Abstract
Purpose of review
Skin type diversity in image datasets refers to the representation of various skin types. This diversity allows for the verification of comparable performance of a trained model across different skin types. A widespread problem in datasets involving human skin is the lack of verifiable diversity in skin types, making it difficult to evaluate whether the performance of the trained models generalizes across different skin types. For example, the diversity issues in skin lesion datasets, which are used to train deep learning-based models, often result in lower accuracy for darker skin types that are typically under-represented in these datasets. Under-representation in datasets results in lower performance in deep learning models for under-represented skin types.
Recent findings
This issue has been discussed in previous works; however, the reporting of skin types, and inherent diversity, have not been fully assessed. Some works report skin types but do not attempt to assess the representation of each skin type in datasets. Others, focusing on skin lesions, identify the issue but do not measure skin type diversity in the datasets examined.
Summary
Effort is needed to address these shortcomings and move towards facilitating verifiable diversity. Building on previous works in skin lesion datasets, this review explores the general issue of skin type diversity by investigating and evaluating skin lesion datasets specifically. The main contributions of this work are an evaluation of publicly available skin lesion datasets and their metadata to assess the frequency and completeness of reporting of skin type and an investigation into the diversity and representation of each skin type within these datasets.
Supplementary Information
The online version contains material available at 10.1007/s13671-024-00440-0.
Keyword: Fitzpatrick skin type · Skin lesion datasets · Skin type diversity · Deep learning
Introduction
Diversity is an important feature in datasets used for training artificial intelligence (AI) based models, as the performance of AI is only as good as its data. In this paper, “skin type diversity” refers to the range and representation of different skin types within the human skin image dataset. It provides the opportunity to verify the comparable performance of a trained model for each skin type. Authors frequently report ethnicity instead of skin type, but ethnicity and skin type are not the same, as many ethnicities can have diverse skin types. There are some other works whose datasets focus on the representation of varied ethnicities [1]. Ethnicity is a wider and more complex concept that refers to groups characterized by shared geographical, ancestral origin, cultural, religious, linguistic, or other shared characteristics [2]. Sufficient diversity should encompass a range of skin tones that adequately represent the population being studied, enabling an assessment of whether a particular skin type is under-represented to a degree that it impacts the reliability of the AI model [3]. Addressing this issue is crucial, as it can result in AI models favoring the majority class, reducing accuracy for the minority class. Techniques like resampling, cost-sensitive learning, over-sampling, under-sampling, and ensemble methods help to balance datasets and improve models’ performance [4, 5]. These approaches are particularly useful for addressing insufficient skin type diversity by ensuring that all skin types are adequately represented and learned by the model, thereby enhancing the model's ability to perform comparably well across different skin types.
In examining the performance of AI on human skin, particularly regarding its lower accuracy for dark-skinned individuals, it is important to recognize that the observed disparities may not solely be due to algorithmic bias. It might be attributed to broader systemic inequities in data collection, demographic characteristics of participants, their socioeconomic status, and other sociological factors [6, 7]. In this paper, the term bias refers to the inadequate representation of skin types in the training datasets and the resulting difference in the performance of trained AI models for certain skin types [8]. This issue can potentially lead to the exclusion of certain groups of people by AI-based models.
The effect of inadequate skin type diversity and under-representation of dark-skinned people in datasets can be seen in many AI-based technologies. For example, AI systems that judge beauty pageant winners are biased against darker-skinned contestants [9]. In a beauty contest run by Beauty.ai, the 44 finalists were judged by the algorithms as the most attractive, except for six who were described as “Asian”, and all were described as “white”. Only one finalist was dark-skinned [10, 11]. Another study investigating the performance of object detection systems on pedestrians with different skin types showed higher precision on lighter skin types than on darker skin types [12]. In another work, bias in face verification applications and datasets was evaluated concerning different skin types, and found that recognition accuracy was reduced for darker-skinned people [13]. The effect of this issue on the performance of robotic systems such as a robot peacekeeper, a self-driving car, and a medical robot was assessed [14]. It was shown that current AI and robotic systems have lower performance for certain skin types.
In AI in healthcare sectors, there are consumer wearable devices that are used for tracking activity, sleep, and other health-related purposes, but due to some limitations, these health products may only be useful for light-skinned people. Findings show that these devices are inaccurate, and even may not work at all for dark-skinned people [1, 15]. While other literature has pointed out potential inaccuracies in pulse oximetry for individuals with darker skin tones, the findings show that the Apple Watch, which employed the Fitzpatrick skin type scale in its model, did not exhibit such limitations seen in traditional pulse oximeters that can be affected by skin pigmentation and performed consistently across different skin types [16]. However, the mentioned examples indicate that the needs of darker skin population groups are not well-represented [17], which can potentially lead to reduced accuracy for dark-skinned groups by deep learning-based models. Several factors play a role in the biased performance of these models towards dark-skinned people.
A significant reason among these is the lack of skin type diversity in datasets used for training AI-based models, the absence of reliable labels for each sample, and consequently, a lack of evaluation of the model's performance on a per skin type basis [1, 15]. There are many reasons for not having enough data from dark-skinned people in datasets used for AI applications. For example, in the case of skin lesion datasets, reasons include low incidence of skin cancer in dark-skinned people [18, 19], unequal access to healthcare [20], poor quality images due to poor quality of care [21, 22] and algorithms with different performance for certain groups of people used in digital cameras as well as computer software [23, 24] contribute to unbalanced datasets. Consequently, dark-skinned people are under-represented in datasets from health services as well as research datasets [20]. Deep learning-based models trained on lighter-skinned subjects are at risk of poor performance for people with darker skin [25].
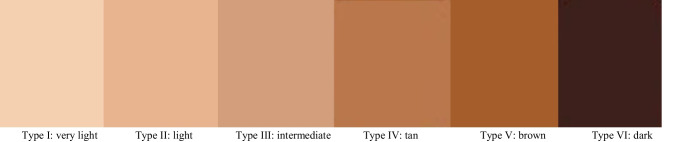
Due to the problems mentioned above, it is necessary to evaluate and quantify skin type diversity to detect under-representation in datasets before using them for training AI systems. Doing this helps to prevent models from having a lower performance for darker-skinned groups of people [26]. The Fitzpatrick scale might be helpful in this regard which provides a skin tone classification based on reaction to exposure to sunlight [27]. While it provides a useful framework for categorizing skin types, it may not fully capture the full spectrum of the human skin diversity needed for training AI models [26]. This scale is used in dermatology to classify skin tones into six numbered categories as shown in Fig. 1. Despite its limitations, the Fitzpatrick skin type scale has previously been used to evaluate skin type diversity in datasets [26].
Fig. 1.
The range of skin tones in the Fitzpatrick skin type scale classifies skin tones into six types
Although the issue of inadequate skin type diversity has been discussed in previous works, these have not attempted to evaluate skin type diversity for datasets. For instance, in the Gender Shades study [28], the Fitzpatrick scale was used to evaluate the PPB, IJB-A, and Adience datasets. However, rather than measuring skin type diversity over six separate Fitzpatrick skin type categories, the authors instead classify the images in these datasets using two aggregate groups—darker and lighter.
To mitigate discrimination against certain groups of people, the FairFace dataset was created, a balanced face image dataset for seven race groups that provides more accurate and consistent modeling across different race and gender groups [29]. However, this work focuses on ethnic diversity and does not report skin type diversity. A new method was proposed using computer simulations to detect biases in face detection using Bayesian parameter search in high dimensional feature space. Although the Fitzpatrick scale was considered for the identification of demographic biases in commercial face application programming interfaces (APIs), skin type diversity was not measured [30].
A new method was introduced for human skin detection, not using color information, but rather using a U-Net-based segmentation network [31]. This method was tested on two datasets containing face images: ECU (Edith Cowan University) and RFW (Racial Faces in the Wild). ECU is an imbalanced dataset created based on six different Fitzpatrick skin types and RFW is a balanced dataset with only the annotation of ethnicity, based on four test subsets: “Caucasian”, “Asian”, “Indian”, and “African”. In the case of the RFW dataset, it is not evaluated based on Fitzpatrick skin type but just based on ethnicity.
Casual Conversations was created, which is a fair and diverse dataset of videos collected from seven countries for AI applications, labeled based on the two skin tone scales of Monk [32] and Fitzpatrick [27]. Nonetheless, the authors do not report any measurement of skin type diversity for their dataset [33]. The SkinCon dataset was created for training models related to skin diseases, which contains labels for different skin types [34]. This dataset was constructed from two skin disease image datasets: Fitzpatrick 17 k [26] and Diverse Dermatology Images (DDI) [35]. Although the Fitzpatrick skin type scale is mentioned in this work, no measurement of skin type diversity is presented. Skin lesion image datasets were assessed for diversity based on their metadata including age, gender, ethnicity, and skin type. The authors mentioned that there is limited reporting on skin type in the metadata and also less representation of darker-skinned people in skin lesion datasets. However, the authors did not measure skin type diversity in any of the skin lesion datasets [1].
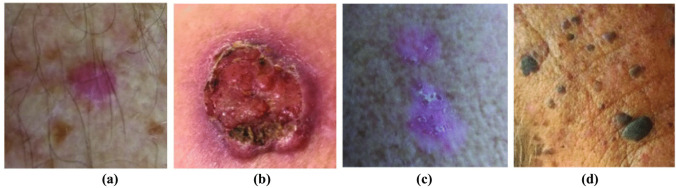
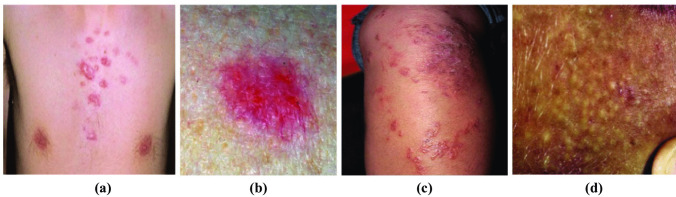
To measure skin type diversity and detect under-representation in datasets used for training deep learning-based models, Fitzpatrick skin type metadata should be included in the datasets [26]. Accessing this information is a crucial step to not only detect under-representation in datasets, but also help to avoid training models on datasets with inadequate skin type diversity, and as a result prevent models from performing poorly for darker-skinned groups of people. According to our investigation, three available skin lesion datasets provide Fitzpatrick scale skin type metadata, labeled by dermatologists: PAD-UFES-20 [36], Fitzpatrick 17k [26], and DDI [35]. To investigate the issue of inadequate skin type diversity in datasets used for training deep learning models, just two datasets—PAD-UFES-20 and Fitzpatrick 17 k—are utilized as examples in this review. DDI was not used because it is a balanced dataset (albeit for three aggregate skin type groups, rather than for all six Fitzpatrick skin types). Sample images from the PAD-UFES-20 and Fitzpatrick 17k datasets are shown in Fig. 2 and Fig. 3, respectively.
Fig. 2.
Some sample images from the PAD-UFES-20 dataset. (a) Skin type I. (b) Skin type II. (c) Skin type III. (d) Skin type IV
Fig. 3.
Some sample images from the Fitzpatrick 17k dataset. (a) Skin type I. (b) Skin type II. (c) Skin type III. (d) Skin type IV
Investigation of metadata in these two datasets is helpful to assess skin type diversity and check to what extent the lack of diversity in the datasets potentially leads to discrimination by models trained on the datasets. The main contributions of this study are an investigation into reporting skin type information in available skin lesions datasets, a significant extension of the work by [1], and an investigation into the diversity and representation of specific skin types within these datasets. Previous similar work by [37] discussed the lack of transparency in medical skin datasets and the necessity of demographic descriptions such as ethnicity and Fitzpatrick skin type for further analysis and deep learning applications. However, the authors do not address the potential limitations in skin type diversity within the investigated datasets, although the Fitzpatrick scale is included. Also, the two publicly available datasets, PAD-UFES-20 and Fitzpatrick 17 k, were published without thoroughly assessing skin type diversity by evaluating the representation of various skin types and ethnicities and ensuring a balanced distribution across different skin tones.
Given the examples of AI model underperformance for individuals with certain skin colors, having a reliable skin type label for each sample can significantly help address the under-representation issue in human skin-related databases, although alternative methods like skin type classification algorithms as a pre-processing step are also viable options [38]. Therefore, this study makes a significant contribution in this regard by:
1. Providing an investigation into publicly available skin lesion datasets to determine the extent of coverage in terms of reporting on skin types compared to other reported metadata in these datasets.
2. Presenting a comprehensive evaluation of skin type diversity level in three datasets where skin type metadata is provided. The results of this analysis are noteworthy, showing an inadequate representation of skin types in these datasets, which can be addressed by technical solutions.
This review emphasizes the danger of implementing algorithms on datasets lacking transparency and diversity, as supported by prior studies [39–41].
Methods
The selection process used in our review to identify papers that used publicly available skin lesion datasets was based on the PRISMA statement [42]. The databases of PubMed, Elsevier, Springer, Google Scholar, and IEEE Xplore were searched. In our initial search, the following search terms were used: “skin cancer detection”, “skin lesion segmentation”, “skin lesion augmentation”, “balancing skin lesion datasets”, “skin lesion datasets”, “Fitzpatrick skin type metadata skin lesion”, “Fitzpatrick skin typology angle”, and “skin type diversity in skin lesion datasets” to identify papers on skin type diversity that make use of skin lesion image datasets. Table 1 provides a summary of which datasets are used in each of the selected papers. Section 3 includes a review of a subset of the identified datasets that match the following criteria: gender, age, ethnicity, and skin type.
Table 1.
A subset of papers identified through the PRISMA process that used publicly available skin lesion datasets. We have attempted to select a subset that spans the majority of the skin lesion datasets used in the full list of identified papers. (C: Clinical images, D: Dermoscopic images)
| Author | Year | Dataset | Image |
|---|---|---|---|
| Mendonça et al [43] | 2013 | PH2 | D |
| Saez et al [44] | 2014 | Interactive Atlas of Dermoscopy | D |
| Sun et al [45] | 2016 | SD-198 | C |
| Liao et al [46] | 2016 | AtlasDerm / Danderm / DermIS / Dermnet / Derma / DermQuest (Derm101) | D |
| Kawahara et al [47] | 2016 | Dermofit Image Library | D |
| Ge et al [48] | 2017 | MoleMap / ISBI-2016 | D |
| Lopez et al [49] | 2017 | Dermofit Image Library / Dermnet / ISBI 2016 Challenge | D |
| Kawahara et al [50] | 2018 | 7-point checklist | C |
| Han et al [51] | 2018 | Asan Dataset / MED-NODE | C |
| Gutman et al. [52] | 2018 | ISIC-MSK-2 | D |
| Han et al [53] | 2018 | Edinburgh Dermofit Image Library / Hallym | C/ D |
| Shoieb and Youssef [54] | 2018 | DermQuest / MED-NODE / DermIS | C/ D |
| Goyal et al [55] | 2018 | ISBI 2017 / PH2 / HAM10000 | D |
| Mendes and da Silva [56] | 2018 | MED-NODE / Atlas / Edinburgh | C/ D |
| Gonzalez-Diaz [57] | 2018 | 2017 ISBI challenge / EDRA / ISIC Archive | D |
| Yang et al [58] | 2019 | SD-198 / SD-260 | |
| Brinker TJ et al [59] | 2019 | MClass-D | C |
| Combalia et al [60] | 2019 | BCN20000 | D |
| Xie et al [61] | 2019 | XiangyaDerm | C |
| He et al [62] | 2019 | Skin-10 / Skin-100 | C |
| Pacheco et al [36] | 2020 | PAD-UFES-20 | C |
| Han et al [63] | 2020 | SNU / Edinburgh | C |
| Han et al [63] | 2020 | Normal / Web | C |
| Milantev et al [64] | 2020 | SD-198 / MED-NODE / PH2 / SKINL2v2 / Seven-Point / Light Field Image | C/ D |
| Andrade et al [65] | 2020 | SMARTSKINS / Dermofit Image Library | C |
| Zhang et al [66] | 2020 | Skin-Cancer-Detection (SCD) / ISIC 2018 | D |
| Hasan et al [67] | 2021 | Skin Cancer Benign vs. Malignant | D |
| Abhishek et al [68] | 2021 | Interactive Atlas of Dermoscopy / MClass-D | D |
| Maron et al [69] | 2021 | HAM10000 / PH2 / SKINL2 / BCN20000/ PROP | D |
| Krohling et al [70] | 2021 | PAD-UFES-20 | C |
| Yao et al [71] | 2021 | ISIC 2018 / Seven-Point Criteria Evaluation (7-PT) | C/ D |
| Groh et al [26] | 2021 | Fitzpatrick 17 k | C |
| Abbas et al [72] | 2021 | Yonsei University Hospital | D |
| Ali et al [73] | 2022 | Monkeypox Skin Lesion Dataset (MSLD) | C |
| Alenezi et al [74] | 2023 | ISIC-2019, 2020 | D |
Results
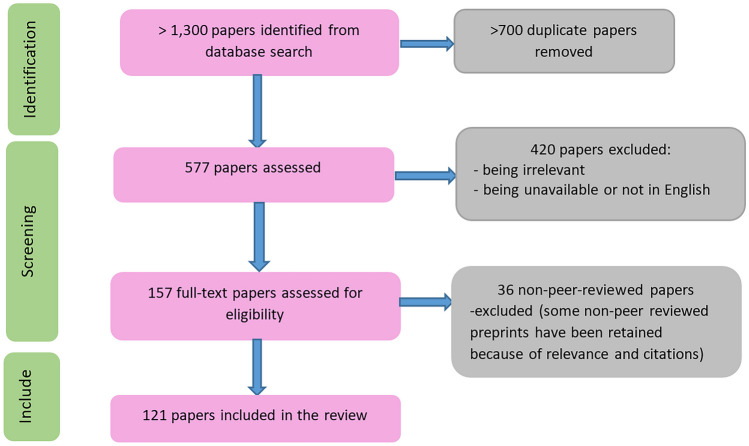
Our initial search (using the search terms listed in Sect. 2) returned over 1,400 publications as shown in Fig. 4. In the first screening, more than 800 duplicate papers were eliminated, leaving 690 papers to be assessed. In the second step, a further 513 publications were excluded due to lack of relevance (did not use skin lesion datasets), or being unavailable (including those not accessible without payment in Technological University Dublin), leaving 177 papers to be assessed for eligibility. Of these, 45 were excluded due to not being peer-reviewed. Ultimately, 132 publications were included in the systematic review.
Fig. 4.
PRISMA flow chart of study selection
The 132 papers identified from the search process used one or more publicly available skin lesion datasets. Table 1 shows a subset of these papers.1
As shown in Table 1, there are overlaps between papers using the same groups of skin lesion datasets. Through the process, 54 different skin lesion datasets were identified from these papers. Table 2 summarizes each dataset’s reporting of the following metadata: age, gender, ethnicity, and Fitzpatrick skin type. The number of images is also shown.
Table 2.
54 different publicly available skin lesion datasets used in publications and their reporting of four main metadata, showing a lack of reporting of skin type information to cover skin type diversity in datasets
| Skin lesion datasets | No. Images | Metadata | |||
|---|---|---|---|---|---|
| Gender | Age | Ethnicity | Skin type | ||
| 7-point criteria evaluation dataset [50] | > 2,000 | ✔ | - | - | - |
| Asan [53] | 120,780 | ✔ | ✔ | ✔ | - |
| Atlas [56] | 3,816 | - | - | - | - |
| AtlasDerm [75] | 9,503 | - | - | ✔ | - |
| BCN20000 [60] | 19,424 | ✔ | ✔ | - | - |
| Cancer Genome Atlas [76] | 2,860 | - | - | - | - |
| Clinical Atlas [77] | 839 | - | - | - | - |
| DanDerm [78] | 1,110 | - | - | ✔ | - |
| Derm7pt [50] | > 2000 | - | - | - | - |
| Derm101 [79] | 107,656 | - | - | ✔ | - |
| Dermatology Dataset [80] | 336 | - | ✔ | - | - |
| DermIS [81, 82] | 7,172 | - | ✔ | ✔ | - |
| Dermnet [46] | 19,500 | - | - | ✔ | - |
| DermNet NZ [75] | 246 | - | - | - | - |
| Dermofit Image Library [83] | 1300 | - | - | ✔ | - |
| Dermoscopic Atlas [77] | 872 | - | - | - | - |
| Dermoscopy Skin Lesion Multispectral Image Database [84] | 30 | - | - | - | - |
| DermQuest [81] | 137 | - | - | - | - |
| DDI [35] | 656 | ✔ | ✔ | - | ✔ |
| Edinburgh [85] | 1,300 | ✔ | ✔ | ✔ | - |
| EDRA Interactive Atlas of Dermoscopy [76] | 1,000 | - | - | - | - |
| Fitzpatrick 17k [26] | 16,577 | - | - | - | ✔ |
| Hallym [51] | 152 | ✔ | ✔ | ✔ | - |
| HAM10000 [77] | 10,015 | ✔ | ✔ | - | - |
| Interactive Atlas of Dermoscopy (IAD) [76] | > 2, 000 | - | - | - | - |
| ISBI 2016 [52] | 1,279 | - | - | - | - |
| ISBI 2017 [86] | 2,750 | - | - | - | - |
| ISIC Challenge 2020 [87] | 33,126 | ✔ | ✔ | ✔ | - |
| ISIC-MSK [52] | 225 | ✔ | ✔ | - | - |
| ISIC-UDA [52] | 557 | - | - | - | - |
| Kaggle [75] | 367 | - | - | - | - |
| Light Field Image [88] | 250 | ✔ | ✔ | - | - |
| MClass [89] | 100 | - | - | - | - |
| MED-NODE [90] | 170 | - | - | - | - |
| MoleMap [82, 91] | 102,451 | - | - | - | - |
| Monkeypox Skin Lesion Dataset (MSLD) [73] | 228 | - | - | ✔ | - |
| Normal [63] | 48,271 | ✔ | ✔ | ✔ | - |
| OLE [46] | 1,300 | - | - | - | - |
| PAD-UFES-20 [36] | 2,299 | ✔ | ✔ | - | ✔ |
| PH2 [43] | 200 | - | - | - | - |
| SD-128 [45] | 5,619 | - | - | ✔ | - |
| SD-198 [45, 92] | 6,584 | ✔ | ✔ | ✔ | - |
| SD-260 [58] | 20,600 | ✔ | ✔ | ✔ | - |
| SIIM-ISIC Melanoma [87] | 33,126 | ✔ | ✔ | - | - |
| Skin-10 [62] | 10,218 | - | - | - | - |
| Skin-100 [62] | 19,807 | - | - | - | - |
| Skin Cancer’ Malignant vs. Benign [93, 94] | 6,594 | - | - | - | - |
| SkinCon [34] | 3230 | - | - | - | - |
| SkinL2 [88] | 376 | - | - | - | - |
| SMARTSKINS [95] | - | ✔ | ✔ | - | - |
| SNU [63] | 2,201 | ✔ | ✔ | ✔ | - |
| Web [63] | 51,459 | ✔ | ✔ | ✔ | - |
| XiangyaDerm [61] | 107,565 | - | - | - | - |
| Yonsei University Health System South Korea [96] | 724 | - | - | - | - |
Ideally, skin lesion datasets should achieve skin type diversity as well as have transparency in their metadata. As a result, not only would their diversity be easily measured, but also any imbalance would be detected before training models using these datasets. As seen in Table 2, only three datasets: PAD-UFES-20, Fitzpatrick 17k, and DDI provide metadata on skin type. They have skin type labels based on the Fitzpatrick rating system [1]. Figure 5 also shows the breakdown of reporting in the metadata for gender, age, ethnicity, and skin type. As shown, skin type metadata is the least frequently provided, being included in just 3 of 54 datasets (5.56%). Age metadata were the most frequently provided, being included in 35.19% of the datasets.
Fig. 5.
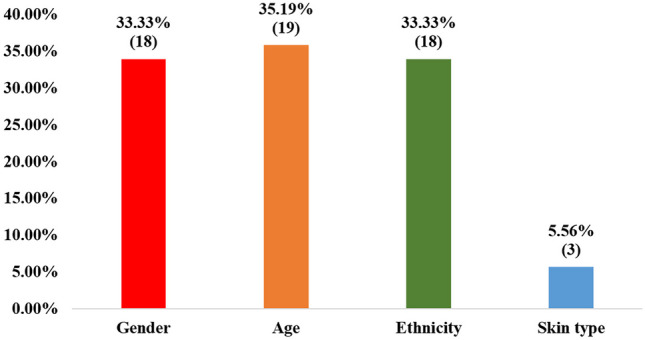
Percentage of the 54 skin lesion datasets that provide metadata for gender, age, ethnicity, and skin type respectively
Although the PAD-UFES-20 and Fitzpatrick 17 k datasets provide skin type metadata, they contain far fewer images of darker skin types (e.g. only 635 out of 16,577 images in Fitzpatrick 17 k are of skin type VI, and only one image of skin type VI in PAD-UFES-20). Thus, apart from the lack of reporting of skin type metadata, even if datasets cover skin type information, there is no guarantee that they have enough representation for darker-skinned groups. Figure 6 and Fig. 7 show the distributions of skin types in the PAD-UFES-20 and Fitzpatrick 17 k datasets respectively. It can be seen that skin type VI accounts for the lowest percentage in both datasets: 0.07% in PAD-UFES-20 and 3.97% in Fitzpatrick 17 k. Note that in the Fitzpatrick 17k dataset, the full number of images is 16,577, but 565 images were excluded because they had unknown Fitzpatrick skin types (labeled “-1”).
Fig. 6.
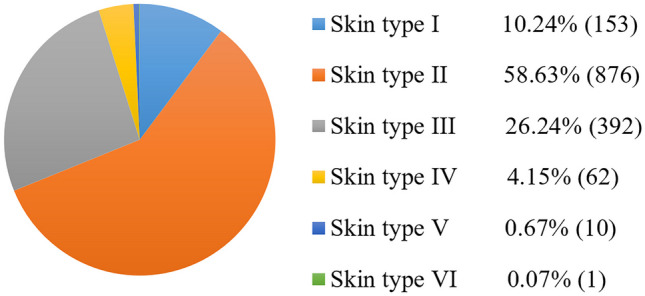
Skin type distribution for 1,494 images in the PAD-UFES-20 dataset [36], according to dermatologist-assigned Fitzpatrick scale labels
Fig. 7.
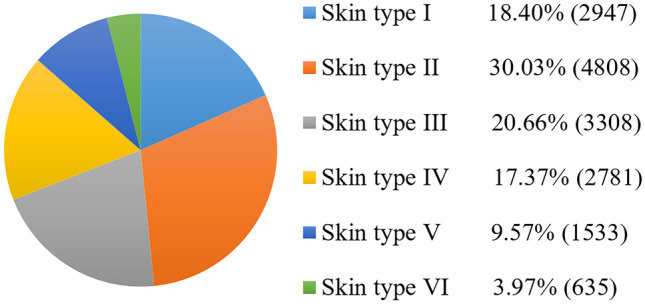
Skin type distribution for 16,012 images in the Fitzpatrick 17 k dataset [26], according to dermatologist-assigned Fitzpatrick scale labels. The original number of images was 16,577, but 565 images had unknown Fitzpatrick skin types
As shown in Fig. 8, the DDI dataset metadata classifies images into three skin type groups, rather than providing exact information for each of the six individual Fitzpatrick skin types. Therefore, although the dataset is balanced concerning these three groups, it does not guarantee that each skin type group is balanced. More importantly, due to its small size, it is not suitable for generalizing deep-learning models for all skin types. In the case of ethnicity labels, it should be noted that ethnicity is different from skin type. To a significant degree, shared ethnicity reflects shared ancestry, but people of the same ethnic group can have a wide range of skin types.
Fig. 8.
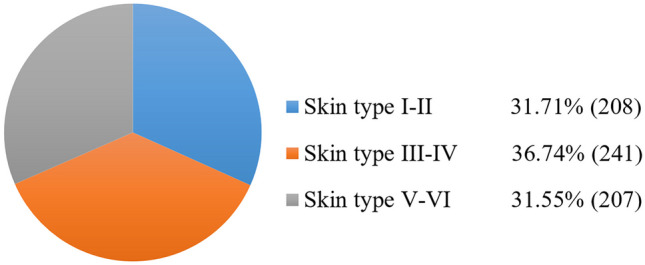
Skin type distribution for the 656 images in the DDI dataset [35], according to dermatologist-assigned Fitzpatrick scale labels
Conclusions
Summary of findings
This study is the first review to date that investigates publicly available skin lesion datasets and their metadata in detail for the important issue of inadequate skin type diversity. As these datasets are used for training deep learning models, inadequate skin type diversity within the datasets could affect the performance of the models, in terms of having low accuracy against specific groups of people [35, 97]. To overcome this issue, it is important that, firstly, information about skin type distribution be provided for datasets, and secondly, that skin type diversity be evaluated in detail to facilitate downstream research and ensure that balanced methods are specified for achieving diverse representation before using the datasets for training models.
The issue of inadequate skin type diversity has been discussed in previous works, but without reporting a measurement for each skin type. For example, in the Gender Shades study [28], although the authors used the Fitzpatrick skin type descriptions for their facial image datasets, they just divided the datasets into two skin type groups: darker and lighter. A balanced dataset, FairFace, was created according to different ethnicities, rather than different skin types [29]. Also, the issue of skin lesion datasets was discussed but did not measure skin type diversity for those datasets [1]. Failure to report the distribution of skin types used in a dataset raises concerns about the extent to which different populations are represented in that dataset, and also about the generalizability of machine learning algorithms that have been trained using it.
Our results showed a lack of skin type reporting in all identified skin lesion datasets, except three: PAD-UFES-20, Fitzpatrick 17k, and DDI. Of the skin lesion datasets used in the papers identified in our review, these three are the only ones that provide information about skin type using the Fitzpatrick scale. The shortage of skin lesion datasets including skin type information compared to the large number of skin lesion datasets without it, raises concerns about the high potential for underperformance in AI models trained on these datasets.
However, as shown in the results, two datasets—PAD-UFES-20 and Fitzpatrick 17k—have considerably less representation of darker skin. The DDI dataset reports skin tone distribution in three aggregate groups, rather than for each of the six Fitzpatrick skin types; therefore, exact information about the number of images belonging to each skin type is unavailable. Furthermore, it is too small for training a generalized model that works for all skin types. Nevertheless, none of these three datasets includes information about the ethnicity corresponding to the skin type of each image. Also, the results showed that the distinction between ethnicity and skin type should be restated as one ethnicity can include different skin types.
Deep learning-based models should be developed with fairness and equity in mind, aiming to include a representative distribution of all skin tones. If achieving this balance is not possible, the limitations should be transparently reported, including details on metadata, the training process, and any associated challenges, to ensure clarity regarding the model's performance across different skin tones [97]. This review has shown that skin type diversity in skin lesion image datasets is either unquantifiable (due to lack of skin type metadata in the vast majority of datasets) or inadequate (in the three datasets where metadata is provided). To facilitate the evaluation of skin type diversity, datasets should ideally include dermatologist-assigned Fitzpatrick skin type labels. Compared to classifications like ethnicity and race, Fitzpatrick skin type is relatively clearly defined and provides a more objective basis for establishing diversity.
Addressing Under-representation
Some metrics such as ISSintra [96] or alternative metrics [97] can be used to measure the skin type diversity of the datasets used for training the AI models. One of the widely used methods in previous works to measure the representation of different skin types in datasets and lack of diversity involves the use of automatic skin type classification methods, such as individual typology angle (ITA). ITA values show an inverse correlation with skin pigmentation and enable the classification of skin color into six groups, ranging from very light to dark skin [98–100].
Finally, to achieve AI models with a fair performance for each skin type, there are methods, including augmentation [101, 102] or adversarial de-biasing [103–105], and balancing datasets [5] to enhance the fairness of the models and create balanced datasets. For example, in addressing dataset imbalance, authors balance minority classes in skin disease datasets through the utilization of class weighting as a data balancing technique [106]. In [5], the authors addressed class imbalance in the clinical dataset using two resampling methods: SMOTE and under-sampling. SMOTE generates synthetic minority examples based on k-nearest neighbors while under-sampling reduces the majority class size to balance the dataset. In the study by Islam et al. [107] normalization, data reduction, and data augmentation are used in pre-processing steps to classify skin lesions from the HAM10000 dataset. In another study, data up-sampling and augmentation methods were used in skin lesion classification using a convolutional neural network (CNN) to improve the classifier's efficiency for the HAM 10000 dataset [108].
To bridge the gap in the under-representation of darker skin tones, [109] used augmentation methods like flipping, cropping, and rotating on two clinical image skin lesion datasets (DermNet NZ and ISIC 2018). This approach increased the inclusion of dark skin tones, resulting in a higher accuracy of 94% for malignancy detection with the augmented datasets. Mohamed et al. [110] showed how balancing the dataset affected skin lesion classification results using two models, MobileNet and DenseNet121, on the HAM10000 dataset. After applying augmentation methods like zooming, rotation, and flipping, the accuracy improved by 20% for DenseNet121 and 10% for MobileNet. Rezk et al. [97] addressed the shortage of dark skin images in dermatology datasets (DermNet NZ, ISIC, Dermatology Atlas) by creating realistic images of darker skin for better diagnosis of skin lesions in people of color. They used style transfer (ST) and deep blending (DB), with ST transferring styles between images and DB blending features from multiple images. Their findings showed that diverse skin color images improved the model's ability to recognize skin tone variations, though geometric transformations alone weren't sufficient to account for all deviations in skin tone distribution in the test set. Rezk et al. [109] used deep learning to generate darker skin tone images from ISIC and DermNet NZ datasets to improve skin cancer detection models. Their results showed that models trained on diverse datasets, including these generated images, provided more accurate diagnoses for people of color. Additionally, other studies have highlighted the benefits of augmentation techniques in balancing datasets and improving diagnostic accuracy [111–114].
Implications for Future Research
In conclusion, this study underscores the need for sufficient representation of all skin types within datasets, emphasizing the importance of accurate skin type labeling. Achieving fair representation is important for mitigating the underperformance of AI models’ performance, particularly concerning darker skin tones. The disparities in model performance across different skin types can lead to inaccuracies, which may adversely affect the diagnostic accuracy and usability of these models in real-world applications. Strategies such as expanding data collection efforts to ensure adequate representation of diverse skin tones, data augmentation to artificially increase the representation of under-represented skin tones, and transparent reporting to clearly convey the diversity of represented skin tones in datasets, could be employed to achieve more balanced datasets.
Supplementary Information
Below is the link to the electronic supplementary material.
Author Contributions
N.A: Wrote the main manuscript, and prepared figures, and tables. T.B, J.C: Reviewed, edited, and supervised the manuscript.
Funding
Open Access funding provided by the IReL Consortium
Data Availability
No datasets were generated or analysed during the current study.
Compliance with Ethical Standards
Human and Animal Rights and Informed Consent
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors.
Competing Interests
The authors declare no competing interests.
Footnotes
The full list of 132 publications is available here: https://arrow.tudublin.ie/engschelecon/15/
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wen D, et al. Characteristics of publicly available skin cancer image datasets: a systematic review. The Lancet Digital Health. 2022;4(1):e64-74. 10.1016/S2589-7500(21)00252-1 [DOI] [PubMed] [Google Scholar]
- 2.Torrelo A. Atopic dermatitis in different skin types. What is to know?. J Eur Acad Dermatol Venereol. 2014;28:2–4. [DOI] [PubMed]
- 3.Yang Y, et al. Enhancing fairness in face detection in computer vision systems by demographic bias mitigation. In: Proceedings of the 2022 AAAI/ACM conference on AI, ethics, and society. 2022. pp. 813–22.
- 4.Laurikkala J. Improving identification of difficult small classes by balancing class distribution. In: Artificial intelligence in medicine: 8th conference on artificial intelligence in medicine in Europe, AIME 2001 Cascais, Portugal, July 1–4, 2001, proceedings 8. Berlin, Heidelberg: Springer; 2001. pp. 63–6.
- 5.Poolsawad N, Kambhampati C, Cleland J. Balancing class for performance of classification with a clinical dataset. In: Proceedings of the world congress on engineering. 2014. vol. 1, pp. 1–6.
- 6.Kostick-Quenet KM, et al. Mitigating racial bias in machine learning. Journal of Law, Medicine & Ethics. 2022;50(1):92–100. 10.1017/jme.2022.13 [DOI] [PubMed] [Google Scholar]
- 7.Obermeyer Z, Powers B, Vogeli C, Mullainathan S. Dissecting racial bias in an algorithm used to manage the health of populations. Science. 2019;366(6464):447–53. 10.1126/science.aax2342 [DOI] [PubMed] [Google Scholar]
- 8.Norori N, et al. Addressing bias in big data and AI for health care: a call for open science. Patterns. 2021;2(10). [DOI] [PMC free article] [PubMed]
- 9.Fuchs DJ. The dangers of human-like bias in machine-learning algorithms. Missouri S&T’s Peer to Peer. 2018;2(1):1. [Google Scholar]
- 10.Jordan P. Why an AI-judged beauty contest picked nearly all white winners. Motherboard. 2016. Available from: https://www.vice.com/en/article/78k7de/why-an-ai-judged-beauty-contest-picked-nearly-all-white-winners.
- 11.Khalil A, Ahmed SG, Khattak AM, Al-Qirim N. Investigating bias in facial analysis systems: A systematic review. IEEE Access. 2020;8:130751–61. 10.1109/ACCESS.2020.3006051 [DOI] [Google Scholar]
- 12.Wilson B, Hoffman J, Morgenstern J. Predictive inequity in object detection. arXiv preprint arXiv:1902.11097. 2019.
- 13.Lu B, Chen JC, Castillo CD, Chellappa R. An experimental evaluation of covariates effects on unconstrained face verification. IEEE Transactions on Biometrics, Behavior, and Identity Science. 2019;1(1):42–55. 10.1109/TBIOM.2018.2890577 [DOI] [Google Scholar]
- 14.Howard A, Borenstein J. The ugly truth about ourselves and our robot creations: the problem of bias and social inequity. Sci Eng Ethics. 2018;24:1521–36. 10.1007/s11948-017-9975-2 [DOI] [PubMed] [Google Scholar]
- 15.Kamulegeya L, et al. Using artificial intelligence on dermatology conditions in Uganda: A case for diversity in training data sets for machine learning. Afr Health Sci. 2023;23(2):753–63. 10.4314/ahs.v23i2.86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pipek LZ, et al. Comparison of SpO2 and heart rate values on Apple Watch and conventional commercial oximeters devices in patients with lung disease. Sci Rep. 2021;11(1):18901. 10.1038/s41598-021-98453-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Myers West S. Discriminating systems: gender, race, and power in artificial intelligence. 2020.
- 18.Diepgen TL, Mahler V. The epidemiology of skin cancer. Br J Dermatol. 2002;146(s61):1–6. 10.1046/j.1365-2133.146.s61.2.x [DOI] [PubMed] [Google Scholar]
- 19.Gloster HM Jr, Neal K. Skin cancer in skin of color. J Am Acad Dermatol. 2006;55(5):741–60. 10.1016/j.jaad.2005.08.063 [DOI] [PubMed] [Google Scholar]
- 20.Hudson K, Lifton R, Patrick-Lake B, Burchard EG, Coles T, Collins R, Conrad A. The precision medicine initiative cohort program—Building a research foundation for 21st century medicine. Precision Medicine Initiative (PMI) Working Group Report to the Advisory Committee to the Director, ed. 2015.
- 21.Betancourt JR, Tan-McGrory A, Flores E, López D. Racial and ethnic disparities in radiology: a call to action. J Am Coll Radiol. 2019;16(4):547–53. 10.1016/j.jacr.2018.12.024 [DOI] [PubMed] [Google Scholar]
- 22.FitzGerald C, Hurst S. Implicit bias in healthcare professionals: a systematic review. BMC Med Ethics. 2017;18(1):1–8. 10.1186/s12910-017-0179-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crutchley M. Book Review: Race after technology: Abolitionist tools for the New Jim Code. London, England: SAGE Publications Sage UK; 2021. [Google Scholar]
- 24.Kraehe AM, Herman D Jr. Racial encounters, ruptures, and reckonings: Art curriculum futurity in the wake of Black Lives Matter. Art Education. 2020;73(5):4–7. 10.1080/00043125.2020.1789413 [DOI] [Google Scholar]
- 25.Marcus G, Davis E. Rebooting AI: building artificial intelligence we can trust. Vintage. 2019.
- 26.Groh M, Harris C, Soenksen L, Lau F, Han R, Kim A, Koochek A, Badri O. Evaluating deep neural networks trained on clinical images in dermatology with the fitzpatrick 17k dataset. In: Proceedings of the IEEE/CVF conference on computer vision and pattern recognition. 2021. pp. 1820–28.
- 27.Fitzpatrick TB. The validity and practicality of sunreactive skin types I through VI. Arehives of Dermatology. 1997;124:868. [DOI] [PubMed] [Google Scholar]
- 28.Buolamwini J, Gebru T. Gender shades: intersectional accuracy disparities in commercial gender classification. In: Conference on fairness, accountability and transparency. PMLR. 2018. pp. 77–91.
- 29.Karkkainen K, Joo J. Fairface: face attribute dataset for balanced race, gender, and age for bias measurement and mitigation. In: Proceedings of the IEEE/CVF winter conference on applications of computer vision 2021. 2021. pp. 1548–58.
- 30.McDuff D, Cheng R, Kapoor A. Identifying bias in AI using simulation. arXiv preprint arXiv:1810.00471. 2018.
- 31.Xu H, Sarkar A, Abbott AL. Color invariant skin segmentation. In: Proceedings of the IEEE/CVF conference on computer vision and pattern recognition. 2022. pp. 2906–15.
- 32.Monk EP Jr. Skin tone stratification among Black Americans, 2001–2003. Soc Forces. 2014;92(4):1313–37. 10.1093/sf/sou007 [DOI] [Google Scholar]
- 33.Porgali B, Albiero V, Ryda J, Ferrer CC, Hazirbas C. The casual conversations v2 dataset. In: Proceedings of the IEEE/CVF conference on computer vision and pattern recognition. 2023. pp. 10–17.
- 34.Daneshjou R, et al. Skincon: A skin disease dataset densely annotated by domain experts for fine-grained debugging and analysis. Adv Neural Inf Process Syst. 2022;35:18157–67. [Google Scholar]
- 35.Daneshjou R, et al. Disparities in dermatology AI performance on a diverse, curated clinical image set. Sci Adv. 2022;8(31):eabq6147. [DOI] [PMC free article] [PubMed]
- 36.Pacheco AG, et al. PAD-UFES-20: A skin lesion dataset composed of patient data and clinical images collected from smartphones. Data Brief. 2020;32: 106221. 10.1016/j.dib.2020.106221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Daneshjou R, et al. Lack of transparency and potential bias in artificial intelligence data sets and algorithms: a scoping review. JAMA Dermatol. 2021;157(11):1362–9. 10.1001/jamadermatol.2021.3129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chang CC et al. Robust skin type classification using convolutional neural networks. In: 2018 13th IEEE Conference on Industrial Electronics and Applications (ICIEA). IEEE; 2018. pp. 2011–4.
- 39.Musselwhite LW, et al. Racial/ethnic disparities in cervical cancer screening and outcomes. Acta Cytol. 2016;60(6):518–26. 10.1159/000452240 [DOI] [PubMed] [Google Scholar]
- 40.Williams DR, Cooper LA. Reducing racial inequities in health: using what we already know to take action. Int J Environ Res Public Health. 2019;16(4):606. 10.3390/ijerph16040606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yu L, Chen H, Dou Q, Qin J, Heng PA. Automated melanoma recognition in dermoscopy images via very deep residual networks. IEEE Trans Med Imaging. 2016;36(4):994–1004. 10.1109/TMI.2016.2642839 [DOI] [PubMed] [Google Scholar]
- 42.Page MJ, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Int J Surg. 2021;88: 105906. 10.1016/j.ijsu.2021.105906 [DOI] [PubMed] [Google Scholar]
- 43.TPH Mendonça. PH 2-A dermoscopic image database for research and benchmarking. In: 2013, 35th annual international conference of the IEEE Engineering in Medicine and Biology Society (EMBC). IEEE; 2013. pp. 5437–40. [DOI] [PubMed]
- 44.Saez A, Serrano C, Acha B. Model-based classification methods of global patterns in dermoscopic images. IEEE Trans Med Imaging. 2014;33(5):1137–47. 10.1109/TMI.2014.2305769 [DOI] [PubMed] [Google Scholar]
- 45.Sun X, Yang J, Sun M, Wang K. A benchmark for automatic visual classification of clinical skin disease images. In: Computer vision–ECCV 2016: 14th European conference, Amsterdam, The Netherlands, proceedings, part VI 14 2016. Springer International Publishing; 2016. pp. 206–22.
- 46.Liao H, Li Y, Luo J. Skin disease classification versus skin lesion characterization: Achieving robust diagnosis using multi-label deep neural networks. In: 2016 23rd International Conference on Pattern Recognition (ICPR). IEEE; 2016. pp. 355–60.
- 47.Kawahara J, BenTaieb A, Hamarneh G. Deep features to classify skin lesions. In: 2016 IEEE 13th International Symposium on Biomedical Imaging (ISBI). 2016. IEEE. pp. 1397–1400.
- 48.Ge Z et al. Exploiting local and generic features for accurate skin lesions classification using clinical and dermoscopy imaging. In: 2017 IEEE 14th International Symposium on Biomedical Imaging (ISBI 2017). IEEE; 2017. pp. 986–90.
- 49.Lopez AR, Giro-i-Nieto X, Burdick J, Marques O. Skin lesion classification from dermoscopic images using deep learning techniques. In: 2017 13th IASTED International Conference on Biomedical Engineering (BioMed). IEEE; 2017. pp. 49–54.
- 50.Kawahara J, Daneshvar S, Argenziano G, Hamarneh G. Seven-point checklist and skin lesion classification using multitask multimodal neural nets. IEEE J Biomed Health Inform. 2018;23(2):538–46. 10.1109/JBHI.2018.2824327 [DOI] [PubMed] [Google Scholar]
- 51.Han SS, Park GH, Lim W, Kim MS, Na JI, Park I, Chang SE. Deep neural networks show an equivalent and often superior performance to dermatologists in onychomycosis diagnosis: Automatic construction of onychomycosis datasets by region-based convolutional deep neural network. PLoS ONE. 2018;13(1): e0191493. 10.1371/journal.pone.0191493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gutman D et al. Skin lesion analysis toward melanoma detection: a challenge at the international symposium on biomedical imaging (ISBI) 2016, hosted by the International Skin Imaging Collaboration (ISIC). arXiv preprint arXiv:1605.01397. 2016.
- 53.Han SS, Kim MS, Lim W, Park GH, Park I, Chang SE. Classification of the clinical images for benign and malignant cutaneous tumors using a deep learning algorithm. J Investig Dermatol. 2018;138(7):1529–38. 10.1016/j.jid.2018.01.028 [DOI] [PubMed] [Google Scholar]
- 54.Shoieb DA, Youssef SM, An enhanced hybrid model for skin diagnosis using deep convolution neural network. In,. 9th Cairo International Biomedical Engineering Conference (CIBEC). IEEE. 2018;2018:37–40. [Google Scholar]
- 55.Goyal M, Yap MH, Hassanpour S, Yap MH. Region of interest detection in dermoscopic images for natural data-augmentation. arXiv preprint arXiv:1807.10711. 2018.
- 56.Mendes DB, da Silva NC. Skin lesions classification using convolutional neural networks in clinical images. arXiv preprint arXiv:1812.02316. 2018.
- 57.Gonzalez-Diaz I. Dermaknet: Incorporating the knowledge of dermatologists to convolutional neural networks for skin lesion diagnosis. IEEE J Biomed Health Inform. 2018;23(2):547–59. 10.1109/JBHI.2018.2806962 [DOI] [PubMed] [Google Scholar]
- 58.Yang J, Wu X, Liang J, Sun X, Cheng MM, Rosin PL, Wang L. Self-paced balance learning for clinical skin disease recognition. IEEE Transactions on Neural Networks and Learning Systems. 2019;31(8):2832–46. 10.1109/TNNLS.2019.2917524 [DOI] [PubMed] [Google Scholar]
- 59.Brinker TJ, et al. Deep learning outperformed 136 of 157 dermatologists in a head-to-head dermoscopic melanoma image classification task. Eur J Cancer. 2019;113:47–54. 10.1016/j.ejca.2019.04.001 [DOI] [PubMed] [Google Scholar]
- 60.Combalia M, et al. Bcn20000: dermoscopic lesions in the wild. arXiv preprint arXiv:1908.02288. 2019. [DOI] [PMC free article] [PubMed]
- 61.Xie B, et al. XiangyaDerm: a clinical image dataset of asian race for skin disease aided diagnosis. In: Large-scale annotation of biomedical data and expert label synthesis and hardware aware learning for medical imaging and computer assisted intervention: international workshops, LABELS. Springer International Publishing; 2019. pp. 22–31.
- 62.He X, et al. Computer-aided clinical skin disease diagnosis using cnn and object detection models. In: 2019 IEEE international conference on big data (Big Data). 2019. pp. 4839–44.
- 63.Han SS, et al. Augmented intelligence dermatology: deep neural networks empower medical professionals in diagnosing skin cancer and predicting treatment options for 134 skin disorders. J Investig Dermatol. 2020;140(9):1753–6. 10.1016/j.jid.2020.01.019 [DOI] [PubMed] [Google Scholar]
- 64.Milantev S, Olyunin V, Bykov I, Milanteva N, Bessmertny I. Skin lesion analysis using ensemble of CNN with dermoscopic images and metadata. In: Majorov International Conference on Software Engineering and Computer Systems (MICSECS). 2020.
- 65.Andrade C, Teixeira LF, Vasconcelos MJ, Rosado L. Data augmentation using adversarial image-to-image translation for the segmentation of mobile-acquired dermatological images. J Imaging. 2020;7(1):2. [DOI] [PMC free article] [PubMed]
- 66.Zhang J, Petitjean C, Ainouz S. Kappa loss for skin lesion segmentation in fully convolutional network. In: 2020 IEEE 17th International Symposium on Biomedical Imaging (ISBI). IEEE; 2020. pp. 2001–4.
- 67.Hasan MR, Fatemi MI, Monirujjaman Khan M, Kaur M, Zaguia A. Comparative analysis of skin cancer (benign vs. malignant) detection using convolutional neural networks. J Healthcare Eng. 2021;5895156. [DOI] [PMC free article] [PubMed]
- 68.Kumar AB, Jeremy KA, Ghassan HA. Predicting the clinical management of skin lesions using deep learning. Sci Rep. 2021;11(1):7769. 10.1038/s41598-021-87064-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Maron RC, et al. Robustness of convolutional neural networks in recognition of pigmented skin lesions. Eur J Cancer. 2021;145:81–91. 10.1016/j.ejca.2020.11.020 [DOI] [PubMed] [Google Scholar]
- 70.Krohling B, Castro PB, Pacheco AG, Krohling RA. A smartphone based application for skin cancer classification using deep learning with clinical images and lesion information. arXiv preprint arXiv:2104.14353. 2021.
- 71.Yao P, et al. Single model deep learning on imbalanced small datasets for skin lesion classification. IEEE Trans Med Imaging. 2021;41(5):1242–54. 10.1109/TMI.2021.3136682 [DOI] [PubMed] [Google Scholar]
- 72.Abbas QA, Ramzan FA, Muhammad US. Acral melanoma detection using dermoscopic images and convolutional neural networks. Visual Computing for Industry, Biomedicine, and Art. 2021;4:1–2. 10.1186/s42492-021-00091-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ali SN, Ahmed MT, Paul JO, Jahan TA, Sani SM, Noor NA, Hasan TA. Monkeypox skin lesion detection using deep learning models: A feasibility study. arXiv preprint arXiv:2207.03342. 2022.
- 74.Fayadh AL, Ammar AR, Kemal PO. A multi-stage melanoma recognition framework with deep residual neural network and hyperparameter optimization-based decision support in dermoscopy images. Expert Syst Appl. 2023;215: 119352. 10.1016/j.eswa.2022.119352 [DOI] [Google Scholar]
- 75.Zhang L, et al. Design and assessment of convolutional neural network based methods for vitiligo diagnosis. Front Med. 2021;8: 754202. 10.3389/fmed.2021.754202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Argenziano G, et al. Interactive atlas of dermoscopy (Book and CD-ROM). 2000.
- 77.Tschandl P, Rosendahl C, Kittler H. The HAM10000 dataset, a large collection of multi-source dermatoscopic images of common pigmented skin lesions. Scientific Data. 2018;5(1):1–9. 10.1038/sdata.2018.161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liao H. A deep learning approach to universal skin disease classification. CSC: University of Rochester Department of Computer Science; 2016. [Google Scholar]
- 79.Boer A, Nischal KC. Get set for the net-www. derm101. com: a growing online resource for learning dermatology and dermatopathology. 2007. [DOI] [PubMed]
- 80.Güvenir HA, Demiröz G, Ilter N. Learning differential diagnosis of erythemato-squamous diseases using voting feature intervals. Artif Intell Med. 1998;13(3):147–65. 10.1016/S0933-3657(98)00028-1 [DOI] [PubMed] [Google Scholar]
- 81.Hosny KM, Kassem MA, Foaud MM. Classification of skin lesions using transfer learning and augmentation with Alex-net. PLoS ONE. 2019;14(5): e0217293. 10.1371/journal.pone.0217293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mikołajczyk A, Grochowski M, Data augmentation for improving deep learning in image classification problem. In,. international interdisciplinary PhD workshop (IIPhDW). IEEE. 2018;2018:117–22. [Google Scholar]
- 83.Fisher R. 2016. Dermofit Image Library. from https://licensing.edinburgh-innovations.ed.ac.uk/product/dermofit-image-library.
- 84.Lézoray O, Revenu M, Desvignes M. Graph-based skin lesion segmentation of multispectral dermoscopic images. In: 2014 IEEE International Conference on Image Processing (ICIP). IEEE; 2014. pp. 897–901.
- 85.Ballerini L, Fisher RB, Aldridge B, Rees J. A color and texture based hierarchical K-NN approach to the classification of non-melanoma skin lesions. In: Color medical image analysis. 2013. pp. 63–86.
- 86.Codella NC et al. Skin lesion analysis toward melanoma detection: a challenge at the 2017 International Symposium on Biomedical Imaging (ISBI), hosted by the International Skin Imaging Collaboration (ISIC). In: 2018 IEEE 15th International Symposium on Biomedical Imaging (ISBI 2018). 2018. pp. 168–72.
- 87.Rotemberg V, et al. A patient-centric dataset of images and metadata for identifying melanomas using clinical context. Scientific Data. 2021;8(1):34. 10.1038/s41597-021-00815-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.de Faria SM et al. Light field image dataset of skin lesions. In: 2019 41st Annual international conference of the IEEE Engineering in Medicine and Biology Society (EMBC). IEEE; 2019. pp. 3905–08. [DOI] [PubMed]
- 89.Brinker TJ, et al. Comparing artificial intelligence algorithms to 157 German dermatologists: the melanoma classification benchmark. Eur J Cancer. 2019;111:30–7. 10.1016/j.ejca.2018.12.016 [DOI] [PubMed] [Google Scholar]
- 90.Giotis I, Molders N, Land S, Biehl M, Jonkman MF, Petkov N. MED-NODE: A computer-assisted melanoma diagnosis system using non-dermoscopic images. Expert Syst Appl. 2015;42(19):6578–85. 10.1016/j.eswa.2015.04.034 [DOI] [Google Scholar]
- 91.Gu Y, Ge Z, Bonnington CP, Zhou J. Progressive transfer learning and adversarial domain adaptation for cross-domain skin disease classification. IEEE J Biomed Health Inform. 2019;24(5):1379–93. 10.1109/JBHI.2019.2942429 [DOI] [PubMed] [Google Scholar]
- 92.Yang J, Sun X, Liang J, Rosin PL. Clinical skin lesion diagnosis using representations inspired by dermatologist criteria. In: Proceedings of the IEEE conference on computer vision and pattern recognition. 2018. pp. 1258–66.
- 93.Fanconi C. Skin cancer: malignant vs. benign. 2019. Available from: https://www.kaggle.com/datasets/fanconic/skin-cancer-malignant-vs-benign.
- 94.Ashim LK, Suresh N, Prasannakumar CV. A comparative analysis of various transfer learning approaches skin cancer detection. In: 2021 5th International Conference on Trends in Electronics and Informatics (ICOEI). IEEE; 2021. pp. 1379–85.
- 95.Vasconcelos MJ, Rosado L, Ferreira M. Principal axes-based asymmetry assessment methodology for skin lesion image analysis. In: International symposium on visual computing. Springer International Publishing; 2014. pp. 21–31.
- 96.Yu C, Yang S, Kim W, Jung J, Chung KY, Lee SW, Oh B. Acral melanoma detection using a convolutional neural network for dermoscopy images. PLoS ONE. 2018;13(3): e0193321. 10.1371/journal.pone.0193321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rezk E, Eltorki M, El-Dakhakhni W. Improving skin color diversity in cancer detection: deep learning approach. JMIR Dermatology. 2022;5(3): e39143. 10.2196/39143 [DOI] [Google Scholar]
- 98.Wu Y, Tanaka T, Akimoto M. Utilization of individual typology angle (ITA) and hue angle in the measurement of skin color on images. Bioimages. 2020;28:1–8. [Google Scholar]
- 99.Kinyanjui NM et al. Estimating skin tone and effects on classification performance in dermatology datasets. arXiv preprint arXiv:1910.13268. 2019.
- 100.Kinyanjui NM et al. Fairness of classifiers across skin tones in dermatology. In: International conference on medical image computing and computer-assisted intervention. Springer International Publishing; 2020. pp. 320–29.
- 101.Saad MM, Rehmani MH, O’Reilly R. A self-attention guided multi-scale gradient GAN for diversified x-ray image synthesis. In: Irish conference on artificial intelligence and cognitive science. Switzerland: Springer Nature; 2022. pp. 18–31.
- 102.Saad MM, O’Reilly R, Rehmani MH. A survey on training challenges in generative adversarial networks for biomedical image analysis. Artif Intell Rev. 2024;57(2):19. 10.1007/s10462-023-10624-y [DOI] [Google Scholar]
- 103.Mikołajczyk A, Majchrowska S, Carrasco Limeros S. The (de) biasing effect of gan-based augmentation methods on skin lesion images. In: International Conference on medical image computing and computer-assisted intervention. Switzerland: Springer Nature; 2022. pp. 437–47.
- 104.Correa-Medero RL, Patel B, Banerjee I. Adversarial Debiasing techniques towards ‘fair’ skin lesion classification. In: 2023 11th International IEEE/EMBS Conference on Neural Engineering (NER). 2023. pp. 1–4.
- 105.Reimers C et al. Towards learning an unbiased classifier from biased data via conditional adversarial debiasing. arXiv preprint arXiv:2103.06179. 2021.
- 106.El Gannour O, et al. Improving skin diseases prediction through data balancing via classes weighting and transfer learning. Bulletin of Electrical Engineering and Informatics. 2024;13(1):628–37. 10.11591/eei.v13i1.5999 [DOI] [Google Scholar]
- 107.Islam MK et al. Melanoma skin lesions classification using deep convolutional neural network with transfer learning. In: 2021 1st International Conference on Artificial Intelligence and Data Analytics (CAIDA). IEEE; 2021. pp. 48–53.
- 108.Sae-Lim W, Wettayaprasit W, Aiyarak P. Convolutional neural networks using MobileNet for skin lesion classification. In: 2019 16th International Joint Conference on Computer Science and Software Engineering (JCSSE). IEEE; 2019. pp. 242–7.
- 109.Rezk E, Eltorki M, El-Dakhakhni W. Leveraging artificial intelligence to improve the diversity of dermatological skin color pathology: Protocol for an algorithm development and validation study. JMIR Research Protocols. 2022;11(3): e34896. 10.2196/34896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mohamed EH, El-Behaidy WH. Enhanced skin lesion classification using deep convolutional networks. In: 2019 Ninth International Conference on Intelligent Computing and Information Systems (ICICIS). IEEE; 2019. pp. 180–8.
- 111.Alam TM, et al. An efficient deep learning-based skin cancer classifier for an imbalanced dataset. Diagnostics. 2022;12(9):2115. 10.3390/diagnostics12092115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Perez F, et al. Data augmentation for skin lesion analysis. In: OR 2.0 context-aware operating theaters, computer assisted robotic endoscopy, clinical image-based procedures, and skin image analysis: first international workshop, OR 2.0 2018, 5th international workshop, CARE 2018, 7th international workshop, proceedings 5. Springer; 2018.
- 113.Qin Z, et al. A GAN-based image synthesis method for skin lesion classification. Comput Methods Prog Biomed. 2020;195:105568. 10.1016/j.cmpb.2020.105568 [DOI] [PubMed] [Google Scholar]
- 114.Al-Masni MA, Kim DH, Kim TS. Multiple skin lesion diagnostics via integrated deep convolutional networks for segmentation and classification. Comput Methods Prog Biomed. 2020;190:105351. 10.1016/j.cmpb.2020.105351 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.