Abstract
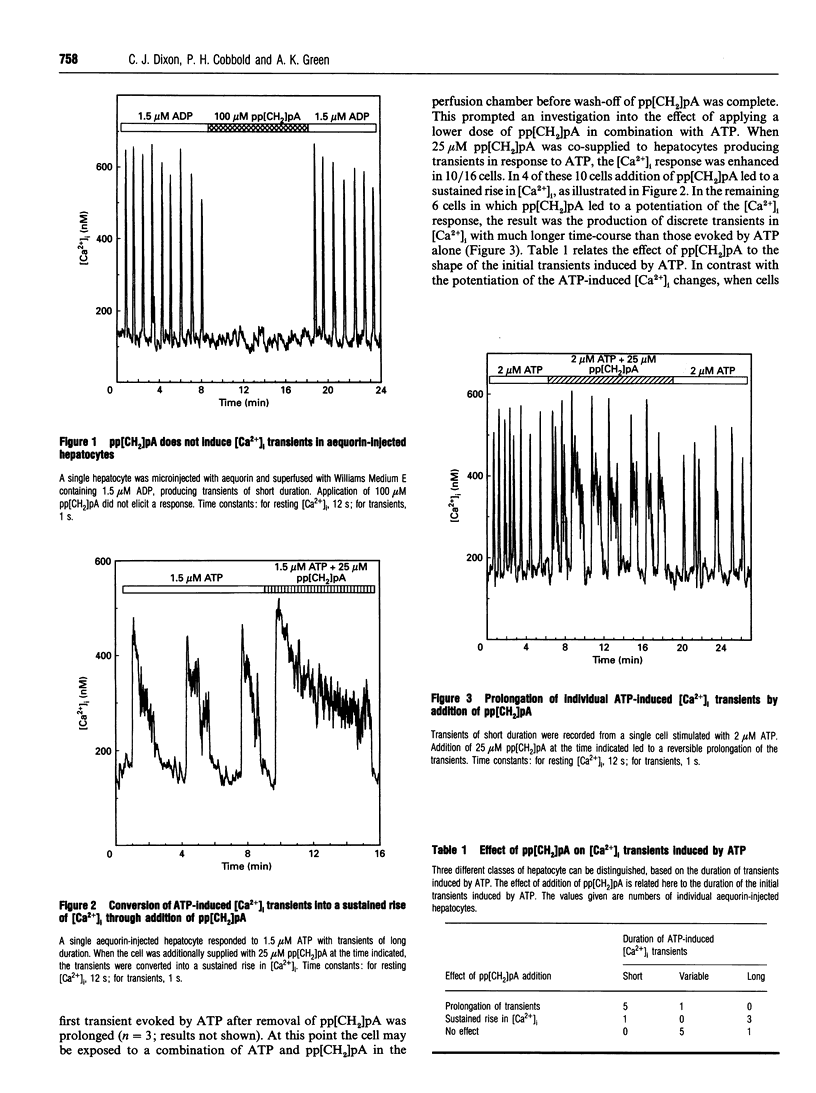
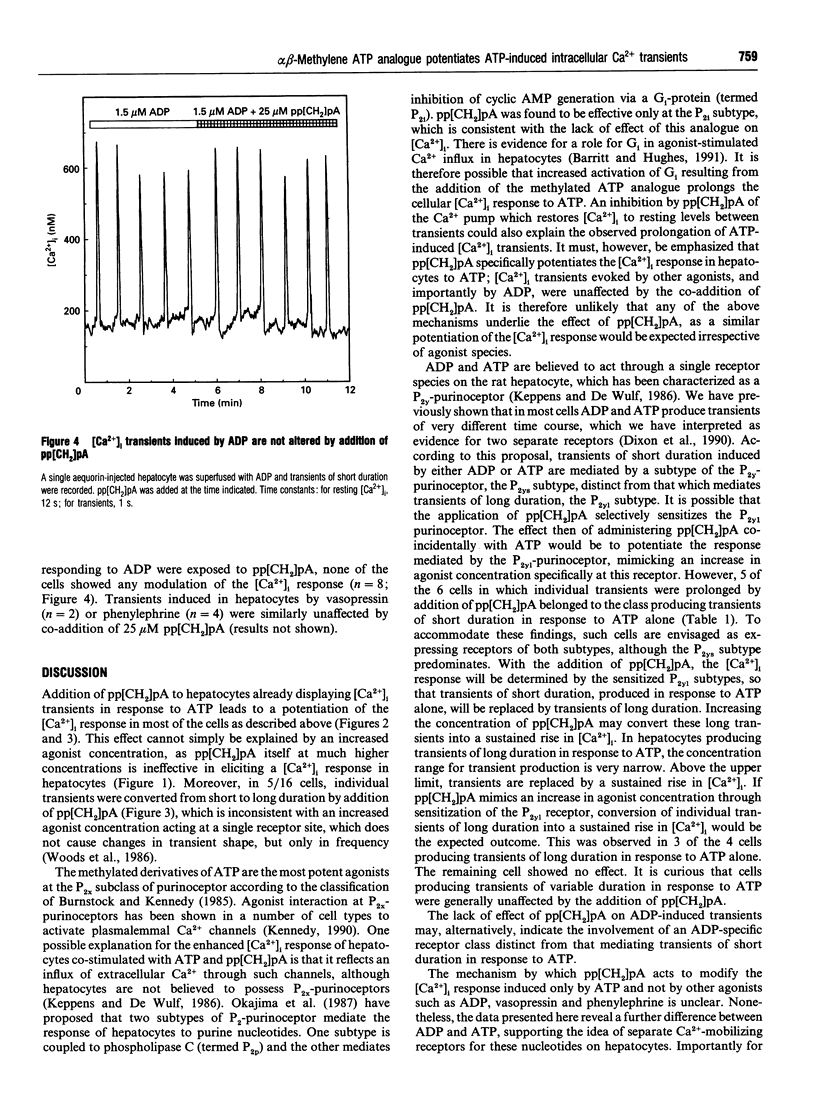
Single rat hepatocytes microinjected with aequorin generate oscillations in cytosolic free Ca2+ concentration ([Ca2+]i) when stimulated with agonists acting through the phosphoinositide signalling pathway. The duration of these transients has been shown to be characteristic of the stimulating agonist, so that transients of very different duration can be induced in the same individual hepatocyte by different agonists. In a previous study we have shown that ADP and ATP, which are believed to act through a single P2y-purinoceptor species, elicit very different [Ca2+]i responses in most of the hepatocytes. We have interpreted this as evidence for two Ca(2+)-mobilizing purinoceptors. The methylated derivative of ATP, adenosine 5'-[alpha beta-methylene]-triphosphate (pp[CH2]pA), is only a weak P2y-purinoceptor agonist. When 100 microM pp[CH2]pA was supplied to aequorin-injected hepatocytes, there was no effect on [Ca2+]i. However, 25 microM pp[CH2]pA co-supplied with ATP causes a potentiation of the [Ca2+]i response in most of the hepatocytes. The effect was specific for ATP-induced transients; [Ca2+]i transients induced by other agonists, and importantly by ADP, were not affected by addition of pp[CH2]pA. This further illustrates differences in the actions of ADP and ATP, strengthening the argument for separate receptors for these nucleotides.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allsup D. J., Boarder M. R. Comparison of P2 purinergic receptors of aortic endothelial cells with those of adrenal medulla: evidence for heterogeneity of receptor subtype and of inositol phosphate response. Mol Pharmacol. 1990 Jul;38(1):84–91. [PubMed] [Google Scholar]
- Bailey S. J., Hourani S. M. A study of the purinoceptors mediating contraction in the rat colon. Br J Pharmacol. 1990 Aug;100(4):753–756. doi: 10.1111/j.1476-5381.1990.tb14087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barritt G. J., Hughes B. P. The nature and mechanism of activation of the hepatocyte receptor-activated Ca2+ inflow system. Cell Signal. 1991;3(4):283–292. doi: 10.1016/0898-6568(91)90056-z. [DOI] [PubMed] [Google Scholar]
- Berridge M. J. Calcium oscillations. J Biol Chem. 1990 Jun 15;265(17):9583–9586. [PubMed] [Google Scholar]
- Brown H. A., Lazarowski E. R., Boucher R. C., Harden T. K. Evidence that UTP and ATP regulate phospholipase C through a common extracellular 5'-nucleotide receptor in human airway epithelial cells. Mol Pharmacol. 1991 Nov;40(5):648–655. [PubMed] [Google Scholar]
- Burnstock G., Kennedy C. Is there a basis for distinguishing two types of P2-purinoceptor? Gen Pharmacol. 1985;16(5):433–440. doi: 10.1016/0306-3623(85)90001-1. [DOI] [PubMed] [Google Scholar]
- Burnstock G., Warland J. J. P2-purinoceptors of two subtypes in the rabbit mesenteric artery: reactive blue 2 selectively inhibits responses mediated via the P2y-but not the P2x-purinoceptor. Br J Pharmacol. 1987 Feb;90(2):383–391. doi: 10.1111/j.1476-5381.1987.tb08968.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charest R., Blackmore P. F., Exton J. H. Characterization of responses of isolated rat hepatocytes to ATP and ADP. J Biol Chem. 1985 Dec 15;260(29):15789–15794. [PubMed] [Google Scholar]
- Cobbold P. H., Sanchez-Bueno A., Dixon C. J. The hepatocyte calcium oscillator. Cell Calcium. 1991 Feb-Mar;12(2-3):87–95. doi: 10.1016/0143-4160(91)90011-3. [DOI] [PubMed] [Google Scholar]
- Cockcroft S., Stutchfield J. The receptors for ATP and fMetLeuPhe are independently coupled to phospholipases C and A2 via G-protein(s). Relationship between phospholipase C and A2 activation and exocytosis in HL60 cells and human neutrophils. Biochem J. 1989 Nov 1;263(3):715–723. doi: 10.1042/bj2630715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson J. S., Wakefield I. K., Sohnius U., van der Merwe P. A., Millar R. P. A novel extracellular nucleotide receptor coupled to phosphoinositidase-C in pituitary cells. Endocrinology. 1990 Jan;126(1):80–87. doi: 10.1210/endo-126-1-80. [DOI] [PubMed] [Google Scholar]
- Dixon C. J., Woods N. M., Cuthbertson K. S., Cobbold P. H. Evidence for two Ca2(+)-mobilizing purinoceptors on rat hepatocytes. Biochem J. 1990 Jul 15;269(2):499–502. doi: 10.1042/bj2690499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubyak G. R., De Young M. B. Intracellular Ca2+ mobilization activated by extracellular ATP in Ehrlich ascites tumor cells. J Biol Chem. 1985 Sep 5;260(19):10653–10661. [PubMed] [Google Scholar]
- Dulon D., Mollard P., Aran J. M. Extracellular ATP elevates cytosolic Ca2+ in cochlear inner hair cells. Neuroreport. 1991 Feb;2(2):69–72. doi: 10.1097/00001756-199102000-00001. [DOI] [PubMed] [Google Scholar]
- Fine J., Cole P., Davidson J. S. Extracellular nucleotides stimulate receptor-mediated calcium mobilization and inositol phosphate production in human fibroblasts. Biochem J. 1989 Oct 15;263(2):371–376. doi: 10.1042/bj2630371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez F. A., Alfonzo R. G., Toro J. R., Heppel L. A. Receptor specific for certain nucleotides stimulates inositol phosphate metabolism and Ca2+ fluxes in A431 cells. J Cell Physiol. 1989 Dec;141(3):606–617. doi: 10.1002/jcp.1041410320. [DOI] [PubMed] [Google Scholar]
- Gonzalez F. A., Rozengurt E., Heppel L. A. Extracellular ATP induces the release of calcium from intracellular stores without the activation of protein kinase C in Swiss 3T6 mouse fibroblasts. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4530–4534. doi: 10.1073/pnas.86.12.4530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon J. L. Extracellular ATP: effects, sources and fate. Biochem J. 1986 Jan 15;233(2):309–319. doi: 10.1042/bj2330309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy C. P1- and P2-purinoceptor subtypes--an update. Arch Int Pharmacodyn Ther. 1990 Jan-Feb;303:30–50. [PubMed] [Google Scholar]
- Keppens S., De Wulf H. Characterization of the biological effects of 2-methylthio-ATP on rat hepatocytes: clear-cut differences with ATP. Br J Pharmacol. 1991 Oct;104(2):301–304. doi: 10.1111/j.1476-5381.1991.tb12426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keppens S., De Wulf H. Characterization of the liver P2-purinoceptor involved in the activation of glycogen phosphorylase. Biochem J. 1986 Dec 1;240(2):367–371. doi: 10.1042/bj2400367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroki M., Takeshige K., Minakami S. ATP-induced calcium mobilization in human neutrophils. Biochim Biophys Acta. 1989 Jun 15;1012(1):103–106. doi: 10.1016/0167-4889(89)90017-7. [DOI] [PubMed] [Google Scholar]
- Martin S. C. ATP activates a Ca(2+)-dependent Cl- current in the rat thyroid cell line, FRTL-5. J Membr Biol. 1992 Feb;125(3):243–253. doi: 10.1007/BF00236437. [DOI] [PubMed] [Google Scholar]
- Merritt J. E., Moores K. E. Human neutrophils have a novel purinergic P2-type receptor linked to calcium mobilization. Cell Signal. 1991;3(3):243–249. doi: 10.1016/0898-6568(91)90050-5. [DOI] [PubMed] [Google Scholar]
- Murphy P. M., Tiffany H. L. Characterization of phagocyte P2 nucleotide receptors expressed in Xenopus oocytes. J Biol Chem. 1990 Jul 15;265(20):11615–11621. [PubMed] [Google Scholar]
- Okajima F., Tokumitsu Y., Kondo Y., Ui M. P2-purinergic receptors are coupled to two signal transduction systems leading to inhibition of cAMP generation and to production of inositol trisphosphate in rat hepatocytes. J Biol Chem. 1987 Oct 5;262(28):13483–13490. [PubMed] [Google Scholar]
- Sage S. O., Reast R., Rink T. J. ADP evokes biphasic Ca2+ influx in fura-2-loaded human platelets. Evidence for Ca2+ entry regulated by the intracellular Ca2+ store. Biochem J. 1990 Feb 1;265(3):675–680. doi: 10.1042/bj2650675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tawada Y., Furukawa K., Shigekawa M. ATP-induced calcium transient in cultured rat aortic smooth muscle cells. J Biochem. 1987 Dec;102(6):1499–1509. doi: 10.1093/oxfordjournals.jbchem.a122197. [DOI] [PubMed] [Google Scholar]
- Wiklund N. P., Gustafsson L. E. Indications for P2-purinoceptor subtypes in guinea pig smooth muscle. Eur J Pharmacol. 1988 Apr 13;148(3):361–370. doi: 10.1016/0014-2999(88)90114-8. [DOI] [PubMed] [Google Scholar]
- Woods N. M., Cuthbertson K. S., Cobbold P. H. Agonist-induced oscillations in cytoplasmic free calcium concentration in single rat hepatocytes. Cell Calcium. 1987 Feb;8(1):79–100. doi: 10.1016/0143-4160(87)90038-8. [DOI] [PubMed] [Google Scholar]
- Woods N. M., Cuthbertson K. S., Cobbold P. H. Repetitive transient rises in cytoplasmic free calcium in hormone-stimulated hepatocytes. Nature. 1986 Feb 13;319(6054):600–602. doi: 10.1038/319600a0. [DOI] [PubMed] [Google Scholar]


