Abstract
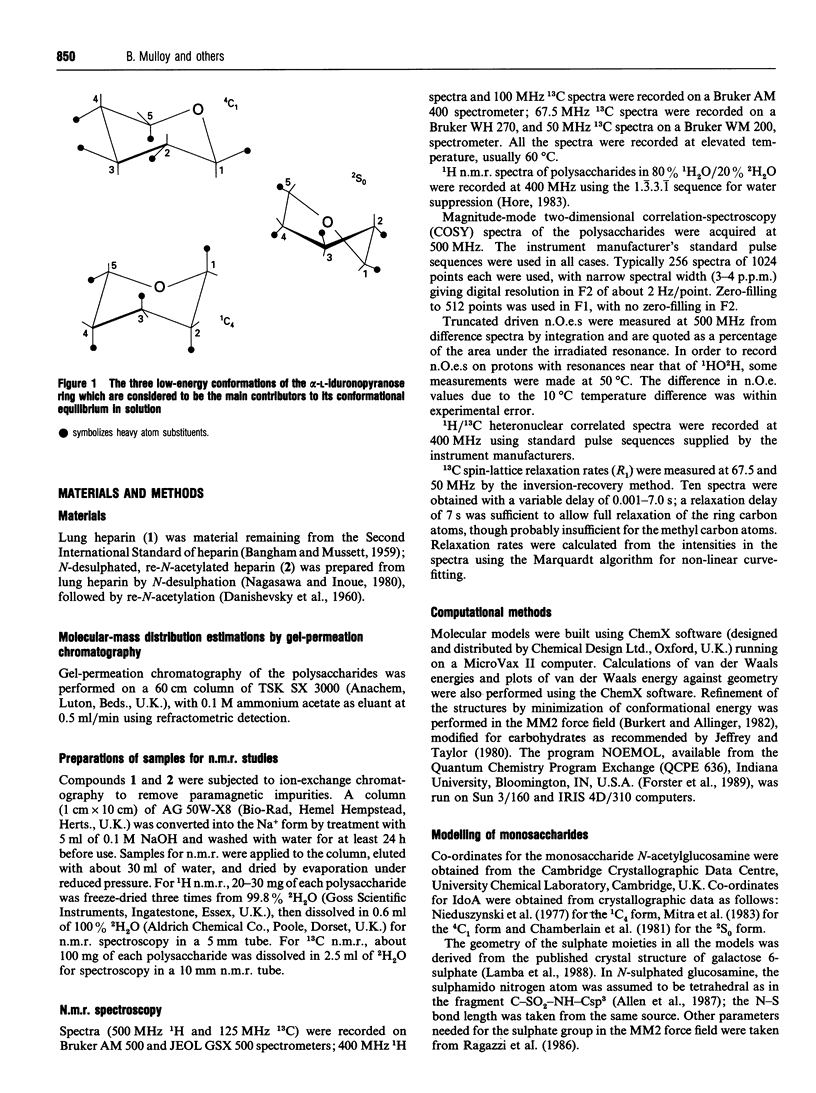
The solution conformations of heparin and de-N-sulphated, re-N-acetylated heparin have been determined by a combination of n.m.r. spectroscopic and molecular-modelling techniques. The 1H- and 13C-n.m.r. spectra of these polysaccharides have been assigned. Observed 1H-1H nuclear Overhauser enhancements (n.O.e.s) have been simulated using the program NOEMOL [Forster, Jones and Mulloy (1989) J. Mol. Graph. 7, 196-201] for molecular models derived from conformational-energy calculations; correlation times for the simulations were chosen to fit experimentally determined 13C spin-lattice relaxation times. In order to achieve good agreement between calculated and observed 1H-1H n.O.e.s it was necessary to assume that the reorientational motion of the polysaccharide molecules was not isotropic, but was that of a symmetric top. The resulting model of heparin in solution is similar to that determined in the fibrous state by X-ray-diffraction techniques [Nieduszynski, Gardner and Atkins (1977) Am. Chem. Soc. Symp. Ser. 48, 73-80].
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkins E. D., Nieduszynski I. A. Crystalline structure of heparin. Adv Exp Med Biol. 1975;52:19–37. doi: 10.1007/978-1-4684-0946-8_2. [DOI] [PubMed] [Google Scholar]
- BANGHAM D. R., MUSSETT M. V. The Second International Standard for heparin. Bull World Health Organ. 1959;20:1201–1208. [PMC free article] [PubMed] [Google Scholar]
- Blackwell J., Schodt K. P., Gelman R. A. Polysaccharide-polypeptide systems as models for heparin interactions. Fed Proc. 1977 Jan;36(1):98–101. [PubMed] [Google Scholar]
- Cardin A. D., Demeter D. A., Weintraub H. J., Jackson R. L. Molecular design and modeling of protein-heparin interactions. Methods Enzymol. 1991;203:556–583. doi: 10.1016/0076-6879(91)03030-k. [DOI] [PubMed] [Google Scholar]
- Cardin A. D., Weintraub H. J. Molecular modeling of protein-glycosaminoglycan interactions. Arteriosclerosis. 1989 Jan-Feb;9(1):21–32. doi: 10.1161/01.atv.9.1.21. [DOI] [PubMed] [Google Scholar]
- Cowan S. W., Bakshi E. N., Machin K. J., Isaacs N. W. Binding of heparin to human platelet factor 4. Biochem J. 1986 Mar 1;234(2):485–488. doi: 10.1042/bj2340485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DANISHEFSKY I., EIBER H. B., CARR J. J. Investigations on the chemistry of heparin. I. Desulfation and acetylation. Arch Biochem Biophys. 1960 Sep;90:114–121. doi: 10.1016/0003-9861(60)90621-4. [DOI] [PubMed] [Google Scholar]
- Damon D. H., Lobb R. R., D'Amore P. A., Wagner J. A. Heparin potentiates the action of acidic fibroblast growth factor by prolonging its biological half-life. J Cell Physiol. 1989 Feb;138(2):221–226. doi: 10.1002/jcp.1041380202. [DOI] [PubMed] [Google Scholar]
- Ferran D. S., Sobel M., Harris R. B. Design and synthesis of a helix heparin-binding peptide. Biochemistry. 1992 Jun 2;31(21):5010–5016. doi: 10.1021/bi00136a014. [DOI] [PubMed] [Google Scholar]
- Ferro D. R., Provasoli A., Ragazzi M., Casu B., Torri G., Bossennec V., Perly B., Sinaÿ P., Petitou M., Choay J. Conformer populations of L-iduronic acid residues in glycosaminoglycan sequences. Carbohydr Res. 1990 Jan 15;195(2):157–167. doi: 10.1016/0008-6215(90)84164-p. [DOI] [PubMed] [Google Scholar]
- Folkman J., Shing Y. Control of angiogenesis by heparin and other sulfated polysaccharides. Adv Exp Med Biol. 1992;313:355–364. doi: 10.1007/978-1-4899-2444-5_34. [DOI] [PubMed] [Google Scholar]
- Forster M., Jones C., Mulloy B. NOEMOL: integrated molecular graphics and the simulation of Nuclear Overhauser effects in NMR spectroscopy. J Mol Graph. 1989 Dec;7(4):196-201, 217. doi: 10.1016/0263-7855(89)80002-5. [DOI] [PubMed] [Google Scholar]
- Gospodarowicz D., Cheng J. Heparin protects basic and acidic FGF from inactivation. J Cell Physiol. 1986 Sep;128(3):475–484. doi: 10.1002/jcp.1041280317. [DOI] [PubMed] [Google Scholar]
- Johnson E. A., Mulloy B. The molecular-weight range of mucosal-heparin preparations. Carbohydr Res. 1976 Oct;51(1):119–127. doi: 10.1016/s0008-6215(00)84041-0. [DOI] [PubMed] [Google Scholar]
- Mitra A. K., Arnott S., Atkins E. D., Isaac D. H. Dermatan sulfate: molecular conformations and interactions in the condensed state. J Mol Biol. 1983 Oct 5;169(4):873–901. doi: 10.1016/s0022-2836(83)80141-7. [DOI] [PubMed] [Google Scholar]
- Pratt C. W., Church F. C. Heparin binding to protein C inhibitor. J Biol Chem. 1992 May 5;267(13):8789–8794. [PubMed] [Google Scholar]
- Ragazzi M., Ferro D. R., Perly B., Sinaÿ P., Petitou M., Choay J. Conformation of the pentasaccharide corresponding to the binding site of heparin for antithrombin III. Carbohydr Res. 1990 Jan 15;195(2):169–185. doi: 10.1016/0008-6215(90)84165-q. [DOI] [PubMed] [Google Scholar]
- Rao B. N., Dua V. K., Bush C. A. Conformations of blood group H-active oligosaccharides of ovarian cyst mucins. Biopolymers. 1985 Dec;24(12):2207–2229. doi: 10.1002/bip.360241205. [DOI] [PubMed] [Google Scholar]
- Rapraeger A. C., Krufka A., Olwin B. B. Requirement of heparan sulfate for bFGF-mediated fibroblast growth and myoblast differentiation. Science. 1991 Jun 21;252(5013):1705–1708. doi: 10.1126/science.1646484. [DOI] [PubMed] [Google Scholar]
- Sanderson P. N., Huckerby T. N., Nieduszynski I. A. Conformational equilibria of alpha-L-iduronate residues in disaccharides derived from heparin. Biochem J. 1987 Apr 1;243(1):175–181. doi: 10.1042/bj2430175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawitzky D., Hampl H., Habermehl K. O. Comparison of heparin-sensitive attachment of pseudorabies virus (PRV) and herpes simplex virus type 1 and identification of heparin-binding PRV glycoproteins. J Gen Virol. 1990 May;71(Pt 5):1221–1225. doi: 10.1099/0022-1317-71-5-1221. [DOI] [PubMed] [Google Scholar]
- Tones M. A., Bootman M. D., Higgins B. F., Lane D. A., Pay G. F., Lindahl U. The effect of heparin on the inositol 1,4,5-trisphosphate receptor in rat liver microsomes. Dependence on sulphate content and chain length. FEBS Lett. 1989 Jul 31;252(1-2):105–108. doi: 10.1016/0014-5793(89)80898-1. [DOI] [PubMed] [Google Scholar]
- Yayon A., Klagsbrun M., Esko J. D., Leder P., Ornitz D. M. Cell surface, heparin-like molecules are required for binding of basic fibroblast growth factor to its high affinity receptor. Cell. 1991 Feb 22;64(4):841–848. doi: 10.1016/0092-8674(91)90512-w. [DOI] [PubMed] [Google Scholar]



