Abstract
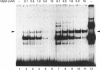
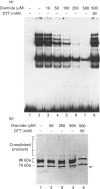
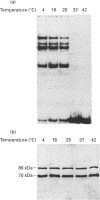
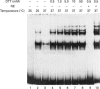
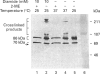
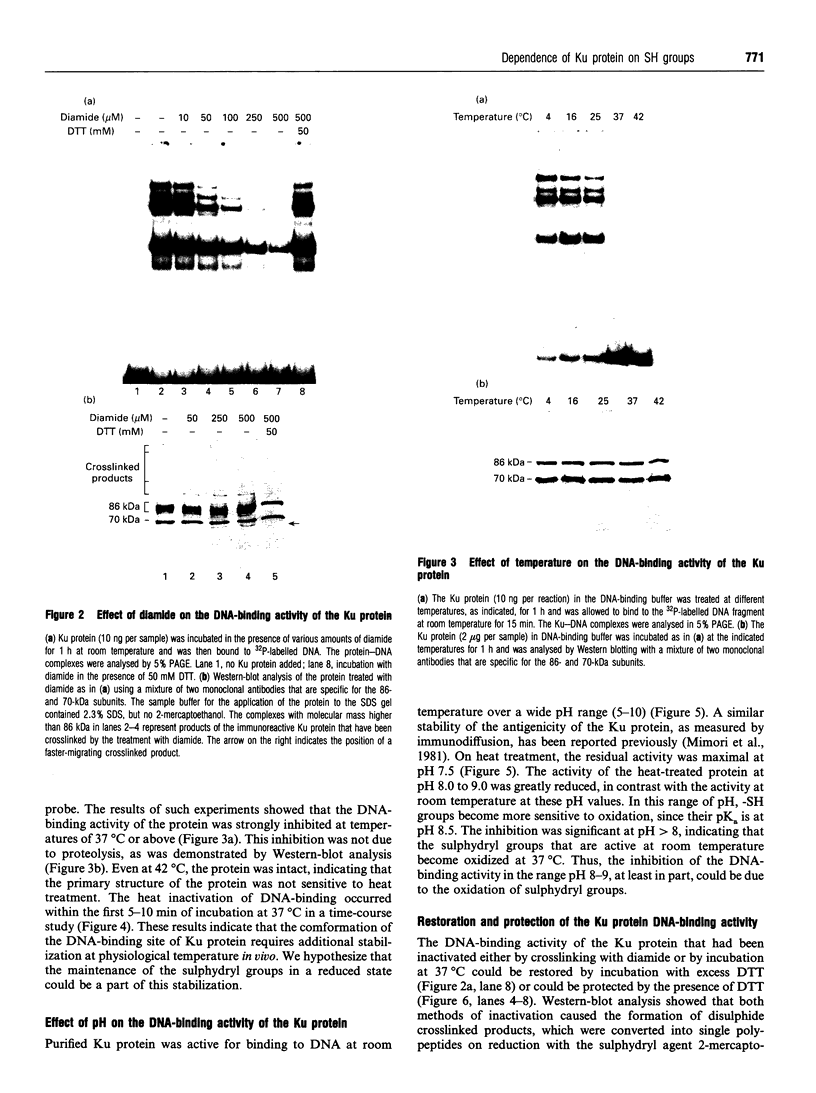
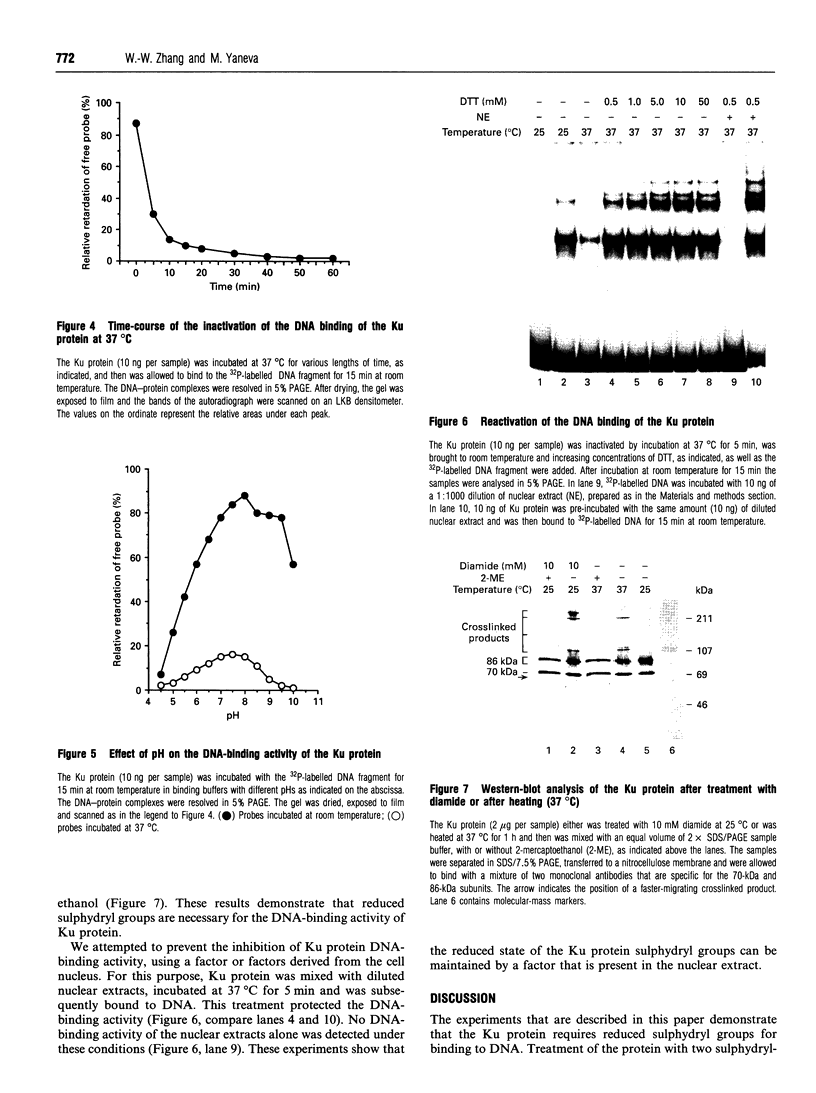
The Ku protein, a DNA-binding complex that is composed of two subunits of 70 kDa and of 86 kDa, has been suggested to play a role in gene transcription. The dependence of the in vitro DNA-binding activity of affinity-purified Ku protein on reduced cysteine residues has been studied using sulphydryl-modifying agents. Inhibition of the DNA-binding activity was caused by alkylation with N-ethylmaleimide and by crosslinking with azadicarboxylic acid bis(dimethylamide). Treatment of the protein with a large excess of N-ethylmaleimide after it had bound to DNA did not completely dissociate the complex from the DNA, suggesting that some cysteines may be in direct contact with DNA. Pre-incubation of the protein at 37 degrees C or above caused rapid inactivation of DNA binding. The elevated temperature azadicarboxylic acid bis(dimethylamide) treatments resulted in the formation of a crosslinked product, which was detected by Western blotting. The effects of azadicarboxylic acid bis(dimethylmaleimide) and heat were completely reversible by treatment with a reducing agent, such as dithiothreitol. These results demonstrate that in vitro DNA-binding activity of the Ku protein requires reduced sulphydryl groups. Interestingly, the DNA-binding activity of Ku protein was protected from heat inactivation by the presence of a HeLa cell nuclear extract, suggesting that a nuclear factor or factors may be responsible for the maintenance of the reduced cysteines of the Ku protein in vivo. Thus, the biochemical function of the Ku protein may be regulated through oxidation-reduction of its cysteine residues.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abate C., Luk D., Curran T. A ubiquitous nuclear protein stimulates the DNA-binding activity of fos and jun indirectly. Cell Growth Differ. 1990 Oct;1(10):455–462. [PubMed] [Google Scholar]
- Abate C., Patel L., Rauscher F. J., 3rd, Curran T. Redox regulation of fos and jun DNA-binding activity in vitro. Science. 1990 Sep 7;249(4973):1157–1161. doi: 10.1126/science.2118682. [DOI] [PubMed] [Google Scholar]
- Abu-Elheiga L., Yaneva M. Antigenic determinants of the 70-kDa subunit of the Ku autoantigen. Clin Immunol Immunopathol. 1992 Aug;64(2):145–152. doi: 10.1016/0090-1229(92)90192-q. [DOI] [PubMed] [Google Scholar]
- Amabis J. M., Amabis D. C., Kaburaki J., Stollar B. D. The presence of an antigen reactive with a human autoantibody in Trichosia pubescens (Diptera: Sciaridae) and its association with certain transcriptionally active regions of the genome. Chromosoma. 1990 Apr;99(2):102–110. doi: 10.1007/BF01735325. [DOI] [PubMed] [Google Scholar]
- Bennett C. F., Spector D. L., Yeoman L. C. Nonhistone protein BA is a glutathione S-transferase localized to interchromatinic regions of the cell nucleus. J Cell Biol. 1986 Feb;102(2):600–609. doi: 10.1083/jcb.102.2.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Cappel R. E., Gilbert H. F. Thiol/disulfide exchange between 3-hydroxy-3-methylglutaryl-CoA reductase and glutathione. A thermodynamically facile dithiol oxidation. J Biol Chem. 1988 Sep 5;263(25):12204–12212. [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvir A., Peterson S. R., Knuth M. W., Lu H., Dynan W. S. Ku autoantigen is the regulatory component of a template-associated protein kinase that phosphorylates RNA polymerase II. Proc Natl Acad Sci U S A. 1992 Dec 15;89(24):11920–11924. doi: 10.1073/pnas.89.24.11920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb T. M., Jackson S. P. The DNA-dependent protein kinase: requirement for DNA ends and association with Ku antigen. Cell. 1993 Jan 15;72(1):131–142. doi: 10.1016/0092-8674(93)90057-w. [DOI] [PubMed] [Google Scholar]
- Gunderson S. I., Knuth M. W., Burgess R. R. The human U1 snRNA promoter correctly initiates transcription in vitro and is activated by PSE1. Genes Dev. 1990 Dec;4(12A):2048–2060. doi: 10.1101/gad.4.12a.2048. [DOI] [PubMed] [Google Scholar]
- Knuth M. W., Gunderson S. I., Thompson N. E., Strasheim L. A., Burgess R. R. Purification and characterization of proximal sequence element-binding protein 1, a transcription activating protein related to Ku and TREF that binds the proximal sequence element of the human U1 promoter. J Biol Chem. 1990 Oct 15;265(29):17911–17920. [PubMed] [Google Scholar]
- May G., Sutton C., Gould H. Purification and characterization of Ku-2, an octamer-binding protein related to the autoantigen Ku. J Biol Chem. 1991 Feb 15;266(5):3052–3059. [PubMed] [Google Scholar]
- Mimori T., Akizuki M., Yamagata H., Inada S., Yoshida S., Homma M. Characterization of a high molecular weight acidic nuclear protein recognized by autoantibodies in sera from patients with polymyositis-scleroderma overlap. J Clin Invest. 1981 Sep;68(3):611–620. doi: 10.1172/JCI110295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimori T., Hardin J. A. Mechanism of interaction between Ku protein and DNA. J Biol Chem. 1986 Aug 5;261(22):10375–10379. [PubMed] [Google Scholar]
- Mimori T., Ohosone Y., Hama N., Suwa A., Akizuki M., Homma M., Griffith A. J., Hardin J. A. Isolation and characterization of cDNA encoding the 80-kDa subunit protein of the human autoantigen Ku (p70/p80) recognized by autoantibodies from patients with scleroderma-polymyositis overlap syndrome. Proc Natl Acad Sci U S A. 1990 Mar;87(5):1777–1781. doi: 10.1073/pnas.87.5.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prywes R., Roeder R. G. Purification of the c-fos enhancer-binding protein. Mol Cell Biol. 1987 Oct;7(10):3482–3489. doi: 10.1128/mcb.7.10.3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves W. H., Sthoeger Z. M. Molecular cloning of cDNA encoding the p70 (Ku) lupus autoantigen. J Biol Chem. 1989 Mar 25;264(9):5047–5052. [PubMed] [Google Scholar]
- Roberts M. R., Miskimins W. K., Ruddle F. H. Nuclear proteins TREF1 and TREF2 bind to the transcriptional control element of the transferrin receptor gene and appear to be associated as a heterodimer. Cell Regul. 1989 Nov;1(1):151–164. doi: 10.1091/mbc.1.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva C. M., Cidlowski J. A. Direct evidence for intra- and intermolecular disulfide bond formation in the human glucocorticoid receptor. Inhibition of DNA binding and identification of a new receptor-associated protein. J Biol Chem. 1989 Apr 25;264(12):6638–6647. [PubMed] [Google Scholar]
- Smyth D. G., Blumenfeld O. O., Konigsberg W. Reactions of N-ethylmaleimide with peptides and amino acids. Biochem J. 1964 Jun;91(3):589–595. doi: 10.1042/bj0910589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaneva M., Busch H. A 10S particle released from deoxyribonuclease-sensitive regions of HeLa cell nuclei contains the 86-kilodalton-70-kilodalton protein complex. Biochemistry. 1986 Sep 9;25(18):5057–5063. doi: 10.1021/bi00366a013. [DOI] [PubMed] [Google Scholar]
- Yaneva M., Ochs R., McRorie D. K., Zweig S., Busch H. Purification of an 86-70 kDa nuclear DNA-associated protein complex. Biochim Biophys Acta. 1985 Jul 26;841(1):22–29. doi: 10.1016/0304-4165(85)90270-3. [DOI] [PubMed] [Google Scholar]
- Yaneva M., Wen J., Ayala A., Cook R. cDNA-derived amino acid sequence of the 86-kDa subunit of the Ku antigen. J Biol Chem. 1989 Aug 15;264(23):13407–13411. [PubMed] [Google Scholar]
- Zhang W. W., Farrés J., Busch H. Purification of a novel 55 kDa HeLa cell nuclear DNA-binding protein. Biochem Biophys Res Commun. 1991 Jan 31;174(2):542–548. doi: 10.1016/0006-291x(91)91451-h. [DOI] [PubMed] [Google Scholar]
- Zhang W. W., Yaneva M. On the mechanisms of Ku protein binding to DNA. Biochem Biophys Res Commun. 1992 Jul 15;186(1):574–579. doi: 10.1016/s0006-291x(05)80847-2. [DOI] [PubMed] [Google Scholar]
- de Vries E., van Driel W., Bergsma W. G., Arnberg A. C., van der Vliet P. C. HeLa nuclear protein recognizing DNA termini and translocating on DNA forming a regular DNA-multimeric protein complex. J Mol Biol. 1989 Jul 5;208(1):65–78. doi: 10.1016/0022-2836(89)90088-0. [DOI] [PubMed] [Google Scholar]