Abstract
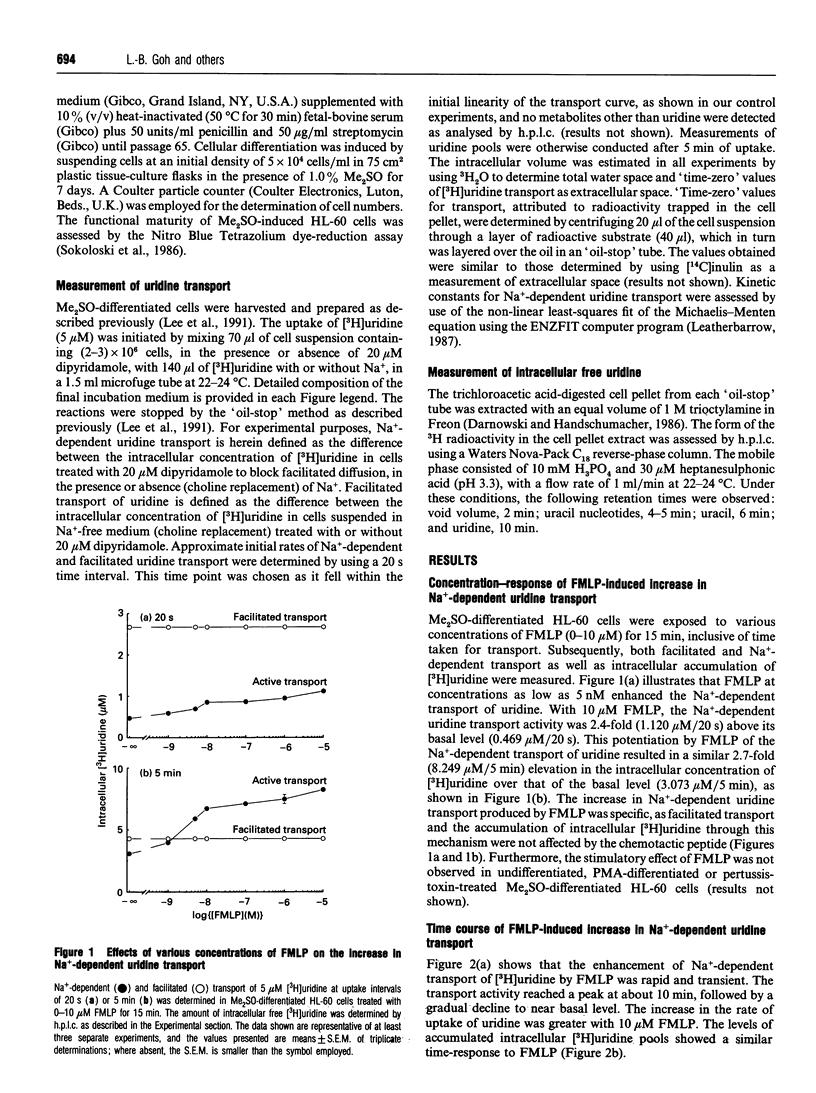
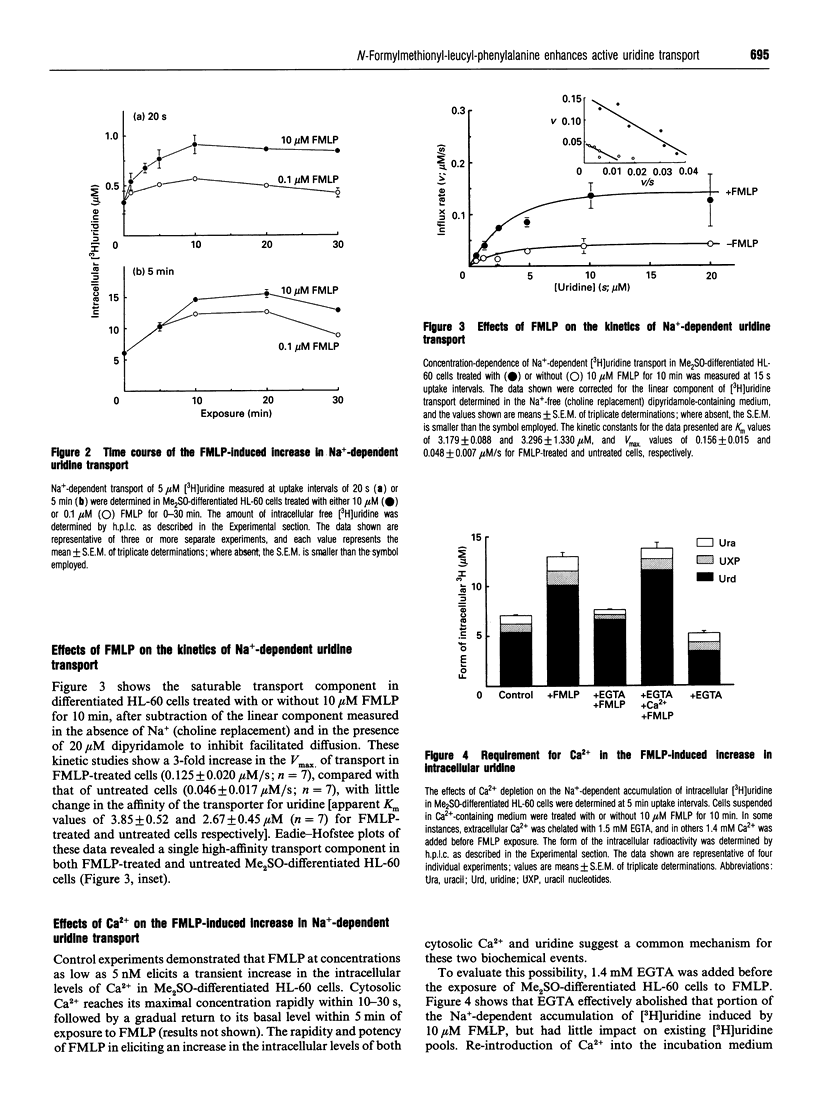
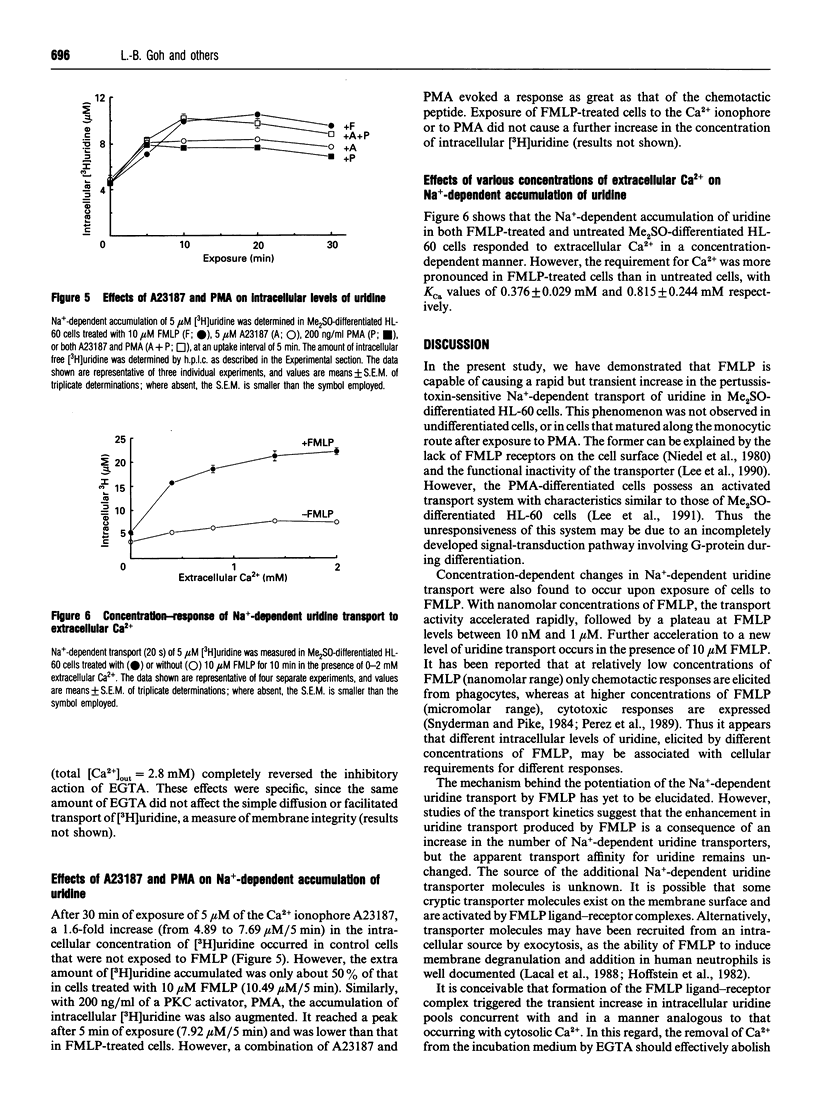
N-Formyl-Met-Leu-Phe (FMLP), at concentrations as low as 5 nM, caused an increase in intracellular uridine pools in dimethyl sulphoxide (Me2SO)-differentiated HL-60 cells. Intracellular uridine pools were elevated rapidly and reached a maximum within 10 min of exposure to 10 microM FMLP, followed by a gradual decline. This enhancement by FMLP was a consequence of a 3-fold increase in the Vmax of pertussis-toxin-sensitive Na(+)-dependent uridine transport system, with no change in the apparent Km. Km values of 2.67 +/- 0.45 and 3.85 +/- 0.52 microM and Vmax. values of 0.046 +/- 0.017 and 0.125 +/- 0.020 microM/s were obtained for untreated and FMLP-treated Me2SO-differentiated cells respectively. The effect of FMLP on the Na(+)-dependent transport of uridine in Me2SO-differentiated HL-60 cells was specific, as the facilitated transport of uridine was unaffected. Furthermore, this phenomenon was not observed in undifferentiated, phorbol 12-myristate 13-acetate (PMA)-differentiated or pertussis-toxin-treated Me2SO-differentiated HL-60 cells. Removal of extracellular Ca2+ with EGTA abolished the FMLP enhancement of uridine transport in a reversible manner, suggesting the involvement of Ca2+. However, the Ca2+ ionophore A23187 only partially mimicked the effect of FMLP. Similarly, with PMA the transport was sub-optimally enhanced, but a full activation was observed in cells treated with both A23187 and PMA. These findings suggest that activation of the Na(+)-dependent uridine transporter by FMLP in Me2SO-differentiated HL-60 cells involves a pertussis-toxin-sensitive G-protein with a bifurcating signal-transduction pathway.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brandt S. J., Dougherty R. W., Lapetina E. G., Niedel J. E. Pertussis toxin inhibits chemotactic peptide-stimulated generation of inositol phosphates and lysosomal enzyme secretion in human leukemic (HL-60) cells. Proc Natl Acad Sci U S A. 1985 May;82(10):3277–3280. doi: 10.1073/pnas.82.10.3277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowen D. S., Baker B., Dubyak G. R. Pertussis toxin produces differential inhibitory effects on basal, P2-purinergic, and chemotactic peptide-stimulated inositol phospholipid breakdown in HL-60 cells and HL-60 cell membranes. J Biol Chem. 1990 Sep 25;265(27):16181–16189. [PubMed] [Google Scholar]
- Darnowski J. W., Handschumacher R. E. Tissue uridine pools: evidence in vivo of a concentrative mechanism for uridine uptake. Cancer Res. 1986 Jul;46(7):3490–3494. [PubMed] [Google Scholar]
- Di Virgilio F., Vicentini L. M., Treves S., Riz G., Pozzan T. Inositol phosphate formation in fMet-Leu-Phe-stimulated human neutrophils does not require an increase in the cytosolic free Ca2+ concentration. Biochem J. 1985 Jul 15;229(2):361–367. doi: 10.1042/bj2290361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty R. W., Godfrey P. P., Hoyle P. C., Putney J. W., Jr, Freer R. J. Secretagogue-induced phosphoinositide metabolism in human leucocytes. Biochem J. 1984 Sep 1;222(2):307–314. doi: 10.1042/bj2220307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffstein S. T., Friedman R. S., Weissmann G. Degranulation, membrane addition, and shape change during chemotactic factor-induced aggregation of human neutrophils. J Cell Biol. 1982 Oct;95(1):234–241. doi: 10.1083/jcb.95.1.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause K. H., Schlegel W., Wollheim C. B., Andersson T., Waldvogel F. A., Lew P. D. Chemotactic peptide activation of human neutrophils and HL-60 cells. Pertussis toxin reveals correlation between inositol trisphosphate generation, calcium ion transients, and cellular activation. J Clin Invest. 1985 Oct;76(4):1348–1354. doi: 10.1172/JCI112109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacal P., Pulido R., Sánchez-Madrid F., Mollinedo F. Intracellular location of T200 and Mo1 glycoproteins in human neutrophils. J Biol Chem. 1988 Jul 15;263(20):9946–9951. [PubMed] [Google Scholar]
- Lee C. W., Sokoloski J. A., Sartorelli A. C., Handschumacher R. E. Induction of the differentiation of HL-60 cells by phorbol 12-myristate 13-acetate activates a Na(+)-dependent uridine-transport system. Involvement of protein kinase C. Biochem J. 1991 Feb 15;274(Pt 1):85–90. doi: 10.1042/bj2740085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marasco W. A., Phan S. H., Krutzsch H., Showell H. J., Feltner D. E., Nairn R., Becker E. L., Ward P. A. Purification and identification of formyl-methionyl-leucyl-phenylalanine as the major peptide neutrophil chemotactic factor produced by Escherichia coli. J Biol Chem. 1984 May 10;259(9):5430–5439. [PubMed] [Google Scholar]
- Niedel J., Kahane I., Lachman L., Cuatrecasas P. A subpopulation of cultured human promyelocytic leukemia cells (HL-60) displays the formyl peptide chemotactic receptor. Proc Natl Acad Sci U S A. 1980 Feb;77(2):1000–1004. doi: 10.1073/pnas.77.2.1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez H. D., Elfman F., Marder S., Lobo E., Ives H. E. Formyl peptide-induced chemotaxis of human polymorphonuclear leukocytes does not require either marked changes in cytosolic calcium or specific granule discharge. Role of formyl peptide receptor reexpression (or recycling). J Clin Invest. 1989 Jun;83(6):1963–1970. doi: 10.1172/JCI114105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozzan T., Lew D. P., Wollheim C. B., Tsien R. Y. Is cytosolic ionized calcium regulating neutrophil activation? Science. 1983 Sep 30;221(4618):1413–1415. doi: 10.1126/science.6310757. [DOI] [PubMed] [Google Scholar]
- Rossi F. The O2- -forming NADPH oxidase of the phagocytes: nature, mechanisms of activation and function. Biochim Biophys Acta. 1986 Nov 4;853(1):65–89. doi: 10.1016/0304-4173(86)90005-4. [DOI] [PubMed] [Google Scholar]
- Schiffmann E., Corcoran B. A., Wahl S. M. N-formylmethionyl peptides as chemoattractants for leucocytes. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1059–1062. doi: 10.1073/pnas.72.3.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Showell H. J., Freer R. J., Zigmond S. H., Schiffmann E., Aswanikumar S., Corcoran B., Becker E. L. The structure-activity relations of synthetic peptides as chemotactic factors and inducers of lysosomal secretion for neutrophils. J Exp Med. 1976 May 1;143(5):1154–1169. doi: 10.1084/jem.143.5.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyderman R., Pike M. C. Chemoattractant receptors on phagocytic cells. Annu Rev Immunol. 1984;2:257–281. doi: 10.1146/annurev.iy.02.040184.001353. [DOI] [PubMed] [Google Scholar]
- Snyderman R., Smith C. D., Verghese M. W. Model for leukocyte regulation by chemoattractant receptors: roles of a guanine nucleotide regulatory protein and polyphosphoinositide metabolism. J Leukoc Biol. 1986 Dec;40(6):785–800. doi: 10.1002/jlb.40.6.785. [DOI] [PubMed] [Google Scholar]
- Sokoloski J. A., Blair O. C., Sartorelli A. C. Alterations in glycoprotein synthesis and guanosine triphosphate levels associated with the differentiation of HL-60 leukemia cells produced by inhibitors of inosine 5'-phosphate dehydrogenase. Cancer Res. 1986 May;46(5):2314–2319. [PubMed] [Google Scholar]
- Sokoloski J. A., Sartorelli A. C., Handschumacher R. E., Lee C. W. Inhibition by pertussis toxin of the activation of Na(+)-dependent uridine transport in dimethyl-sulphoxide-induced HL-60 leukaemia cells. Biochem J. 1991 Dec 1;280(Pt 2):515–519. doi: 10.1042/bj2800515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams L. T., Snyderman R., Pike M. C., Lefkowitz R. J. Specific receptor sites for chemotactic peptides on human polymorphonuclear leukocytes. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1204–1208. doi: 10.1073/pnas.74.3.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]


