Abstract
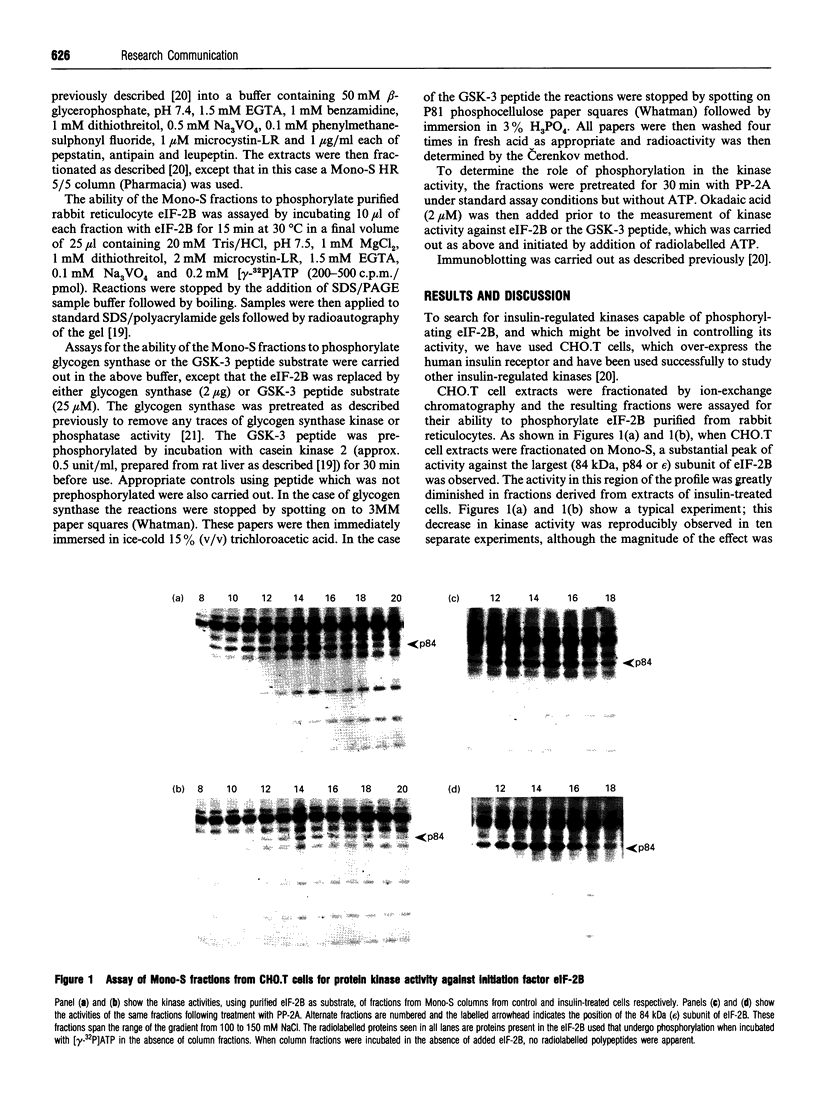
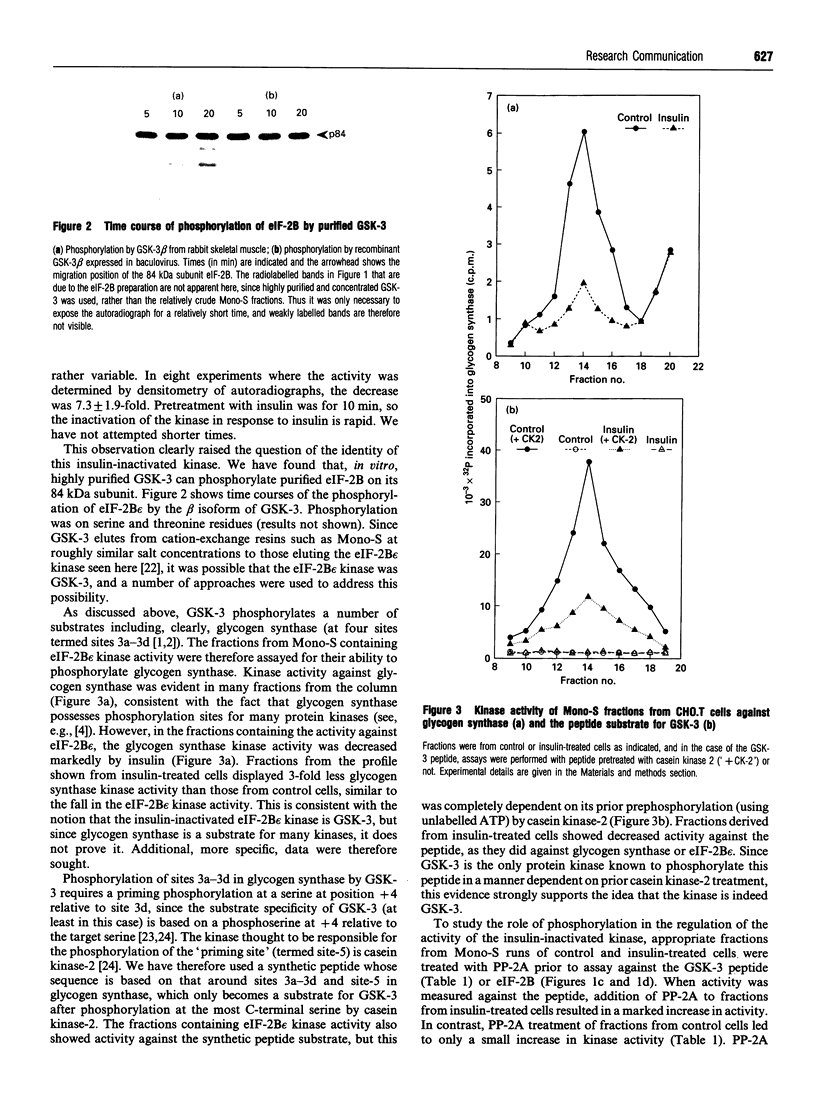
We have studied the control of insulin-regulated protein kinases in Chinese hamster ovary cells transfected with the human insulin receptor (CHO.T cells). Among these enzymes is one that is obtained after chromatography of cell extracts on Mono-S, whose activity is decreased (7.3 +/- 1.9-fold) within 10 min of insulin treatment. This enzyme phosphorylates glycogen synthase and the largest subunit of protein synthesis eukaryotic initiation factor (eIF)-2B (the guanine nucleotide exchange factor). The kinase appears to be glycogen synthase kinase-3 (GSK-3), on the basis of: (1) its ability to phosphorylate a peptide based on the phosphorylation sites for GSK-3 in glycogen synthase, and (2) the finding that the fractions possessing this activity contain immunoreactive GSK-3, whose peak is coincident with that of kinase activity, as judged by immunoblotting using antibodies specific for the alpha- and beta-isoforms of GSK-3. The decrease in kinase activity induced by insulin was reversed by treatment of the column fractions with protein phosphatase-2A. These data indicate that insulin rapidly causes inactivation of GSK-3 and that this is due to phosphorylation of GSK-3. The implications of these findings for the control of glycogen and protein metabolism are discussed.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blackshear P. J., Haupt D. M., Stumpo D. J. Insulin activation of protein kinase C: a reassessment. J Biol Chem. 1991 Jun 15;266(17):10946–10952. [PubMed] [Google Scholar]
- Blackshear P. J. Insulin-stimulated protein biosynthesis as a paradigm of protein kinase C-independent growth factor action. Clin Res. 1989 Jan;37(1):15–25. [PubMed] [Google Scholar]
- Bourouis M., Moore P., Ruel L., Grau Y., Heitzler P., Simpson P. An early embryonic product of the gene shaggy encodes a serine/threonine protein kinase related to the CDC28/cdc2+ subfamily. EMBO J. 1990 Sep;9(9):2877–2884. doi: 10.1002/j.1460-2075.1990.tb07477.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle W. J., Smeal T., Defize L. H., Angel P., Woodgett J. R., Karin M., Hunter T. Activation of protein kinase C decreases phosphorylation of c-Jun at sites that negatively regulate its DNA-binding activity. Cell. 1991 Feb 8;64(3):573–584. doi: 10.1016/0092-8674(91)90241-p. [DOI] [PubMed] [Google Scholar]
- Cooper D. R., Konda T. S., Standaert M. L., Davis J. S., Pollet R. J., Farese R. V. Insulin increases membrane and cytosolic protein kinase C activity in BC3H-1 myocytes. J Biol Chem. 1987 Mar 15;262(8):3633–3639. [PubMed] [Google Scholar]
- Dent P., Lavoinne A., Nakielny S., Caudwell F. B., Watt P., Cohen P. The molecular mechanism by which insulin stimulates glycogen synthesis in mammalian skeletal muscle. Nature. 1990 Nov 22;348(6299):302–308. doi: 10.1038/348302a0. [DOI] [PubMed] [Google Scholar]
- Dickens M., Chin J. E., Roth R. A., Ellis L., Denton R. M., Tavaré J. M. Characterization of insulin-stimulated protein serine/threonine kinases in CHO cells expressing human insulin receptors with point and deletion mutations. Biochem J. 1992 Oct 1;287(Pt 1):201–209. doi: 10.1042/bj2870201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Embi N., Rylatt D. B., Cohen P. Glycogen synthase kinase-3 from rabbit skeletal muscle. Separation from cyclic-AMP-dependent protein kinase and phosphorylase kinase. Eur J Biochem. 1980 Jun;107(2):519–527. [PubMed] [Google Scholar]
- Farese R. V., Cooper D. R. Potential role of phospholipid-signaling systems in insulin action and states of clinical insulin resistance. Diabetes Metab Rev. 1989 Aug;5(5):455–474. doi: 10.1002/dmr.5610050504. [DOI] [PubMed] [Google Scholar]
- Fiol C. J., Mahrenholz A. M., Wang Y., Roeske R. W., Roach P. J. Formation of protein kinase recognition sites by covalent modification of the substrate. Molecular mechanism for the synergistic action of casein kinase II and glycogen synthase kinase 3. J Biol Chem. 1987 Oct 15;262(29):14042–14048. [PubMed] [Google Scholar]
- Goode N., Hughes K., Woodgett J. R., Parker P. J. Differential regulation of glycogen synthase kinase-3 beta by protein kinase C isotypes. J Biol Chem. 1992 Aug 25;267(24):16878–16882. [PubMed] [Google Scholar]
- Hemmings B. A., Aitken A., Cohen P., Rymond M., Hofmann F. Phosphorylation of the type-II regulatory subunit of cyclic-AMP-dependent protein kinase by glycogen synthase kinase 3 and glycogen synthase kinase 5. Eur J Biochem. 1982 Oct;127(3):473–481. doi: 10.1111/j.1432-1033.1982.tb06896.x. [DOI] [PubMed] [Google Scholar]
- Hemmings B. A., Yellowlees D., Kernohan J. C., Cohen P. Purification of glycogen synthase kinase 3 from rabbit skeletal muscle. Copurification with the activating factor (FA) of the (Mg-ATP) dependent protein phosphatase. Eur J Biochem. 1981 Oct;119(3):443–451. doi: 10.1111/j.1432-1033.1981.tb05628.x. [DOI] [PubMed] [Google Scholar]
- Hughes K., Nikolakaki E., Plyte S. E., Totty N. F., Woodgett J. R. Modulation of the glycogen synthase kinase-3 family by tyrosine phosphorylation. EMBO J. 1993 Feb;12(2):803–808. doi: 10.1002/j.1460-2075.1993.tb05715.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes K., Pulverer B. J., Theocharous P., Woodgett J. R. Baculovirus-mediated expression and characterisation of rat glycogen synthase kinase-3 beta, the mammalian homologue of the Drosophila melanogaster zeste-white 3sgg homeotic gene product. Eur J Biochem. 1992 Jan 15;203(1-2):305–311. doi: 10.1111/j.1432-1033.1992.tb19860.x. [DOI] [PubMed] [Google Scholar]
- Hughes K., Ramakrishna S., Benjamin W. B., Woodgett J. R. Identification of multifunctional ATP-citrate lyase kinase as the alpha-isoform of glycogen synthase kinase-3. Biochem J. 1992 Nov 15;288(Pt 1):309–314. doi: 10.1042/bj2880309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizuka T., Cooper D. R., Hernandez H., Buckley D., Standaert M., Farese R. V. Effects of insulin on diacylglycerol-protein kinase C signaling in rat diaphragm and soleus muscles and relationship to glucose transport. Diabetes. 1990 Feb;39(2):181–190. doi: 10.2337/diab.39.2.181. [DOI] [PubMed] [Google Scholar]
- Nakielny S., Campbell D. G., Cohen P. The molecular mechanism by which adrenalin inhibits glycogen synthesis. Eur J Biochem. 1991 Aug 1;199(3):713–722. doi: 10.1111/j.1432-1033.1991.tb16175.x. [DOI] [PubMed] [Google Scholar]
- Oldfield S., Proud C. G. Purification, phosphorylation and control of the guanine-nucleotide-exchange factor from rabbit reticulocyte lysates. Eur J Biochem. 1992 Aug 15;208(1):73–81. doi: 10.1111/j.1432-1033.1992.tb17160.x. [DOI] [PubMed] [Google Scholar]
- Parker P. J., Caudwell F. B., Cohen P. Glycogen synthase from rabbit skeletal muscle; effect of insulin on the state of phosphorylation of the seven phosphoserine residues in vivo. Eur J Biochem. 1983 Jan 17;130(1):227–234. doi: 10.1111/j.1432-1033.1983.tb07140.x. [DOI] [PubMed] [Google Scholar]
- Poulter L., Ang S. G., Gibson B. W., Williams D. H., Holmes C. F., Caudwell F. B., Pitcher J., Cohen P. Analysis of the in vivo phosphorylation state of rabbit skeletal muscle glycogen synthase by fast-atom-bombardment mass spectrometry. Eur J Biochem. 1988 Aug 15;175(3):497–510. doi: 10.1111/j.1432-1033.1988.tb14222.x. [DOI] [PubMed] [Google Scholar]
- Proud C. G. Protein phosphorylation in translational control. Curr Top Cell Regul. 1992;32:243–369. doi: 10.1016/b978-0-12-152832-4.50008-2. [DOI] [PubMed] [Google Scholar]
- Ramakrishna S., Benjamin W. B. Cyclic nucleotide-independent protein kinase from rat liver. Purification and characterization of a multifunctional protein kinase. J Biol Chem. 1985 Oct 5;260(22):12280–12286. [PubMed] [Google Scholar]
- Ramakrishna S., Benjamin W. B. Insulin action rapidly decreases multifunctional protein kinase activity in rat adipose tissue. J Biol Chem. 1988 Sep 5;263(25):12677–12681. [PubMed] [Google Scholar]
- Ramakrishna S., Murthy K. S., Benjamin W. B. Effect of insulin on ATP-citrate lyase phosphorylation: regulation of peptide A and peptide B phosphorylations. Biochemistry. 1989 Jan 24;28(2):856–860. doi: 10.1021/bi00428a067. [DOI] [PubMed] [Google Scholar]
- Rhoads R. E. Regulation of eukaryotic protein synthesis by initiation factors. J Biol Chem. 1993 Feb 15;268(5):3017–3020. [PubMed] [Google Scholar]
- Siegfried E., Perkins L. A., Capaci T. M., Perrimon N. Putative protein kinase product of the Drosophila segment-polarity gene zeste-white3. Nature. 1990 Jun 28;345(6278):825–829. doi: 10.1038/345825a0. [DOI] [PubMed] [Google Scholar]
- Vandenheede J. R., Yang S. D., Goris J., Merlevede W. ATP x Mg-dependent protein phosphatase from rabbit skeletal muscle. II. Purification of the activating factor and its characterization as a bifunctional protein also displaying synthase kinase activity. J Biol Chem. 1980 Dec 25;255(24):11768–11774. [PubMed] [Google Scholar]
- Villa-Moruzzi E. Stimulation of FA and phosphatase-1 activities by insulin in 3T3-L1 cells. FEBS Lett. 1989 Dec 4;258(2):208–210. doi: 10.1016/0014-5793(89)81654-0. [DOI] [PubMed] [Google Scholar]
- Welsh G. I., Proud C. G. Evidence for a role for protein kinase C in the stimulation of protein synthesis by insulin in swiss 3T3 fibroblasts. FEBS Lett. 1993 Feb 1;316(3):241–246. doi: 10.1016/0014-5793(93)81300-o. [DOI] [PubMed] [Google Scholar]
- Welsh G. I., Proud C. G. Regulation of protein synthesis in Swiss 3T3 fibroblasts. Rapid activation of the guanine-nucleotide-exchange factor by insulin and growth factors. Biochem J. 1992 May 15;284(Pt 1):19–23. doi: 10.1042/bj2840019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodgett J. R., Cohen P. Multisite phosphorylation of glycogen synthase. Molecular basis for the substrate specificity of glycogen synthase kinase-3 and casein kinase-II (glycogen synthase kinase-5). Biochim Biophys Acta. 1984 Aug 14;788(3):339–347. doi: 10.1016/0167-4838(84)90047-5. [DOI] [PubMed] [Google Scholar]
- Woodgett J. R. Molecular cloning and expression of glycogen synthase kinase-3/factor A. EMBO J. 1990 Aug;9(8):2431–2438. doi: 10.1002/j.1460-2075.1990.tb07419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S. D., Ho L. T., Fung T. J., Yu J. S. Insulin induces activation of kinase FA in membranes and thereby promotes activation of ATP.Mg-dependent phosphatase in adipocytes. Biochem Biophys Res Commun. 1989 Feb 15;158(3):762–768. doi: 10.1016/0006-291x(89)92787-3. [DOI] [PubMed] [Google Scholar]