Abstract
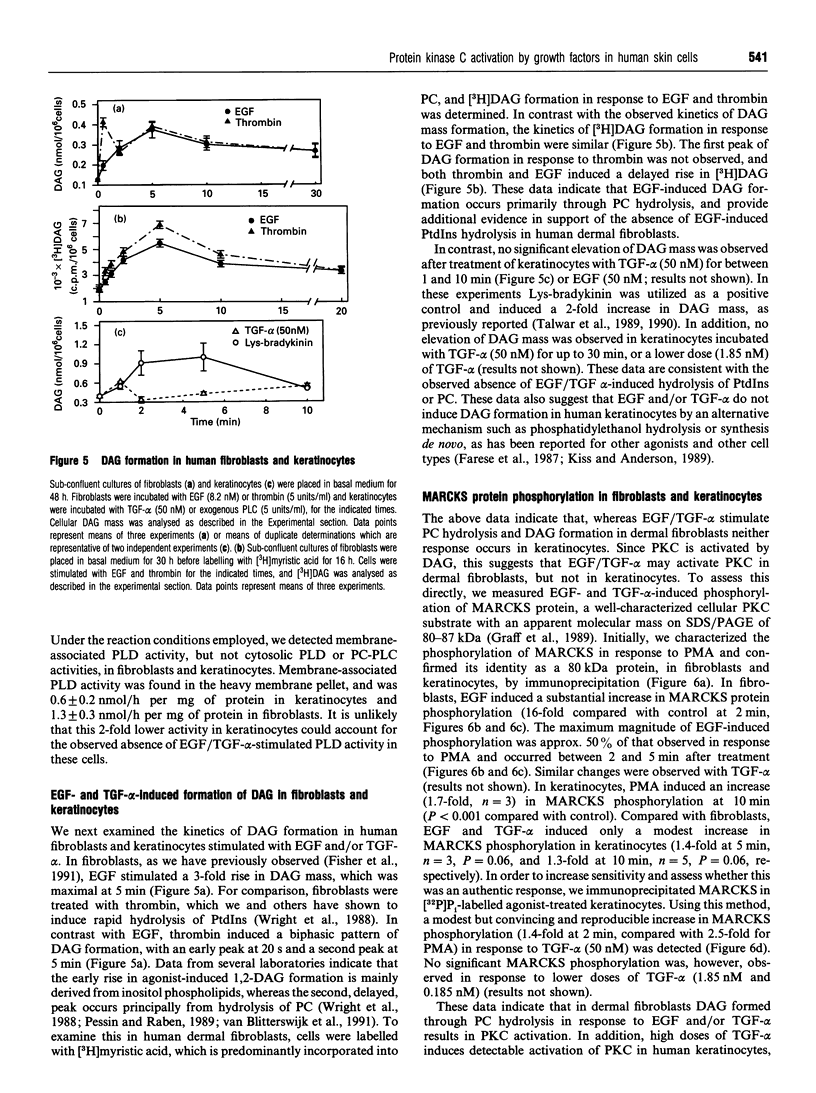
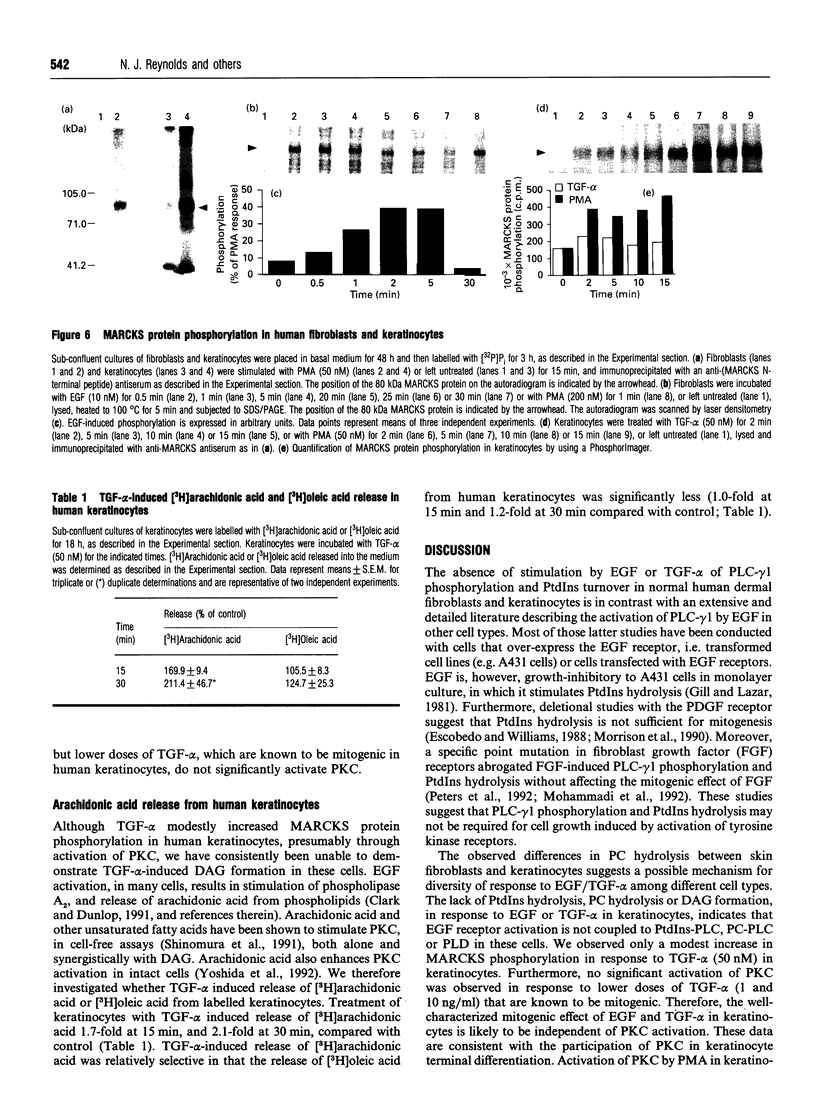
We have investigated coupling between the epidermal growth factor (EGF) receptor and the phospholipase C (PLC)/protein kinase C (PKC) signal-transduction system in normal skin fibroblasts and keratinocytes, for which EGF and transforming growth factor alpha (TGF-alpha) are mitogenic. EGF and TGF-alpha induced a rapid increase in tyrosine phosphorylation of the EGF receptor, in both fibroblasts and keratinocytes, but failed to induce tyrosine phosphorylation of PLC-gamma 1 or detectable phosphoinositide hydrolysis, as measured by two sensitive assays. In fibroblasts, EGF induced phosphatidylcholine (PC) hydrolysis, resulting in increased diacylglycerol (DAG). In contrast, in keratinocytes, there was no detectable PC hydrolysis or elevation of DAG in response to EGF or TGF-alpha. EGF and TGF-alpha activated PKC in fibroblasts, as evidenced by increased phosphorylation of a specific cellular PKC substrate (myristoylated alanine-rich C-kinase substrate, 'MARCKS'). In keratinocytes, TGF-alpha and EGF induced only a modest increase in MARCKS protein phosphorylation. This apparent modest activation of PKC, in the absence of detectable DAG formation, may have been mediated by arachidonic acid, which was released from keratinocytes in response to TGF-alpha, and has been shown to stimulate PKC activity in vitro. These data demonstrate that (1) in dermal fibroblasts and keratinocytes, which express normal levels of EGF receptors, EGF receptor activation is not coupled to tyrosine phosphorylation of PLC-gamma 1 or PtdIns hydrolysis, suggesting that these events are not required for the mitogenic activity of EGF or TGF-alpha in these cells, (2) coupling of EGF receptor to PC hydrolysis is cell-type specific, and (3) in skin fibroblasts, DAG, formed through EGF-induced PC hydrolysis, is capable of activating PKC.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrandon Y., Green H. Cell migration is essential for sustained growth of keratinocyte colonies: the roles of transforming growth factor-alpha and epidermal growth factor. Cell. 1987 Sep 25;50(7):1131–1137. doi: 10.1016/0092-8674(87)90179-6. [DOI] [PubMed] [Google Scholar]
- Besterman J. M., Watson S. P., Cuatrecasas P. Lack of association of epidermal growth factor-, insulin-, and serum-induced mitogenesis with stimulation of phosphoinositide degradation in BALB/c 3T3 fibroblasts. J Biol Chem. 1986 Jan 15;261(2):723–727. [PubMed] [Google Scholar]
- Billah M. M., Eckel S., Mullmann T. J., Egan R. W., Siegel M. I. Phosphatidylcholine hydrolysis by phospholipase D determines phosphatidate and diglyceride levels in chemotactic peptide-stimulated human neutrophils. Involvement of phosphatidate phosphohydrolase in signal transduction. J Biol Chem. 1989 Oct 15;264(29):17069–17077. [PubMed] [Google Scholar]
- Bocckino S. B., Wilson P. B., Exton J. H. Ca2+-mobilizing hormones elicit phosphatidylethanol accumulation via phospholipase D activation. FEBS Lett. 1987 Dec 10;225(1-2):201–204. doi: 10.1016/0014-5793(87)81157-2. [DOI] [PubMed] [Google Scholar]
- Carpenter G., Cohen S. Epidermal growth factor. J Biol Chem. 1990 May 15;265(14):7709–7712. [PubMed] [Google Scholar]
- Chen W. S., Lazar C. S., Poenie M., Tsien R. Y., Gill G. N., Rosenfeld M. G. Requirement for intrinsic protein tyrosine kinase in the immediate and late actions of the EGF receptor. 1987 Aug 27-Sep 2Nature. 328(6133):820–823. doi: 10.1038/328820a0. [DOI] [PubMed] [Google Scholar]
- Clark S., Dunlop M. Modulation of phospholipase A2 activity by epidermal growth factor (EGF) in CHO cells transfected with human EGF receptor. Role of receptor cytoplasmic subdomain. Biochem J. 1991 Mar 15;274(Pt 3):715–721. doi: 10.1042/bj2740715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffey R. J., Jr, Derynck R., Wilcox J. N., Bringman T. S., Goustin A. S., Moses H. L., Pittelkow M. R. Production and auto-induction of transforming growth factor-alpha in human keratinocytes. 1987 Aug 27-Sep 2Nature. 328(6133):817–820. doi: 10.1038/328817a0. [DOI] [PubMed] [Google Scholar]
- Cook P. W., Pittelkow M. R., Shipley G. D. Growth factor-independent proliferation of normal human neonatal keratinocytes: production of autocrine- and paracrine-acting mitogenic factors. J Cell Physiol. 1991 Feb;146(2):277–289. doi: 10.1002/jcp.1041460213. [DOI] [PubMed] [Google Scholar]
- Decker S. J., Ellis C., Pawson T., Velu T. Effects of substitution of threonine 654 of the epidermal growth factor receptor on epidermal growth factor-mediated activation of phospholipase C. J Biol Chem. 1990 Apr 25;265(12):7009–7015. [PubMed] [Google Scholar]
- Dennis E. A., Rhee S. G., Billah M. M., Hannun Y. A. Role of phospholipase in generating lipid second messengers in signal transduction. FASEB J. 1991 Apr;5(7):2068–2077. doi: 10.1096/fasebj.5.7.1901288. [DOI] [PubMed] [Google Scholar]
- Downes C. P., Mussat M. C., Michell R. H. The inositol trisphosphate phosphomonoesterase of the human erythrocyte membrane. Biochem J. 1982 Apr 1;203(1):169–177. doi: 10.1042/bj2030169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebner R., Derynck R. Epidermal growth factor and transforming growth factor-alpha: differential intracellular routing and processing of ligand-receptor complexes. Cell Regul. 1991 Aug;2(8):599–612. doi: 10.1091/mbc.2.8.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder J. T., Fisher G. J., Lindquist P. B., Bennett G. L., Pittelkow M. R., Coffey R. J., Jr, Ellingsworth L., Derynck R., Voorhees J. J. Overexpression of transforming growth factor alpha in psoriatic epidermis. Science. 1989 Feb 10;243(4892):811–814. doi: 10.1126/science.2916128. [DOI] [PubMed] [Google Scholar]
- Escobedo J. A., Williams L. T. A PDGF receptor domain essential for mitogenesis but not for many other responses to PDGF. Nature. 1988 Sep 1;335(6185):85–87. doi: 10.1038/335085a0. [DOI] [PubMed] [Google Scholar]
- Exton J. H. Signaling through phosphatidylcholine breakdown. J Biol Chem. 1990 Jan 5;265(1):1–4. [PubMed] [Google Scholar]
- Farese R. V., Konda T. S., Davis J. S., Standaert M. L., Pollet R. J., Cooper D. R. Insulin rapidly increases diacylglycerol by activating de novo phosphatidic acid synthesis. Science. 1987 May 1;236(4801):586–589. doi: 10.1126/science.3107122. [DOI] [PubMed] [Google Scholar]
- Fisher G. J., Henderson P. A., Voorhees J. J., Baldassare J. J. Epidermal growth factor-induced hydrolysis of phosphatidylcholine by phospholipase D and phospholipase C in human dermal fibroblasts. J Cell Physiol. 1991 Feb;146(2):309–317. doi: 10.1002/jcp.1041460216. [DOI] [PubMed] [Google Scholar]
- Fu T., Okano Y., Nozawa Y. Bradykinin-induced generation of inositol 1,4,5-trisphosphate in fibroblasts and neuroblastoma cells: effect of pertussis toxin, extracellular calcium, and down-regulation of protein kinase C. Biochem Biophys Res Commun. 1988 Dec 30;157(3):1429–1435. doi: 10.1016/s0006-291x(88)81035-0. [DOI] [PubMed] [Google Scholar]
- Gill G. N., Lazar C. S. Increased phosphotyrosine content and inhibition of proliferation in EGF-treated A431 cells. Nature. 1981 Sep 24;293(5830):305–307. doi: 10.1038/293305a0. [DOI] [PubMed] [Google Scholar]
- Gottlieb A. B., Chang C. K., Posnett D. N., Fanelli B., Tam J. P. Detection of transforming growth factor alpha in normal, malignant, and hyperproliferative human keratinocytes. J Exp Med. 1988 Feb 1;167(2):670–675. doi: 10.1084/jem.167.2.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graff J. M., Young T. N., Johnson J. D., Blackshear P. J. Phosphorylation-regulated calmodulin binding to a prominent cellular substrate for protein kinase C. J Biol Chem. 1989 Dec 25;264(36):21818–21823. [PubMed] [Google Scholar]
- Harper R. A., Savage C. R., Jr Vitamin A potentiates the mitogenic effect of epidermal growth factor in cultures of normal adult human skin fibroblasts. Endocrinology. 1980 Dec;107(6):2113–2114. doi: 10.1210/endo-107-6-2113. [DOI] [PubMed] [Google Scholar]
- Hawley-Nelson P., Stanley J. R., Schmidt J., Gullino M., Yuspa S. H. The tumor promoter, 12-O-tetradecanoylphorbol-13-acetate accelerates keratinocyte differentiation and stimulates growth of an unidentified cell type in cultured human epidermis. Exp Cell Res. 1982 Jan;137(1):155–167. doi: 10.1016/0014-4827(82)90017-9. [DOI] [PubMed] [Google Scholar]
- Hepler J. R., Earp H. S., Harden T. K. Long-term phorbol ester treatment down-regulates protein kinase C and sensitizes the phosphoinositide signaling pathway to hormone and growth factor stimulation. Evidence for a role of protein kinase C in agonist-induced desensitization. J Biol Chem. 1988 Jun 5;263(16):7610–7619. [PubMed] [Google Scholar]
- Issandou M., Rozengurt E. Bradykinin transiently activates protein kinase C in Swiss 3T3 cells. Distinction from activation by bombesin and vasopressin. J Biol Chem. 1990 Jul 15;265(20):11890–11896. [PubMed] [Google Scholar]
- Jetten A. M., George M. A., Pettit G. R., Herald C. L., Rearick J. I. Action of phorbol esters, bryostatins, and retinoic acid on cholesterol sulfate synthesis: relation to the multistep process of differentiation in human epidermal keratinocytes. J Invest Dermatol. 1989 Jul;93(1):108–115. doi: 10.1111/1523-1747.ep12277374. [DOI] [PubMed] [Google Scholar]
- Johnson R. M., Garrison J. C. Epidermal growth factor and angiotensin II stimulate formation of inositol 1,4,5- and inositol 1,3,4-trisphosphate in hepatocytes. Differential inhibition by pertussis toxin and phorbol 12-myristate 13-acetate. J Biol Chem. 1987 Dec 25;262(36):17285–17293. [PubMed] [Google Scholar]
- Khandke L., Krane J. F., Ashinoff R., Staiano-Coico L., Granelli-Piperno A., Luster A. D., Carter D. M., Krueger J. G., Gottlieb A. B. Cyclosporine in psoriasis treatment. Inhibition of keratinocyte cell-cycle progression in G1 independent of effects on transforming growth factor alpha/epidermal growth factor receptor pathways. Arch Dermatol. 1991 Aug;127(8):1172–1179. doi: 10.1001/archderm.127.8.1172. [DOI] [PubMed] [Google Scholar]
- Kiss Z., Anderson W. B. Phorbol ester stimulates the hydrolysis of phosphatidylethanolamine in leukemic HL-60, NIH 3T3, and baby hamster kidney cells. J Biol Chem. 1989 Jan 25;264(3):1483–1487. [PubMed] [Google Scholar]
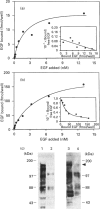
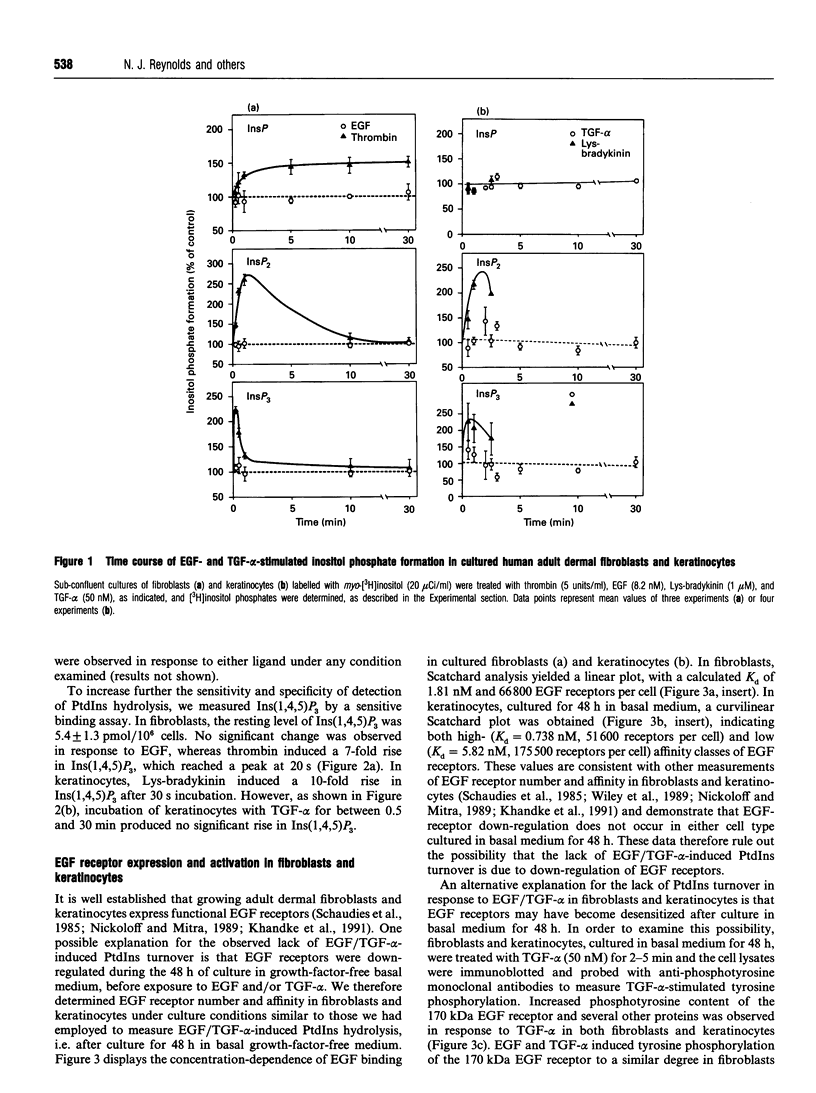
- Klein S. B., Fisher G. J., Jensen T. C., Mendelsohn J., Voorhees J. J., Elder J. T. Regulation of TGF-alpha expression in human keratinocytes: PKC-dependent and -independent pathways. J Cell Physiol. 1992 May;151(2):326–336. doi: 10.1002/jcp.1041510214. [DOI] [PubMed] [Google Scholar]
- Larrodera P., Cornet M. E., Diaz-Meco M. T., Lopez-Barahona M., Diaz-Laviada I., Guddal P. H., Johansen T., Moscat J. Phospholipase C-mediated hydrolysis of phosphatidylcholine is an important step in PDGF-stimulated DNA synthesis. Cell. 1990 Jun 15;61(6):1113–1120. doi: 10.1016/0092-8674(90)90074-o. [DOI] [PubMed] [Google Scholar]
- Leach K. L., Ruff V. A., Wright T. M., Pessin M. S., Raben D. M. Dissociation of protein kinase C activation and sn-1,2-diacylglycerol formation. Comparison of phosphatidylinositol- and phosphatidylcholine-derived diglycerides in alpha-thrombin-stimulated fibroblasts. J Biol Chem. 1991 Feb 15;266(5):3215–3221. [PubMed] [Google Scholar]
- Lobaugh L. A., Blackshear P. J. Neuropeptide Y stimulation of myosin light chain phosphorylation in cultured aortic smooth muscle cells. J Biol Chem. 1990 Oct 25;265(30):18393–18399. [PubMed] [Google Scholar]
- Martin T. W. Formation of diacylglycerol by a phospholipase D-phosphatidate phosphatase pathway specific for phosphatidylcholine in endothelial cells. Biochim Biophys Acta. 1988 Oct 14;962(3):282–296. doi: 10.1016/0005-2760(88)90258-5. [DOI] [PubMed] [Google Scholar]
- Massagué J. Epidermal growth factor-like transforming growth factor. I. Isolation, chemical characterization, and potentiation by other transforming factors from feline sarcoma virus-transformed rat cells. J Biol Chem. 1983 Nov 25;258(22):13606–13613. [PubMed] [Google Scholar]
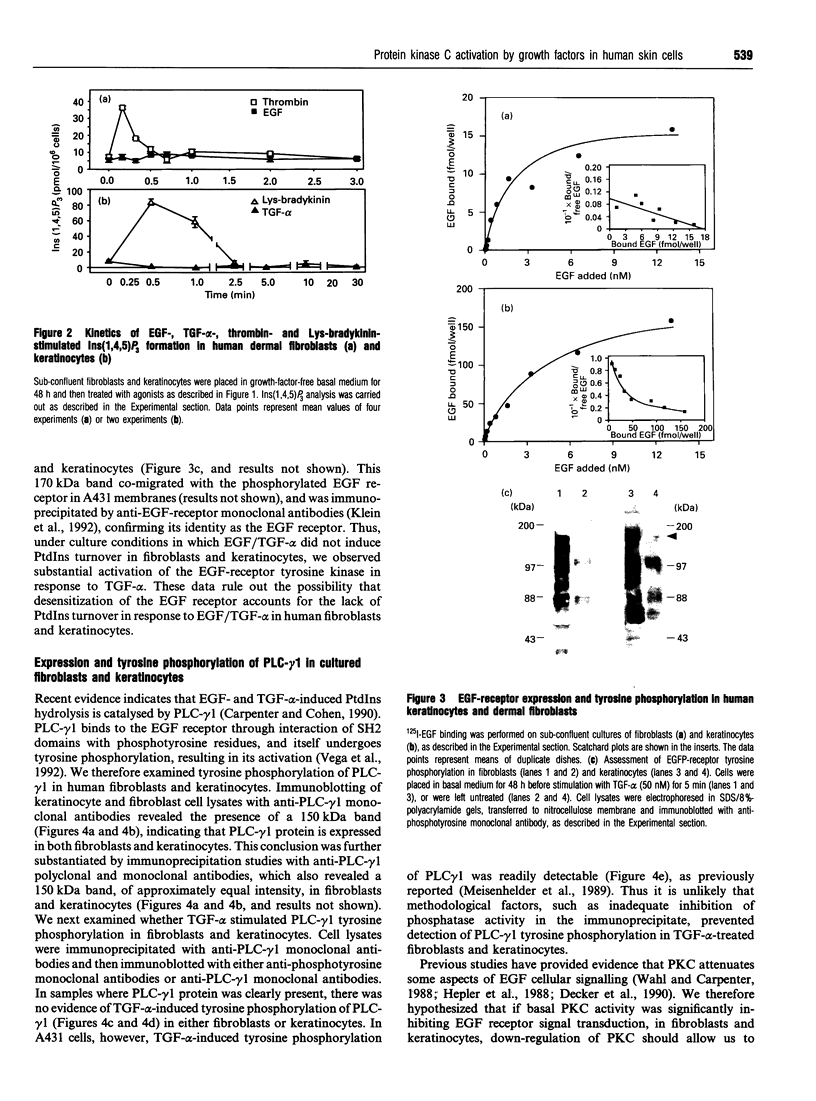
- Meisenhelder J., Suh P. G., Rhee S. G., Hunter T. Phospholipase C-gamma is a substrate for the PDGF and EGF receptor protein-tyrosine kinases in vivo and in vitro. Cell. 1989 Jun 30;57(7):1109–1122. doi: 10.1016/0092-8674(89)90048-2. [DOI] [PubMed] [Google Scholar]
- Mohammadi M., Dionne C. A., Li W., Li N., Spivak T., Honegger A. M., Jaye M., Schlessinger J. Point mutation in FGF receptor eliminates phosphatidylinositol hydrolysis without affecting mitogenesis. Nature. 1992 Aug 20;358(6388):681–684. doi: 10.1038/358681a0. [DOI] [PubMed] [Google Scholar]
- Morrison D. K., Kaplan D. R., Rhee S. G., Williams L. T. Platelet-derived growth factor (PDGF)-dependent association of phospholipase C-gamma with the PDGF receptor signaling complex. Mol Cell Biol. 1990 May;10(5):2359–2366. doi: 10.1128/mcb.10.5.2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscat J., Molloy C. J., Fleming T. P., Aaronson S. A. Epidermal growth factor activates phosphoinositide turnover and protein kinase C in BALB/MK keratinocytes. Mol Endocrinol. 1988 Sep;2(9):799–805. doi: 10.1210/mend-2-9-799. [DOI] [PubMed] [Google Scholar]
- Myrdal S. E., Twardzik D. R., Auersperg N. Cell-mediated co-action of transforming growth factors: incubation of type beta with normal rat kidney cells produces a soluble activity that prolongs the ruffling response to type alpha. J Cell Biol. 1986 Apr;102(4):1230–1234. doi: 10.1083/jcb.102.4.1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickoloff B. J., Mitra R. S. Inhibition of 125I-epidermal growth factor binding to cultured keratinocytes by antiproliferative molecules gamma interferon, cyclosporin A, and transforming growth factor-beta. J Invest Dermatol. 1989 Dec;93(6):799–803. doi: 10.1111/1523-1747.ep12284427. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. Studies and perspectives of protein kinase C. Science. 1986 Jul 18;233(4761):305–312. doi: 10.1126/science.3014651. [DOI] [PubMed] [Google Scholar]
- Pessin M. S., Baldassare J. J., Raben D. M. Molecular species analysis of mitogen-stimulated 1,2-diglycerides in fibroblasts. Comparison of alpha-thrombin, epidermal growth factor, and platelet-derived growth factor. J Biol Chem. 1990 May 15;265(14):7959–7966. [PubMed] [Google Scholar]
- Pessin M. S., Raben D. M. Molecular species analysis of 1,2-diglycerides stimulated by alpha-thrombin in cultured fibroblasts. J Biol Chem. 1989 May 25;264(15):8729–8738. [PubMed] [Google Scholar]
- Peters K. G., Marie J., Wilson E., Ives H. E., Escobedo J., Del Rosario M., Mirda D., Williams L. T. Point mutation of an FGF receptor abolishes phosphatidylinositol turnover and Ca2+ flux but not mitogenesis. Nature. 1992 Aug 20;358(6388):678–681. doi: 10.1038/358678a0. [DOI] [PubMed] [Google Scholar]
- Pittelkow M. R., Lindquist P. B., Abraham R. T., Graves-Deal R., Derynck R., Coffey R. J., Jr Induction of transforming growth factor-alpha expression in human keratinocytes by phorbol esters. J Biol Chem. 1989 Mar 25;264(9):5164–5171. [PubMed] [Google Scholar]
- Preiss J. E., Loomis C. R., Bell R. M., Niedel J. E. Quantitative measurement of sn-1,2-diacylglycerols. Methods Enzymol. 1987;141:294–300. doi: 10.1016/0076-6879(87)41077-x. [DOI] [PubMed] [Google Scholar]
- Sako T., Yuspa S. H., Herald C. L., Pettit G. R., Blumberg P. M. Partial parallelism and partial blockade by bryostatin 1 of effects of phorbol ester tumor promoters on primary mouse epidermal cells. Cancer Res. 1987 Oct 15;47(20):5445–5450. [PubMed] [Google Scholar]
- Schaudies R. P., Harper R. A., Savage C. R., Jr 125I-EGF binding to responsive and nonresponsive cells in culture: loss of cell-associated radioactivity relates to growth induction. J Cell Physiol. 1985 Sep;124(3):493–498. doi: 10.1002/jcp.1041240320. [DOI] [PubMed] [Google Scholar]
- Schreiber A. B., Winkler M. E., Derynck R. Transforming growth factor-alpha: a more potent angiogenic mediator than epidermal growth factor. Science. 1986 Jun 6;232(4755):1250–1253. doi: 10.1126/science.2422759. [DOI] [PubMed] [Google Scholar]
- Shinomura T., Asaoka Y., Oka M., Yoshida K., Nishizuka Y. Synergistic action of diacylglycerol and unsaturated fatty acid for protein kinase C activation: its possible implications. Proc Natl Acad Sci U S A. 1991 Jun 15;88(12):5149–5153. doi: 10.1073/pnas.88.12.5149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla S. D., Halenda S. P. Phospholipase D in cell signalling and its relationship to phospholipase C. Life Sci. 1991;48(9):851–866. doi: 10.1016/0024-3205(91)90031-6. [DOI] [PubMed] [Google Scholar]
- Staiano-Coico L., Khandke L., Krane J. F., Sharif S., Gottlieb A. B., Krueger J. G., Heim L., Rigas B., Higgins P. J. TGF-alpha and TGF-beta expression during sodium-N-butyrate-induced differentiation of human keratinocytes: evidence for subpopulation-specific up-regulation of TGF-beta mRNA in suprabasal cells. Exp Cell Res. 1990 Dec;191(2):286–291. doi: 10.1016/0014-4827(90)90016-4. [DOI] [PubMed] [Google Scholar]
- Strulovici B., Daniel-Issakani S., Baxter G., Knopf J., Sultzman L., Cherwinski H., Nestor J., Jr, Webb D. R., Ransom J. Distinct mechanisms of regulation of protein kinase C epsilon by hormones and phorbol diesters. J Biol Chem. 1991 Jan 5;266(1):168–173. [PubMed] [Google Scholar]
- Takasu N., Takasu M., Yamada T., Shimizu Y. Epidermal growth factor (EGF) produces inositol phosphates and increases cytoplasmic free calcium in cultured porcine thyroid cells. Biochem Biophys Res Commun. 1988 Feb 29;151(1):530–534. doi: 10.1016/0006-291x(88)90626-2. [DOI] [PubMed] [Google Scholar]
- Talwar H. S., Fisher G. J., Harris V. A., Voorhees J. J. Agonist-induced hydrolysis of phosphoinositides and formation of 1,2-diacylglycerol in adult human keratinocytes. J Invest Dermatol. 1989 Aug;93(2):241–245. doi: 10.1111/1523-1747.ep12277581. [DOI] [PubMed] [Google Scholar]
- Talwar H. S., Fisher G. J., Voorhees J. J. Bradykinin induces phosphoinositide turnover, 1,2-diglyceride formation, and growth in cultured adult human keratinocytes. J Invest Dermatol. 1990 Dec;95(6):705–710. doi: 10.1111/1523-1747.ep12514507. [DOI] [PubMed] [Google Scholar]
- Thompson N. T., Tateson J. E., Randall R. W., Spacey G. D., Bonser R. W., Garland L. G. The temporal relationship between phospholipase activation, diradylglycerol formation and superoxide production in the human neutrophil. Biochem J. 1990 Oct 1;271(1):209–213. doi: 10.1042/bj2710209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao M. S., Earp H. S., Grisham J. W. The effects of epidermal growth factor and the state of confluence on enzymatic activities of cultured rat liver epithelial cells. J Cell Physiol. 1986 Feb;126(2):167–173. doi: 10.1002/jcp.1041260204. [DOI] [PubMed] [Google Scholar]
- Vassar R., Fuchs E. Transgenic mice provide new insights into the role of TGF-alpha during epidermal development and differentiation. Genes Dev. 1991 May;5(5):714–727. doi: 10.1101/gad.5.5.714. [DOI] [PubMed] [Google Scholar]
- Vega Q. C., Cochet C., Filhol O., Chang C. P., Rhee S. G., Gill G. N. A site of tyrosine phosphorylation in the C terminus of the epidermal growth factor receptor is required to activate phospholipase C. Mol Cell Biol. 1992 Jan;12(1):128–135. doi: 10.1128/mcb.12.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl M., Carpenter G. Regulation of epidermal growth factor-stimulated formation of inositol phosphates in A-431 cells by calcium and protein kinase C. J Biol Chem. 1988 Jun 5;263(16):7581–7590. [PubMed] [Google Scholar]
- Wang P., Anthes J. C., Siegel M. I., Egan R. W., Billah M. M. Existence of cytosolic phospholipase D. Identification and comparison with membrane-bound enzyme. J Biol Chem. 1991 Aug 15;266(23):14877–14880. [PubMed] [Google Scholar]
- Whitman M., Cantley L. Phosphoinositide metabolism and the control of cell proliferation. Biochim Biophys Acta. 1989 Feb;948(3):327–344. doi: 10.1016/0304-419x(89)90005-x. [DOI] [PubMed] [Google Scholar]
- Wiley H. S., Walsh B. J., Lund K. A. Global modulation of the epidermal growth factor receptor is triggered by occupancy of only a few receptors. Evidence for a binary regulatory system in normal human fibroblasts. J Biol Chem. 1989 Nov 15;264(32):18912–18920. [PubMed] [Google Scholar]
- Wolf R. A., Gross R. W. Identification of neutral active phospholipase C which hydrolyzes choline glycerophospholipids and plasmalogen selective phospholipase A2 in canine myocardium. J Biol Chem. 1985 Jun 25;260(12):7295–7303. [PubMed] [Google Scholar]
- Wright T. M., Rangan L. A., Shin H. S., Raben D. M. Kinetic analysis of 1,2-diacylglycerol mass levels in cultured fibroblasts. Comparison of stimulation by alpha-thrombin and epidermal growth factor. J Biol Chem. 1988 Jul 5;263(19):9374–9380. [PubMed] [Google Scholar]
- Yavin E. Regulation of phospholipid metabolism in differentiating cells from rat brain cerebral hemispheres in culture. Patterns of acetylcholine phosphocholine, and choline phosphoglycerides labeling from (methyl-14C)choline. J Biol Chem. 1976 Mar 10;251(5):1392–1397. [PubMed] [Google Scholar]
- Yoshida K., Asaoka Y., Nishizuka Y. Platelet activation by simultaneous actions of diacylglycerol and unsaturated fatty acids. Proc Natl Acad Sci U S A. 1992 Jul 15;89(14):6443–6446. doi: 10.1073/pnas.89.14.6443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachary I., Sinnett-Smith J. W., Rozengurt E. Early events elicited by bombesin and structurally related peptides in quiescent Swiss 3T3 cells. I. Activation of protein kinase C and inhibition of epidermal growth factor binding. J Cell Biol. 1986 Jun;102(6):2211–2222. doi: 10.1083/jcb.102.6.2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Blitterswijk W. J., Hilkmann H., de Widt J., van der Bend R. L. Phospholipid metabolism in bradykinin-stimulated human fibroblasts. I. Biphasic formation of diacylglycerol from phosphatidylinositol and phosphatidylcholine, controlled by protein kinase C. J Biol Chem. 1991 Jun 5;266(16):10337–10343. [PubMed] [Google Scholar]