Abstract
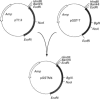
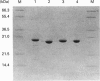
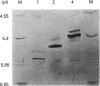
Two cDNA clones encoding a new Mu class glutathione S-transferase (GST) have been isolated from a human testis cDNA library. Both clones are incomplete and appear to result from alternative splicing. One clone is missing the sequence encoding exon 4 and the other is missing exon 8. The complete sequence of the previously undescribed isoenzyme can be deduced from the two cDNA clones. This is the first report of alternative splicing in a GST transcript and may represent either a novel form of regulation in this multigene family or illegitimate transcription and experimental alternative splicing as part of the evolutionary process. By combining components from each clone a complete cDNA has been constructed and the encoded protein expressed in Escherichia coli. In general, the recombinant enzyme has relatively low activity when compared with all the previously described human Mu class GST isoenzymes.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andreadis A., Gallego M. E., Nadal-Ginard B. Generation of protein isoform diversity by alternative splicing: mechanistic and biological implications. Annu Rev Cell Biol. 1987;3:207–242. doi: 10.1146/annurev.cb.03.110187.001231. [DOI] [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Board P. G., Mannervik B. The contribution of the C-terminal sequence to the catalytic activity of GST2, a human alpha-class glutathione transferase. Biochem J. 1991 Apr 1;275(Pt 1):171–174. doi: 10.1042/bj2750171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Board P. G., Suzuki T., Shaw D. C. Human muscle glutathione S-transferase (GST-4) shows close homology to human liver GST-1. Biochim Biophys Acta. 1988 Apr 14;953(3):214–217. doi: 10.1016/0167-4838(88)90027-1. [DOI] [PubMed] [Google Scholar]
- Board P., Coggan M., Johnston P., Ross V., Suzuki T., Webb G. Genetic heterogeneity of the human glutathione transferases: a complex of gene families. Pharmacol Ther. 1990;48(3):357–369. doi: 10.1016/0163-7258(90)90054-6. [DOI] [PubMed] [Google Scholar]
- Brophy P. M., Southan C., Barrett J. Glutathione transferases in the tapeworm Moniezia expansa. Biochem J. 1989 Sep 15;262(3):939–946. doi: 10.1042/bj2620939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell E., Takahashi Y., Abramovitz M., Peretz M., Listowsky I. A distinct human testis and brain mu-class glutathione S-transferase. Molecular cloning and characterization of a form present even in individuals lacking hepatic type mu isoenzymes. J Biol Chem. 1990 Jun 5;265(16):9188–9193. [PubMed] [Google Scholar]
- Chelly J., Concordet J. P., Kaplan J. C., Kahn A. Illegitimate transcription: transcription of any gene in any cell type. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2617–2621. doi: 10.1073/pnas.86.8.2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel V., Sharon R., Bensimon A. Regulatory elements controlling the basal and drug-inducible expression of glutathione S-transferase Ya subunit gene. DNA. 1989 Jul-Aug;8(6):399–408. doi: 10.1089/dna.1.1989.8.399. [DOI] [PubMed] [Google Scholar]
- DeJong J. L., Chang C. M., Whang-Peng J., Knutsen T., Tu C. P. The human liver glutathione S-transferase gene superfamily: expression and chromosome mapping of an Hb subunit cDNA. Nucleic Acids Res. 1988 Sep 12;16(17):8541–8554. doi: 10.1093/nar/16.17.8541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonknechten N., Chelly J., Lepercq J., Kahn A., Kaplan J. C., Kitzis A., Chomel J. C. CFTR illegitimate transcription in lymphoid cells: quantification and applications to the investigation of pathological transcripts. Hum Genet. 1992 Mar;88(5):508–512. doi: 10.1007/BF00219336. [DOI] [PubMed] [Google Scholar]
- Habig W. H., Jakoby W. B. Assays for differentiation of glutathione S-transferases. Methods Enzymol. 1981;77:398–405. doi: 10.1016/s0076-6879(81)77053-8. [DOI] [PubMed] [Google Scholar]
- Hussey A. J., Kerr L. A., Cronshaw A. D., Harrison D. J., Hayes J. D. Variation in the expression of Mu-class glutathione S-transferase isoenzymes from human skeletal muscle. Evidence for the existence of heterodimers. Biochem J. 1991 Jan 15;273(Pt 2):323–332. doi: 10.1042/bj2730323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lai H. C., Qian B., Grove G., Tu C. P. Gene expression of rat glutathione S-transferases. Evidence for gene conversion in the evolution of the Yb multigene family. J Biol Chem. 1988 Aug 15;263(23):11389–11395. [PubMed] [Google Scholar]
- Maniatis T. Mechanisms of alternative pre-mRNA splicing. Science. 1991 Jan 4;251(4989):33–34. doi: 10.1126/science.1824726. [DOI] [PubMed] [Google Scholar]
- Mannervik B., Alin P., Guthenberg C., Jensson H., Tahir M. K., Warholm M., Jörnvall H. Identification of three classes of cytosolic glutathione transferase common to several mammalian species: correlation between structural data and enzymatic properties. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7202–7206. doi: 10.1073/pnas.82.21.7202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannervik B., Awasthi Y. C., Board P. G., Hayes J. D., Di Ilio C., Ketterer B., Listowsky I., Morgenstern R., Muramatsu M., Pearson W. R. Nomenclature for human glutathione transferases. Biochem J. 1992 Feb 15;282(Pt 1):305–306. doi: 10.1042/bj2820305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannervik B., Danielson U. H. Glutathione transferases--structure and catalytic activity. CRC Crit Rev Biochem. 1988;23(3):283–337. doi: 10.3109/10409238809088226. [DOI] [PubMed] [Google Scholar]
- Mannervik B., Guthenberg C. Glutathione transferase (human placenta). Methods Enzymol. 1981;77:231–235. doi: 10.1016/s0076-6879(81)77030-7. [DOI] [PubMed] [Google Scholar]
- McKeown M. Alternative mRNA splicing. Annu Rev Cell Biol. 1992;8:133–155. doi: 10.1146/annurev.cb.08.110192.001025. [DOI] [PubMed] [Google Scholar]
- Morrow C. S., Chiu J., Cowan K. H. Posttranscriptional control of glutathione S-transferase pi gene expression in human breast cancer cells. J Biol Chem. 1992 May 25;267(15):10544–10550. [PubMed] [Google Scholar]
- Okuda A., Imagawa M., Maeda Y., Sakai M., Muramatsu M. Structural and functional analysis of an enhancer GPEI having a phorbol 12-O-tetradecanoate 13-acetate responsive element-like sequence found in the rat glutathione transferase P gene. J Biol Chem. 1989 Oct 5;264(28):16919–16926. [PubMed] [Google Scholar]
- Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977 Dec;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Porter S., Mintz B. Multiple alternatively spliced transcripts of the mouse tyrosinase-encoding gene. Gene. 1991 Jan 15;97(2):277–282. doi: 10.1016/0378-1119(91)90063-h. [DOI] [PubMed] [Google Scholar]
- Rushmore T. H., Pickett C. B. Transcriptional regulation of the rat glutathione S-transferase Ya subunit gene. Characterization of a xenobiotic-responsive element controlling inducible expression by phenolic antioxidants. J Biol Chem. 1990 Aug 25;265(24):14648–14653. [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Barrell B. G., Smith A. J., Roe B. A. Cloning in single-stranded bacteriophage as an aid to rapid DNA sequencing. J Mol Biol. 1980 Oct 25;143(2):161–178. doi: 10.1016/0022-2836(80)90196-5. [DOI] [PubMed] [Google Scholar]
- Seidegård J., De Pierre J. W., Birberg W., Pilotti A., Pero R. W. Characterization of soluble glutathione transferase activity in resting mononuclear leukocytes from human blood. Biochem Pharmacol. 1984 Oct 1;33(19):3053–3058. doi: 10.1016/0006-2952(84)90608-7. [DOI] [PubMed] [Google Scholar]
- Seidegård J., Guthenberg C., Pero R. W., Mannervik B. The trans-stilbene oxide-active glutathione transferase in human mononuclear leucocytes is identical with the hepatic glutathione transferase mu. Biochem J. 1987 Sep 15;246(3):783–785. doi: 10.1042/bj2460783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidegård J., Pero R. W. The hereditary transmission of high glutathione transferase activity towards trans-stilbene oxide in human mononuclear leukocytes. Hum Genet. 1985;69(1):66–68. doi: 10.1007/BF00295531. [DOI] [PubMed] [Google Scholar]
- Seidegård J., Vorachek W. R., Pero R. W., Pearson W. R. Hereditary differences in the expression of the human glutathione transferase active on trans-stilbene oxide are due to a gene deletion. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7293–7297. doi: 10.1073/pnas.85.19.7293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singhal S. S., Ahmad H., Sharma R., Gupta S., Haque A. K., Awasthi Y. C. Purification and characterization of human muscle glutathione S-transferases: evidence that glutathione S-transferase zeta corresponds to a locus distinct from GST1, GST2, and GST3. Arch Biochem Biophys. 1991 Feb 15;285(1):64–73. doi: 10.1016/0003-9861(91)90329-h. [DOI] [PubMed] [Google Scholar]
- Smith C. W., Patton J. G., Nadal-Ginard B. Alternative splicing in the control of gene expression. Annu Rev Genet. 1989;23:527–577. doi: 10.1146/annurev.ge.23.120189.002523. [DOI] [PubMed] [Google Scholar]
- Soma Y., Satoh K., Sato K. Purification and subunit-structural and immunological characterization of five glutathione S-transferases in human liver, and the acidic form as a hepatic tumor marker. Biochim Biophys Acta. 1986 Feb 14;869(3):247–258. doi: 10.1016/0167-4838(86)90064-6. [DOI] [PubMed] [Google Scholar]
- Suzuki T., Coggan M., Shaw D. C., Board P. G. Electrophoretic and immunological analysis of human glutathione S-transferase isozymes. Ann Hum Genet. 1987 May;51(Pt 2):95–106. doi: 10.1111/j.1469-1809.1987.tb01051.x. [DOI] [PubMed] [Google Scholar]
- Suzuki T., Shaw D. C., Board P. G. Purification and characterization of acidic glutathione S-transferase 6 from human brain. Biochem J. 1991 Mar 1;274(Pt 2):405–408. doi: 10.1042/bj2740405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J. B., Oliver J., Sherrington R., Pemble S. E. Structure of human glutathione S-transferase class Mu genes. Biochem J. 1991 Mar 1;274(Pt 2):587–593. doi: 10.1042/bj2740587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchida S., Maki T., Sato K. Purification and characterization of glutathione transferases with an activity toward nitroglycerin from human aorta and heart. Multiplicity of the human class Mu forms. J Biol Chem. 1990 May 5;265(13):7150–7157. [PubMed] [Google Scholar]
- Tsuchida S., Sato K. Glutathione transferases and cancer. Crit Rev Biochem Mol Biol. 1992;27(4-5):337–384. doi: 10.3109/10409239209082566. [DOI] [PubMed] [Google Scholar]
- Vander Jagt D. L., Hunsaker L. A., Garcia K. B., Royer R. E. Isolation and characterization of the multiple glutathione S-transferases from human liver. Evidence for unique heme-binding sites. J Biol Chem. 1985 Sep 25;260(21):11603–11610. [PubMed] [Google Scholar]
- Vorachek W. R., Pearson W. R., Rule G. S. Cloning, expression, and characterization of a class-mu glutathione transferase from human muscle, the product of the GST4 locus. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4443–4447. doi: 10.1073/pnas.88.10.4443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warholm M., Guthenberg C., Mannervik B. Molecular and catalytic properties of glutathione transferase mu from human liver: an enzyme efficiently conjugating epoxides. Biochemistry. 1983 Jul 19;22(15):3610–3617. doi: 10.1021/bi00284a011. [DOI] [PubMed] [Google Scholar]