Abstract
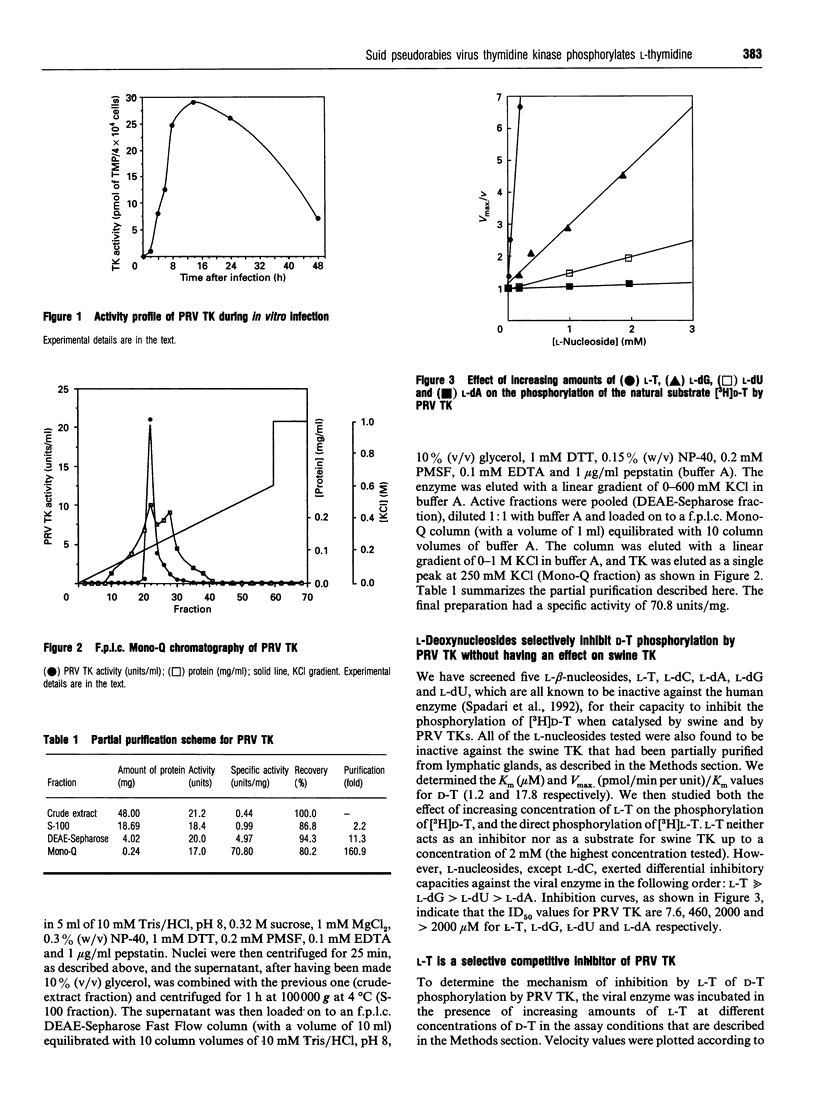
We have partially purified suid pseudorabies virus (PRV) thymidine kinase from infected thymidine kinase- mouse cells, and cytosolic swine thymidine kinase from lymphatic glands, and we have found that PRV thymidine kinase, unlike the host enzyme, shows no stereospecificity for D- and L-beta-nucleosides. In vitro, unnatural L-enantiomers, except L-deoxycytidine, function as specific inhibitors for the viral enzyme in the order: L-thymidine >> L-deoxyguanosine > L-deoxyuridine > L-deoxyadenosine. Contrary to human and swine thymidine kinases and like herpes simplex virus-1 and -2 thymidine kinases, PRV thymidine kinase phosphorylates both the natural (D-) and the unnatural (L-) thymidine enantiomers to their corresponding monophosphates with comparable efficiency. The kinetic parameters Vmax/Km for D- and L-thymidine are 3.7 and 2.3 respectively. Our results demonstrate that the lack of stereospecificity might be a common feature of the thymidine kinases that are encoded by human and animal herpes viruses. These observations could lead to the development of a novel class of antiviral drugs.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cornish-Bowden A., Eisenthal R. Statistical considerations in the estimation of enzyme kinetic parameters by the direct linear plot andother methods. Biochem J. 1974 Jun;139(3):721–730. doi: 10.1042/bj1390721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Clercq E., Descamps J., De Somer P., Barr P. J., Jones A. S., Walker R. T. (E)-5-(2-Bromovinyl)-2'-deoxyuridine: a potent and selective anti-herpes agent. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2947–2951. doi: 10.1073/pnas.76.6.2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Clercq E., Desgranges C., Herdewijn P., Sim I. S., Jones A. S., McLean M. J., Walker R. T. Synthesis and antiviral activity of (E)-5-(2-bromovinyl)uracil and (E)-5-(2-bromovinyl)uridine. J Med Chem. 1986 Feb;29(2):213–217. doi: 10.1021/jm00152a008. [DOI] [PubMed] [Google Scholar]
- Derse D., Cheng Y. C., Furman P. A., St Clair M. H., Elion G. B. Inhibition of purified human and herpes simplex virus-induced DNA polymerases by 9-(2-hydroxyethoxymethyl)guanine triphosphate. Effects on primer-template function. J Biol Chem. 1981 Nov 25;256(22):11447–11451. [PubMed] [Google Scholar]
- Elion G. B., Furman P. A., Fyfe J. A., de Miranda P., Beauchamp L., Schaeffer H. J. Selectivity of action of an antiherpetic agent, 9-(2-hydroxyethoxymethyl) guanine. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5716–5720. doi: 10.1073/pnas.74.12.5716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field H. J. Chemotherapy of Aujeszky's disease (pseudorabies) in the mouse by means of nucleoside analogues: bromovinyldeoxyuridine, acyclovir, and dihydroxypropoxymethylguanine. Antiviral Res. 1985 Jun;5(3):157–168. doi: 10.1016/0166-3542(85)90048-8. [DOI] [PubMed] [Google Scholar]
- Focher F., Hildebrand C., Freese S., Ciarrocchi G., Noonan T., Sangalli S., Brown N., Spadari S., Wright G. N2-phenyldeoxyguanosine: a novel selective inhibitor of herpes simplex thymidine kinase. J Med Chem. 1988 Aug;31(8):1496–1500. doi: 10.1021/jm00403a004. [DOI] [PubMed] [Google Scholar]
- Focher F., Sandoli D., Hildebrand C., Sangalli S., Ciarrocchi G., Rebuzzini A., Pedrali-Noy G., Manservigi R., Wright G., Brown N. Nucleoside analogs as non-substrate inhibitors of herpes simplex viruses thymidine kinase. Methods Find Exp Clin Pharmacol. 1989 Sep;11(9):577–582. [PubMed] [Google Scholar]
- Gambino J., Focher F., Hildebrand C., Maga G., Noonan T., Spadari S., Wright G. Quantitative structure-activity relationships of N2-phenylguanines as inhibitors of herpes simplex virus thymidine kinases. J Med Chem. 1992 Aug 7;35(16):2979–2983. doi: 10.1021/jm00094a007. [DOI] [PubMed] [Google Scholar]
- Hildebrand C., Sandoli D., Focher F., Gambino J., Ciarrocchi G., Spadari S., Wright G. Structure-activity relationships of N2-substituted guanines as inhibitors of HSV1 and HSV2 thymidine kinases. J Med Chem. 1990 Jan;33(1):203–206. doi: 10.1021/jm00163a033. [DOI] [PubMed] [Google Scholar]
- Kit S., Kit M., Pirtle E. C. Attenuated properties of thymidine kinase-negative deletion mutant of pseudorabies virus. Am J Vet Res. 1985 Jun;46(6):1359–1367. [PubMed] [Google Scholar]
- Leib D. A., Ruffner K. L., Hildebrand C., Schaffer P. A., Wright G. E., Coen D. M. Specific inhibitors of herpes simplex virus thymidine kinase diminish reactivation of latent virus from explanted murine ganglia. Antimicrob Agents Chemother. 1990 Jun;34(6):1285–1286. doi: 10.1128/aac.34.6.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGregor S., Easterday B. C., Kaplan A. S., Ben-Porat T. Vaccination of swine with thymidine kinase-deficient mutants of pseudorabies virus. Am J Vet Res. 1985 Jul;46(7):1494–1497. [PubMed] [Google Scholar]
- Nsiah Y. A., Tolman R. L., Karkas J. D., Rapp F. Suppression of herpes simplex virus type 1 reactivation from latency by (+-)-9-([(Z)-2-(hydroxymethyl)cyclohexyl]methyl) guanine (L-653,180) in vitro. Antimicrob Agents Chemother. 1990 Aug;34(8):1551–1555. doi: 10.1128/aac.34.8.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutter L. M., Grill S. P., Dutschman G. E., Sharma R. A., Bobek M., Cheng Y. C. Demonstration of viral thymidine kinase inhibitor and its effect on deoxynucleotide metabolism in cells infected with herpes simplex virus. Antimicrob Agents Chemother. 1987 Mar;31(3):368–374. doi: 10.1128/aac.31.3.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PRUSOFF W. H. Synthesis and biological activities of iododeoxyuridine, an analog of thymidine. Biochim Biophys Acta. 1959 Mar;32(1):295–296. doi: 10.1016/0006-3002(59)90597-9. [DOI] [PubMed] [Google Scholar]
- Shibata I., Inaba Y., Akashi H. Avirulent ts and thymidine kinase-deficient mutant of Aujeszky's disease virus. J Vet Med Sci. 1991 Aug;53(4):663–670. doi: 10.1292/jvms.53.663. [DOI] [PubMed] [Google Scholar]
- Spadari S., Maga G., Focher F., Ciarrocchi G., Manservigi R., Arcamone F., Capobianco M., Carcuro A., Colonna F., Iotti S. L-thymidine is phosphorylated by herpes simplex virus type 1 thymidine kinase and inhibits viral growth. J Med Chem. 1992 Oct 30;35(22):4214–4220. doi: 10.1021/jm00100a029. [DOI] [PubMed] [Google Scholar]
- Veerisetty V., Balasubramaniam N. K., Gentry G. A. Equine herpes virus 1 and pseudorabies virus resistance to 2'-fluoropyrimidine analogs and to bromovinyldeoxyuridine: implications for dTMP kinase activity. Acta Virol. 1990 Dec;34(6):568–573. [PubMed] [Google Scholar]
- Volz D. M., Lager K. M., Mengeling W. L. Latency of a thymidine kinase-negative pseudorabies vaccine virus detected by the polymerase chain reaction. Arch Virol. 1992;122(3-4):341–348. doi: 10.1007/BF01317195. [DOI] [PubMed] [Google Scholar]


