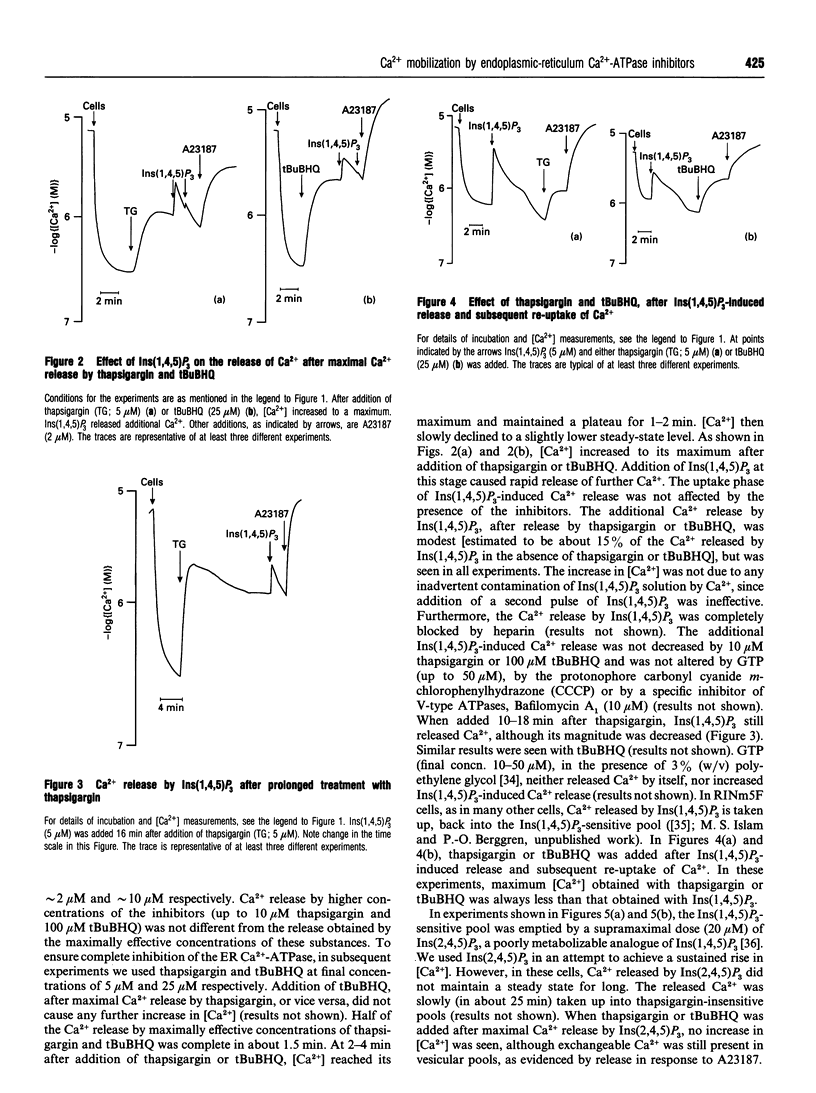
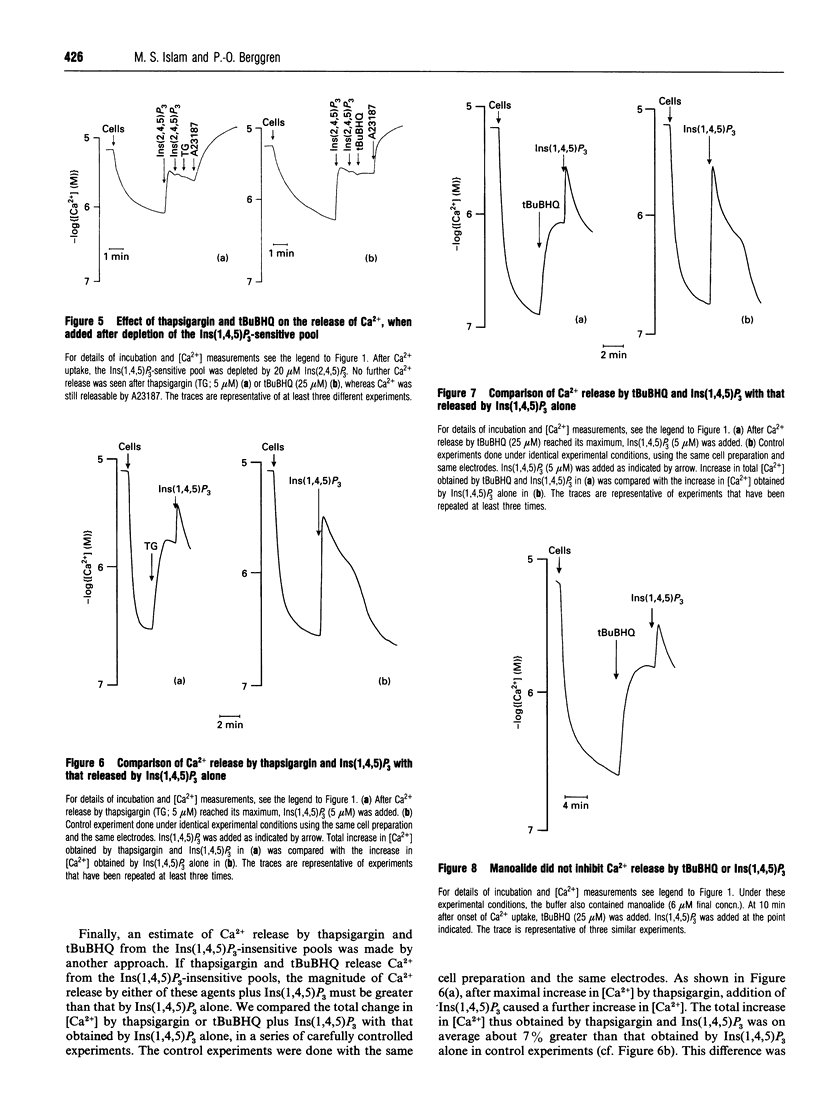
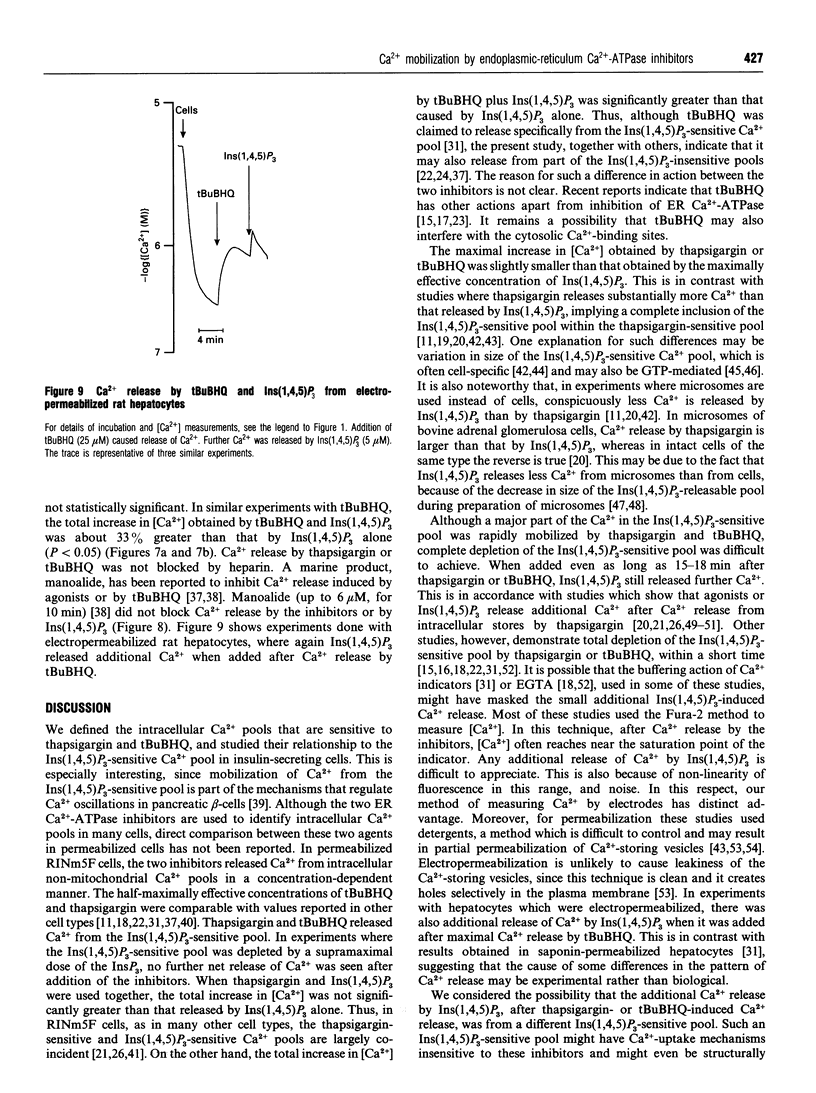
We characterized and directly compared the Ca(2+)-releasing actions of two inhibitors of endoplasmic-reticulum (ER) Ca(2+)-ATPase, thapsigargin and 2,5-di-(t-butyl)-1,4-benzohydroquinone (tBuBHQ), in electropermeabilized insulin-secreting RINm5F cells. Ambient free calcium concentration ([Ca2+]) was monitored by Ca(2+)-selective mini-electrodes. After ATP-dependent Ca2+ uptake, thapsigargin and tBuBHQ released Ca2+ with and EC50 of approximately 37 nM and approximately 2 microM respectively. Both agents mobilized Ca2+ predominantly from the Ins(1,4,5)P3-sensitive Ca2+ pool, and in this respect thapsigargin was more specific than tBuBHQ. The total increase in [Ca2+] obtained with thapsigargin and Ins(1,4,5)P3 was, on the average, only 7% greater than that with Ins(1,4,5)P3 alone. In contrast, the total increase in [Ca2+] obtained with tBuBHQ and Ins(1,4,5)P3 was 33% greater than that obtained with only InsP3 (P < 0.05). Although Ca2+ was rapidly mobilized by thapsigargin and tBuBHQ, complete depletion of the Ins(1,4,5)P3-sensitive Ca2+ pool was difficult to achieve. After the release by thapsigargin or tBuBHQ, Ins(1,4,5)P3 induced additional Ca2+ release. The additional Ins(1,4,5)P3-induced Ca2+ release was not altered by supramaximal concentrations of thapsigargin and tBuBHQ, or by Bafilomycin A1, an inhibitor of V-type ATPases, but was decreased by prolonged treatment with the ER Ca(2+)-ATPase inhibitors. These results suggest the existence of distinct uptake and release compartments within the Ins(1,4,5)P3-sensitive Ca2+ pool. When treated with the inhibitors, the two compartments became distinguishable on the basis of their Ca2+ permeability. Apparently, thapsigargin and tBuBHQ readily mobilized Ca2+ from the uptake compartment, whereas Ca2+ from the release compartment could be mobilized only very slowly, in the absence of Ins(1,4,5)P3.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ammälä C., Larsson O., Berggren P. O., Bokvist K., Juntti-Berggren L., Kindmark H., Rorsman P. Inositol trisphosphate-dependent periodic activation of a Ca(2+)-activated K+ conductance in glucose-stimulated pancreatic beta-cells. Nature. 1991 Oct 31;353(6347):849–852. doi: 10.1038/353849a0. [DOI] [PubMed] [Google Scholar]
- Baker P. F., Knight D. E., Umbach J. A. Calcium clamp of the intracellular environment. Cell Calcium. 1985 Apr;6(1-2):5–14. doi: 10.1016/0143-4160(85)90030-2. [DOI] [PubMed] [Google Scholar]
- Berridge M. J. Calcium oscillations. J Biol Chem. 1990 Jun 15;265(17):9583–9586. [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol phosphates and cell signalling. Nature. 1989 Sep 21;341(6239):197–205. doi: 10.1038/341197a0. [DOI] [PubMed] [Google Scholar]
- Bian J. H., Ghosh T. K., Wang J. C., Gill D. L. Identification of intracellular calcium pools. Selective modification by thapsigargin. J Biol Chem. 1991 May 15;266(14):8801–8806. [PubMed] [Google Scholar]
- Biden T. J., Prentki M., Irvine R. F., Berridge M. J., Wollheim C. B. Inositol 1,4,5-trisphosphate mobilizes intracellular Ca2+ from permeabilized insulin-secreting cells. Biochem J. 1984 Oct 15;223(2):467–473. doi: 10.1042/bj2230467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biden T. J., Wollheim C. B., Schlegel W. Inositol 1,4,5-trisphosphate and intracellular Ca2+ homeostasis in clonal pituitary cells (GH3). Translocation of Ca2+ into mitochondria from a functionally discrete portion of the nonmitochondrial store. J Biol Chem. 1986 Jun 5;261(16):7223–7229. [PubMed] [Google Scholar]
- Booth C., Koch G. L. Perturbation of cellular calcium induces secretion of luminal ER proteins. Cell. 1989 Nov 17;59(4):729–737. doi: 10.1016/0092-8674(89)90019-6. [DOI] [PubMed] [Google Scholar]
- Bowman E. J., Siebers A., Altendorf K. Bafilomycins: a class of inhibitors of membrane ATPases from microorganisms, animal cells, and plant cells. Proc Natl Acad Sci U S A. 1988 Nov;85(21):7972–7976. doi: 10.1073/pnas.85.21.7972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brüne B., Ullrich V. Calcium mobilization in human platelets by receptor agonists and calcium-ATPase inhibitors. FEBS Lett. 1991 Jun 17;284(1):1–4. doi: 10.1016/0014-5793(91)80747-q. [DOI] [PubMed] [Google Scholar]
- Brüne B., Ullrich V. Different calcium pools in human platelets and their role in thromboxane A2 formation. J Biol Chem. 1991 Oct 15;266(29):19232–19237. [PubMed] [Google Scholar]
- Burgoyne R. D., Cheek T. R., Morgan A., O'Sullivan A. J., Moreton R. B., Berridge M. J., Mata A. M., Colyer J., Lee A. G., East J. M. Distribution of two distinct Ca2+-ATPase-like proteins and their relationships to the agonist-sensitive calcium store in adrenal chromaffin cells. Nature. 1989 Nov 2;342(6245):72–74. doi: 10.1038/342072a0. [DOI] [PubMed] [Google Scholar]
- Corkey B. E., Tornheim K., Deeney J. T., Glennon M. C., Parker J. C., Matschinsky F. M., Ruderman N. B., Prentki M. Linked oscillations of free Ca2+ and the ATP/ADP ratio in permeabilized RINm5F insulinoma cells supplemented with a glycolyzing cell-free muscle extract. J Biol Chem. 1988 Mar 25;263(9):4254–4258. [PubMed] [Google Scholar]
- Dawson A. P. GTP enhances inositol trisphosphate-stimulated Ca2+ release from rat liver microsomes. FEBS Lett. 1985 Jun 3;185(1):147–150. doi: 10.1016/0014-5793(85)80759-6. [DOI] [PubMed] [Google Scholar]
- Dawson A. P., Irvine R. F. Inositol (1,4,5)trisphosphate-promoted Ca2+ release from microsomal fractions of rat liver. Biochem Biophys Res Commun. 1984 May 16;120(3):858–864. doi: 10.1016/s0006-291x(84)80186-2. [DOI] [PubMed] [Google Scholar]
- Demaurex N., Lew D. P., Krause K. H. Cyclopiazonic acid depletes intracellular Ca2+ stores and activates an influx pathway for divalent cations in HL-60 cells. J Biol Chem. 1992 Feb 5;267(4):2318–2324. [PubMed] [Google Scholar]
- Ely J. A., Ambroz C., Baukal A. J., Christensen S. B., Balla T., Catt K. J. Relationship between agonist- and thapsigargin-sensitive calcium pools in adrenal glomerulosa cells. Thapsigargin-induced Ca2+ mobilization and entry. J Biol Chem. 1991 Oct 5;266(28):18635–18641. [PubMed] [Google Scholar]
- Endo M. Calcium release from the sarcoplasmic reticulum. Physiol Rev. 1977 Jan;57(1):71–108. doi: 10.1152/physrev.1977.57.1.71. [DOI] [PubMed] [Google Scholar]
- Foskett J. K., Wong D. Calcium oscillations in parotid acinar cells induced by microsomal Ca(2+)-ATPase inhibition. Am J Physiol. 1992 Mar;262(3 Pt 1):C656–C663. doi: 10.1152/ajpcell.1992.262.3.C656. [DOI] [PubMed] [Google Scholar]
- Gazdar A. F., Chick W. L., Oie H. K., Sims H. L., King D. L., Weir G. C., Lauris V. Continuous, clonal, insulin- and somatostatin-secreting cell lines established from a transplantable rat islet cell tumor. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3519–3523. doi: 10.1073/pnas.77.6.3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh T. K., Mullaney J. M., Tarazi F. I., Gill D. L. GTP-activated communication between distinct inositol 1,4,5-trisphosphate-sensitive and -insensitive calcium pools. Nature. 1989 Jul 20;340(6230):236–239. doi: 10.1038/340236a0. [DOI] [PubMed] [Google Scholar]
- Inesi G., Sumbilla C., Kirtley M. E. Relationships of molecular structure and function in Ca2(+)-transport ATPase. Physiol Rev. 1990 Jul;70(3):749–760. doi: 10.1152/physrev.1990.70.3.749. [DOI] [PubMed] [Google Scholar]
- Islam M. S., Rorsman P., Berggren P. O. Ca(2+)-induced Ca2+ release in insulin-secreting cells. FEBS Lett. 1992 Jan 27;296(3):287–291. doi: 10.1016/0014-5793(92)80306-2. [DOI] [PubMed] [Google Scholar]
- Jackson T. R., Patterson S. I., Thastrup O., Hanley M. R. A novel tumour promoter, thapsigargin, transiently increases cytoplasmic free Ca2+ without generation of inositol phosphates in NG115-401L neuronal cells. Biochem J. 1988 Jul 1;253(1):81–86. doi: 10.1042/bj2530081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph S. K., Williams R. J., Corkey B. E., Matschinsky F. M., Williamson J. R. The effect of inositol trisphosphate on Ca2+ fluxes in insulin-secreting tumor cells. J Biol Chem. 1984 Nov 10;259(21):12952–12955. [PubMed] [Google Scholar]
- Kass G. E., Duddy S. K., Moore G. A., Orrenius S. 2,5-Di-(tert-butyl)-1,4-benzohydroquinone rapidly elevates cytosolic Ca2+ concentration by mobilizing the inositol 1,4,5-trisphosphate-sensitive Ca2+ pool. J Biol Chem. 1989 Sep 15;264(26):15192–15198. [PubMed] [Google Scholar]
- Knight D. E., Scrutton M. C. Gaining access to the cytosol: the technique and some applications of electropermeabilization. Biochem J. 1986 Mar 15;234(3):497–506. doi: 10.1042/bj2340497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshiyama H., Tashjian A. H., Jr Evidence for multiple intracellular calcium pools in GH4C1 cells: investigations using thapsigargin. Biochem Biophys Res Commun. 1991 May 31;177(1):551–558. doi: 10.1016/0006-291x(91)92019-g. [DOI] [PubMed] [Google Scholar]
- Kwan C. Y., Takemura H., Obie J. F., Thastrup O., Putney J. W., Jr Effects of MeCh, thapsigargin, and La3+ on plasmalemmal and intracellular Ca2+ transport in lacrimal acinar cells. Am J Physiol. 1990 Jun;258(6 Pt 1):C1006–C1015. doi: 10.1152/ajpcell.1990.258.6.C1006. [DOI] [PubMed] [Google Scholar]
- Lanini L., Bachs O., Carafoli E. The calcium pump of the liver nuclear membrane is identical to that of endoplasmic reticulum. J Biol Chem. 1992 Jun 5;267(16):11548–11552. [PubMed] [Google Scholar]
- Law G. J., Pachter J. A., Thastrup O., Hanley M. R., Dannies P. S. Thapsigargin, but not caffeine, blocks the ability of thyrotropin-releasing hormone to release Ca2+ from an intracellular store in GH4C1 pituitary cells. Biochem J. 1990 Apr 15;267(2):359–364. doi: 10.1042/bj2670359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llopis J., Chow S. B., Kass G. E., Gahm A., Orrenius S. Comparison between the effects of the microsomal Ca(2+)-translocase inhibitors thapsigargin and 2,5-di-(t-butyl)-1,4-benzohydroquinone on cellular calcium fluxes. Biochem J. 1991 Jul 15;277(Pt 2):553–556. doi: 10.1042/bj2770553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lytton J., Westlin M., Hanley M. R. Thapsigargin inhibits the sarcoplasmic or endoplasmic reticulum Ca-ATPase family of calcium pumps. J Biol Chem. 1991 Sep 15;266(26):17067–17071. [PubMed] [Google Scholar]
- Mason M. J., Garcia-Rodriguez C., Grinstein S. Coupling between intracellular Ca2+ stores and the Ca2+ permeability of the plasma membrane. Comparison of the effects of thapsigargin, 2,5-di-(tert-butyl)-1,4-hydroquinone, and cyclopiazonic acid in rat thymic lymphocytes. J Biol Chem. 1991 Nov 5;266(31):20856–20862. [PubMed] [Google Scholar]
- Menniti F. S., Bird G. S., Takemura H., Thastrup O., Potter B. V., Putney J. W., Jr Mobilization of calcium by inositol trisphosphates from permeabilized rat parotid acinar cells. Evidence for translocation of calcium from uptake to release sites within the inositol 1,4,5-trisphosphate- and thapsigargin-sensitive calcium pool. J Biol Chem. 1991 Jul 25;266(21):13646–13653. [PubMed] [Google Scholar]
- Moore G. A., McConkey D. J., Kass G. E., O'Brien P. J., Orrenius S. 2,5-Di(tert-butyl)-1,4-benzohydroquinone--a novel inhibitor of liver microsomal Ca2+ sequestration. FEBS Lett. 1987 Nov 30;224(2):331–336. doi: 10.1016/0014-5793(87)80479-9. [DOI] [PubMed] [Google Scholar]
- Muallem S., Loessberg P., Sachs G., Wheeler L. A. Agonist-sensitive and -insensitive intracellular Ca2+ pools. Separate Ca(2+)-releasing mechanisms revealed by manoalide and benzohydroquinone. Biochem J. 1991 Oct 15;279(Pt 2):367–375. doi: 10.1042/bj2790367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicotera P., Orrenius S., Nilsson T., Berggren P. O. An inositol 1,4,5-trisphosphate-sensitive Ca2+ pool in liver nuclei. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6858–6862. doi: 10.1073/pnas.87.17.6858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson T., Arkhammar P., Hallberg A., Hellman B., Berggren P. O. Characterization of the inositol 1,4,5-trisphosphate-induced Ca2+ release in pancreatic beta-cells. Biochem J. 1987 Dec 1;248(2):329–336. doi: 10.1042/bj2480329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oie H. K., Gazdar A. F., Minna J. D., Weir G. C., Baylin S. B. Clonal analysis of insulin and somatostatin secretion and L-dopa decarboxylase expression by a rat islet cell tumor. Endocrinology. 1983 Mar;112(3):1070–1075. doi: 10.1210/endo-112-3-1070. [DOI] [PubMed] [Google Scholar]
- Oldershaw K. A., Taylor C. W. 2,5-Di-(tert-butyl)-1,4-benzohydroquinone mobilizes inositol 1,4,5-trisphosphate-sensitive and -insensitive Ca2+ stores. FEBS Lett. 1990 Nov 12;274(1-2):214–216. doi: 10.1016/0014-5793(90)81366-v. [DOI] [PubMed] [Google Scholar]
- Papp B., Enyedi A., Kovács T., Sarkadi B., Wuytack F., Thastrup O., Gárdos G., Bredoux R., Levy-Toledano S., Enouf J. Demonstration of two forms of calcium pumps by thapsigargin inhibition and radioimmunoblotting in platelet membrane vesicles. J Biol Chem. 1991 Aug 5;266(22):14593–14596. [PubMed] [Google Scholar]
- Pepperell J. R., Behrman H. R. The calcium-mobilizing agent, thapsigargin, inhibits progesterone production in rat luteal cells by a calcium-independent mechanism. Endocrinology. 1990 Oct;127(4):1818–1824. doi: 10.1210/endo-127-4-1818. [DOI] [PubMed] [Google Scholar]
- Polokoff M. A., Bencen G. H., Vacca J. P., deSolms S. J., Young S. D., Huff J. R. Metabolism of synthetic inositol trisphosphate analogs. J Biol Chem. 1988 Aug 25;263(24):11922–11927. [PubMed] [Google Scholar]
- Robinson I. M., Burgoyne R. D. A distinct 2,5-di-(tert-butyl)-1,4-benzohydroquinone-sensitive calcium store in bovine adrenal chromaffin cells. FEBS Lett. 1991 Sep 9;289(2):151–154. doi: 10.1016/0014-5793(91)81057-f. [DOI] [PubMed] [Google Scholar]
- Rooney T. A., Renard D. C., Sass E. J., Thomas A. P. Oscillatory cytosolic calcium waves independent of stimulated inositol 1,4,5-trisphosphate formation in hepatocytes. J Biol Chem. 1991 Jul 5;266(19):12272–12282. [PubMed] [Google Scholar]
- Rossier M. F., Bird G. S., Putney J. W., Jr Subcellular distribution of the calcium-storing inositol 1,4,5-trisphosphate-sensitive organelle in rat liver. Possible linkage to the plasma membrane through the actin microfilaments. Biochem J. 1991 Mar 15;274(Pt 3):643–650. doi: 10.1042/bj2740643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuttleworth T. J. Ca2+ release from inositol trisphosphate-sensitive stores is not modulated by intraluminal [Ca2+]. J Biol Chem. 1992 Feb 25;267(6):3573–3576. [PubMed] [Google Scholar]
- Takemura H., Hughes A. R., Thastrup O., Putney J. W., Jr Activation of calcium entry by the tumor promoter thapsigargin in parotid acinar cells. Evidence that an intracellular calcium pool and not an inositol phosphate regulates calcium fluxes at the plasma membrane. J Biol Chem. 1989 Jul 25;264(21):12266–12271. [PubMed] [Google Scholar]
- Thastrup O., Cullen P. J., Drøbak B. K., Hanley M. R., Dawson A. P. Thapsigargin, a tumor promoter, discharges intracellular Ca2+ stores by specific inhibition of the endoplasmic reticulum Ca2(+)-ATPase. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2466–2470. doi: 10.1073/pnas.87.7.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thastrup O., Foder B., Scharff O. The calcium mobilizing tumor promoting agent, thapsigargin elevates the platelet cytoplasmic free calcium concentration to a higher steady state level. A possible mechanism of action for the tumor promotion. Biochem Biophys Res Commun. 1987 Feb 13;142(3):654–660. doi: 10.1016/0006-291x(87)91464-1. [DOI] [PubMed] [Google Scholar]
- Thastrup O. Role of Ca2(+)-ATPases in regulation of cellular Ca2+ signalling, as studied with the selective microsomal Ca2(+)-ATPase inhibitor, thapsigargin. Agents Actions. 1990 Jan;29(1-2):8–15. doi: 10.1007/BF01964706. [DOI] [PubMed] [Google Scholar]
- Thomas A. P. Enhancement of the inositol 1,4,5-trisphosphate-releasable Ca2+ pool by GTP in permeabilized hepatocytes. J Biol Chem. 1988 Feb 25;263(6):2704–2711. [PubMed] [Google Scholar]
- Thévenod F., Dehlinger-Kremer M., Kemmer T. P., Christian A. L., Potter B. V., Schulz I. Characterization of inositol 1,4,5-trisphosphate-sensitive (IsCaP) and -insensitive (IisCaP) nonmitochondrial Ca2+ pools in rat pancreatic acinar cells. J Membr Biol. 1989 Jul;109(2):173–186. doi: 10.1007/BF01870856. [DOI] [PubMed] [Google Scholar]
- Tsien R. Y., Rink T. J. Ca2+-selective electrodes: a novel PVC-gelled neutral carrier mixture compared with other currently available sensors. J Neurosci Methods. 1981 Jun;4(1):73–86. doi: 10.1016/0165-0270(81)90020-0. [DOI] [PubMed] [Google Scholar]
- Tsien R. Y., Rink T. J. Neutral carrier ion-selective microelectrodes for measurement of intracellular free calcium. Biochim Biophys Acta. 1980 Jul;599(2):623–638. doi: 10.1016/0005-2736(80)90205-9. [DOI] [PubMed] [Google Scholar]
- Verma A., Hirsch D. J., Hanley M. R., Thastrup O., Christensen S. B., Snyder S. H. Inositol trisphosphate and thapsigargin discriminate endoplasmic reticulum stores of calcium in rat brain. Biochem Biophys Res Commun. 1990 Oct 30;172(2):811–816. doi: 10.1016/0006-291x(90)90747-b. [DOI] [PubMed] [Google Scholar]
- Volpe P., Krause K. H., Hashimoto S., Zorzato F., Pozzan T., Meldolesi J., Lew D. P. "Calciosome," a cytoplasmic organelle: the inositol 1,4,5-trisphosphate-sensitive Ca2+ store of nonmuscle cells? Proc Natl Acad Sci U S A. 1988 Feb;85(4):1091–1095. doi: 10.1073/pnas.85.4.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler L. A., Sachs G., De Vries G., Goodrum D., Woldemussie E., Muallem S. Manoalide, a natural sesterterpenoid that inhibits calcium channels. J Biol Chem. 1987 May 15;262(14):6531–6538. [PubMed] [Google Scholar]
- Wollheim C. B., Sharp G. W. Regulation of insulin release by calcium. Physiol Rev. 1981 Oct;61(4):914–973. doi: 10.1152/physrev.1981.61.4.914. [DOI] [PubMed] [Google Scholar]
- Zacchetti D., Clementi E., Fasolato C., Lorenzon P., Zottini M., Grohovaz F., Fumagalli G., Pozzan T., Meldolesi J. Intracellular Ca2+ pools in PC12 cells. A unique, rapidly exchanging pool is sensitive to both inositol 1,4,5-trisphosphate and caffeine-ryanodine. J Biol Chem. 1991 Oct 25;266(30):20152–20158. [PubMed] [Google Scholar]
- de Meis L., Inesi G. Functional evidence of a transmembrane channel within the Ca2+ transport ATPase of sarcoplasmic reticulum. FEBS Lett. 1992 Mar 24;299(1):33–35. doi: 10.1016/0014-5793(92)80093-v. [DOI] [PubMed] [Google Scholar]
- de Smedt H., Eggermont J. A., Wuytack F., Parys J. B., Van den Bosch L., Missiaen L., Verbis J., Casteels R. Isoform switching of the sarco(endo)plasmic reticulum Ca2+ pump during differentiation of BC3H1 myoblasts. J Biol Chem. 1991 Apr 15;266(11):7092–7095. [PubMed] [Google Scholar]


