Abstract
Proline is a unique amino acid in that its side-chain is cyclised to the backbone, thus giving proline an exceptional rigidity and a considerably restricted conformational space. Polyproline forms two well-characterized helical structures: a left-handed polyproline helix (PPII) and a right-handed polyproline helix (PPI). Usually, sequences made only of prolyl residues are in PPII conformation, but even sequences not rich in proline but which are rich in glycine, lysine, glutamate, or aspartate have also a tendency to form PPII helices. Currently, the only way to study unambiguously PPII structure in solution is to use spectroscopies based on optical activity such as circular dichroism, vibrational circular dichroism and Raman optical activity. The importance of the PPII structure is emphasized by its ubiquitous presence in different organisms from yeast to human beings where proline-rich motifs and their binding domains are believed to be involved in vital biological processes. Some of the domains that are bound by proline-rich motifs include SH3 domains, WW domains, GYF domains and UEV domains, etc. The PPII structure has been demonstrated to be essential to biological activities such as signal transduction, transcription, cell motility, and immune response.
Keywords: Proline, PPI, PPII, Amino acid, Biological activity
Introduction
Proline is a special amino acid because its side-chain is cyclised to the backbone, which gives it an exceptional rigidity and a highly restricted conformational space.(Moradi et al. 2009) In biological systems, proline adopts two isomeric conformations, the cis and the trans conformations which both have high abundances.(Fanni et al. 2023; Gurung et al. 2023) This can be explained by the fact that the difference in energy due to steric hindrance for the two conformations is relatively smaller in comparison to the ones between the cis and trans conformations of linear amino acids which rather prefer the trans conformation due to its low level of steric hindrance.(Gurung et al. 2023) However, it has been observed that the cyclic hexapeptides c-(-DXaa-Ser-Pro-DXaa-Lys-Pro-) adopt preferably the cis-Pro/cis-Pro conformation under physiological conditions.(Malešević et al. 2012) In these peptides where the D-amino acids are either phenylalanine or alanine, the cis propensity for prolyl residues also increases when serine is either phosphorylated or replaced with tyrosine.(Malešević et al. 2012) On the other hand, the linear counterparts have reduced inclination for the formation of the cis-Pro/cis-Pro conformation.(Malešević et al. 2012) The cis–trans isomerization of proline greatly regulates the folding and function of many proteins.(William J. et al. 2002; Fanni et al. 2023; Gurung et al. 2023) It also serves as a special case of post-translational modification that regulates numerous biological pathways such as DNA repair, cell cycle regulation, ion channel gating and T-cell activation.(Gurung et al. 2023).
Polyproline forms two characteristic helical structures: PPII and PPI. PPII is left-handed and is formed when all backbone residues adopt backbone dihedral angles (ϕ, ψ) of approximately (−75°, 146°) (Table 1), with all prolyl peptide bonds in the trans-isomer conformation (i.e. backbone dihedral angle ω = 180°).(Horng and Raines 2006; Moradi et al. 2009; Zanna et al. 2016) As for PPI, it is right-handed and it is formed when all backbone residues adopt backbone dihedral angles (ϕ, ψ) of approximately (−75°, 160°) (Table 1) and all prolyl peptide bonds assume a cis-isomer conformation (i.e. backbone dihedral angle ω = 0°).(Horng and Raines 2006; Moradi et al. 2009; Zanna et al. 2016) PPII is extended and has 3 residues per turn whereas PPI is more compact and has 3.3 residues per turn.(Moradi et al. 2009) The unit height of PPI is 1.9 Å(Traub, W. and Shmueli, U. 1963) whereas the unit height of a PPII helix is generally around 3.1 Å.(Cowan and McGavin 1955; Adzhubei and Sternberg 1993).
Table 1.
Comparison of properties of PPI and PPII
| Parameters | PPI | PPII |
|---|---|---|
| Favorable solvent | Methanol, ethanol and 1-propanol.(Kakinoki et al. 2005; Shi et al. 2016; El-Baba et al. 2019) | Water and trifluoroethanol (TFE). (Kakinoki et al. 2005; Shi et al. 2016; El-Baba et al. 2019) |
| Interactions between residues | Inter-residue interactions more favourable.(Moradi et al. 2009) | Inter-residue interactions less favourable.(Moradi et al. 2009) |
| Unit height | 1.9 Å.(Traub, W. and Shmueli, U. 1963) | 3.1 Å.(Cowan, and McGavin 1955; Adzhubei and Sternberg 1993) |
| Structure type | Compact structure.(Liu et al. 2004b) | Extended structure.(Liu et al. 2004b) |
| Geometry (helical structure) | Right-handed.(Horng and Raines 2006; Moradi et al. 2009) | Left-handed.(Horng and Raines 2006; Moradi et al. 2009) |
| Dihedral angles (ϕ, ψ, ω) | (-75°, 160°, 0°).(Horng and Raines 2006; Moradi et al. 2009; Zanna et al. 2016) | (-75°, 146°, 180°).(Horng and Raines 2006; Moradi et al. 2009; Zanna et al. 2016) |
| Residues per turn | 3.3 residues per turn.(Moradi et al. 2009; Zanna et al. 2016) | 3 residues per turn.(Moradi et al. 2009; Zanna et al. 2016) |
| Vibrational circular dichroism (VCD) bands |
- Medium intensity negative (198–200 nm) - Strong positive (214–215 nm) - Weak negative (231–232 nm).(Kakinoki et al. 2005) |
- Strong negative (202–206 nm) - Weak positive (225–229 nm).(Kakinoki et al. 2005) |
| Puckering conformations of proline residues | Partially mixed puckering (up-puckered and down-puckered conformations).(Kang et al. 2006) | All-down puckering in solutions.(Kang et al. 2006) |
The PPII conformation is not only formed by sequences made of only prolyl residues because even polyglutamate, polylysine, polyglycine and polyaspartate can also form this structure.(Kelly et al. 2001; Beck et al. 2008; Brown and Zondlo 2012; Kumar and Bansal 2016) PPII structure in solution is studied using spectroscopies based on optical activity.(Bochicchio and Tamburro 2002; Pollastrini et al. 2022) The characteristic bands of PPII, observed using circular dichroism, appear as a strong negative band at 202–206 nm and a weak positive band at 225–229 nm.(Kakinoki et al. 2005) As for PPI, it is characterised by three bands, a medium intensity negative band at 198–200 nm, a strong positive band at 214–215 nm and a weak negative band at 231–232 nm.(Kakinoki et al. 2005).
Proline-rich motifs and proline-rich motif recognition domains are ubiquitous in eukaryotic organisms and are involved in many signalling mechanisms that govern cellular behaviour.(Freund et al. 2008) In fact, the PPII structure has been demonstrated to be essential to a variety of biological activities including for example signal transduction, transcription, energy production, cell motility, cell growth, stress reduction and immune response.(Kelly et al. 2001; Saibi et al. 2015; Christgen and Becker 2019; Milorey et al. 2021) Identification of proline-rich (PxxP) regions particular to a certain pathogen can be exploited to develop drugs targeting that organism. Some pathogenic microorganisms use proline-rich proteins to bind the host-cell SH3 domains.(Bliska 1996) The Src-homology 3 (SH3) domain is a conserved region composed of approximately 60 residues folded into 5 β-strands connected by 3 loops and a 310-helix segment.(Zafra-Ruano and Luque 2012) SH3 domains are believed to be involved in cell polarization and subcellular localisation, signal transduction and regulation of tyrosine kinase activity.(Musacchio et al. 1994) Generally, SH3 domains bind to ligands rich in proline and PxxP sequence is considered the minimal consensus target.Ravi Chandra et al. (2004) and Saksela and Permi (2012) studied the proteome of human, mouse, yeast, Plasmodium falciparum and Mycobacterium tuberculosis and found out that PxxP regions are very common in all of these organisms. Interestingly, they also found that P. falciparum and M. tuberculosis possess some PxxP motifs unique to them, which can be exploited to design peptidomimetics which can serve as leads for the development of drugs targeting those organisms.
The basis of PPII stability
PPII structure is characterized by the absence of intramolecular hydrogen bonds, which makes it difficult to distinguish PPII from irregular structures using 1H-NMR spectroscopy.(Bochicchio and Tamburro 2002) There are various factors which have influence on the formation of PPII and these include the nature of the monomer, the chain length, the solvent, ionic strength, the temperature, the presence of denaturants, etc. (Brown and Zondlo 2012; Zondlo 2022; Rajewski et al. 2023) Kakinoki et al. (2005) have used circular dichroism and molecular mechanics to study the propensity of proline oligomers to form either PPII or PPI helices with respect to the chain length and the solvent. In their experimental study which was carried out on proline homopolymers of 13 (P13), 6 (P6) and 4 (P4) residues respectively, they found out that the formation of PPII or PPI helices depended on the chain length and on the solvent. At 5 °C, while PPII was favoured by water and trifluoroethanol (TFE), PPI was favoured by methanol and 1-propanol. This is not in perfect accordance with the findings of a recent study by (El-Baba et al. 2019) who on their side found that PPII was stabilized by polar solvents (water and methanol) at all temperatures. Referring back to the study of Kakinoki et al. (2005), each of the PPII and PPI conformations in their study was stabilized by an increasing number of residues as long as the solvent was favourable to that conformation. The dependence on chain length was for example evidenced by the fact that a time-dependent conformational transition from PPII to PPI could be observed when P13 was dissolved in methanol and 1-propanol, whereas P4 and P6 stayed in PPII conformation in methanol and only P4 stayed in PPII conformation in 1-propanol. Their theoretical studies which took into consideration the energy difference between PPI and PPII helices per residues showed that inter-residue interactions are more favourable in PPI than in PPII. Their findings are also supported by another theoretical study by Moradi et al. (2009) who studied in silico a hexamer, a nanomer and a tridecamer polyproline in gas phase and in implicit water. They have also studied the nanomer with explicit hexane and 1-propanol. Their results corresponding to gas phase, implicit water and explicit 1-propanol media all supported the experimental results of Kakinoki et al. (2005). In an effort to explain the difference in behaviour of polyproline in different solvents, Moradi et al. (2009) suggested two features which are involved in this behaviour. First, they suggested that the characteristic ring of polyproline prevents the nitrogen atom of the prolyl bond from hydrogen bonding, and that this affects both the structure of the helices and the interaction with the solvents. Although this argument may hold for polyproline, it is likely not appropriate to explain the formation of PPII by other amino acids such as polyglycine, polylysine, etc. which do not have conformational restrictions. Then, they argue that the cis–trans conformations give different orientations of the carboxyl groups, and that while these groups are shielded from the solvent by proline rings due to the almost parallel orientation of these carboxyl groups with respect to the axis of the helix in PPI, they are more exposed to the solvent in PPII due to their mainly perpendicular orientation with respect to the axis of the PPII helix. They also add that the compact structure of PPI and the extended structure of PPII respectively contribute to the shielding or the exposition of the carbonyl groups to the solvent, thus leading to different behaviour of polyproline in different solvents. It is noteworthy that Liu et al. (2004b) had previously noticed that in water and short-chain aliphatic alcohols (methanol, ethanol and 2-propanol), the PPII content of AcGGAGGNH2, the model of a neutral fragment with minimal constraints, decreased with the decrease in the solvent’s empirical overall polarity rather than with the dielectric constant or any other single solvent property.
Water is thought to be a very good solvent for PPII stability. There are different views about forces which stabilize PPII in water, and while many authors believe that hydrogen bonds mediated by water molecules to form water bridges are the main stabilizing force, there are also a few others who have views which differ from that one. Sreerama and Woody (1999) have used molecular dynamics simulations of alanine octapeptide in water to investigate the PPII conformation behaviour and the role played by water in stabilizing PPII conformation in the absence of constraints due to pyrrolidine ring. Adzhubei and Sternberg (1993) had previously suggested that PPII segments found on the surface of the proteins were stabilized by hydrogen bonds between water molecules and PPII backbone. When Sreerama and Woody (1999) compared α, β, and PPII conformations, they noticed that α-helix is stabilized by intra-molecular hydrogen bonds while β-conformation and PPII lack these interactions, which gives them more flexibility than α-helix. Moreover, they found out that in the absence of intermolecular hydrogen bonds, PPII was approximately twice more populated than β-conformation in water. Since water can form bridges connecting the backbone atoms of both β-strand and PPII, it was suggested that PPII structure is stabilized by water bridges which connect two consecutive oxygen atoms of the peptide backbone, a kind of hydrogen-bonding not found in a β-strand. However, it should be noticed that here they referred to a single β-strand and not to β-conformation in β-sheets which are stabilized by interstrand hydrogen bonds. Simulations by Mezei et al. (2004) gave different results. While, they could observe that PPII displayed more hydrogen bonds than α-helix or β-strand, there were no water bridges in PPII or α-helix mediated by a single water molecule, a feature widely present in β-strands..Mezei et al. (2004) argued that PPII geometry is not favourable to bridge formation and that as these bridges would disrupt the overall organization of water molecules surrounding a PPII helix, then the stability of PPII in comparison to a β-strand can be explained by the fact that PPII does not form the entropically disfavoured water bridges found in β-strands. Kentsis et al. (2004) share the same view with Mezei et al. (2004) about the entropic origin of PPII stabilization by water.
The research on the origin of PPII stability stays a contentious subject. A relatively recent study by Mirkin and Krimm (2012) came to the same conclusion as some of previous researches that water plays a great role in stabilizing PPII helix in comparison to β-conformation by means of hydrogen bonds. Mirkin and Krimm (2012) used a model of alanine dipeptide surrounded with an increasing number (n = 4, 6 or 12) of explicit water molecules with and without an external force field (polarized continuum model) and by comparing the relative energies of solvated PPII and β-conformations, they noticed that although β-conformation is intrinsically more stable than PPII, the interaction energy difference between PPII and β-systems are very negative, thus resulting in a negative energy difference between PPII and β-systems. Although the role played by other factors such as electrostatic interactions and side chains interactions is not ignored, the importance of water in stabilizing PPII in comparison to β-conformation is demonstrated by the interaction energy difference which becomes increasingly negative as the number of water molecules included in the model increases. Mirkin and Krimm (2012) suggest that the interaction energies involved are due to hydrogen bonds of water molecules to the peptide C = O and NH as well as between them. However, a number of other authors investigating the role of water bridges in PPII stability had previously come to the conclusion that water bridges are not the primary source of PPII stability. Law and Daggett (2010) analysed frequencies of water bridges in three data sets, including native state and unfolding molecular dynamics (MD) simulations of about 67% of all known protein structures as well as a collection of 2351 high-resolution crystal structures. Surprisingly, they realised that out of the four major conformations (αR, αL, PPII and β), the frequency of water bridges around PPII was the lowest in native MD while it was third lowest in crystal structures and again third lowest in denatured MD. This led them to concluding that water bridges are not a major source of PPII stability, arguing that if they were, then they would have been frequent around PPII. Drozdov et al. (2004) also used Monte Carlo simulations to study 16 low-energy conformers of the alanine dipeptide (N-acetylalanine-N'-methyl amide) in liquid water and came to the conclusion that the preference of PPII conformation is not a consequence of favourable interactions between the peptide and water, but that rather peptide-water interactions, by overcoming intradipeptide attractive electrostatic interactions, help unmask underlying conformational preferences of the peptide which are a consequence of minimizing intradipeptide steric conflicts. In another differing view, Pappu and Rose (2002) analysed the potential energy landscape of certain alanine-based peptides in order to shed light on the physical basis of PPII helices in peptide and protein unfolded states and they suggested that PPII preference is not simply a consequence of hydrogen-bonding, but that it results from the general trend of unfolded states to minimize chain packing density when the solvent is favourable to that. Moreover, Bochicchio and Tamburro (2002) suggested that chain-chain associations either by hydrogen bonds or by hydrophobic interactions could also play a vital role in the stabilization of PPII helices in aqueous solvent.
Stereoelectronic effects are also important in PPII stability. Bretscher et al. (2001), based on their results from a study of collagen mimics (Pro, (4R)-hydroxy- and (4S)-hydroxy-L-proline, (4R)-fluoro and (4S)-hydroxy-L-proline), concluded that a substituent at the γ-position of proline can enhance conformational stability by favouring the trans-isomer, thus pushing the strands into a conformation similar to the one of the collagen triple helix. They also found that the stereochemistry at γ-position was important for conformational stability. Previously, Eberhardt et al. (1996) had shown that inductive effects exerted by hydroxyl group in position Cγ stabilize collagen triple helix whose all peptide bonds are in trans-conformation. Further studies showed that electron-withdrawing groups placed in position (4R) on prolyl residues favour trans peptide bonds, thus promoting PPII while electron-withdrawing groups placed in position (4S) favour cis peptide bonds, thus promoting PPI.(Horng and Raines 2006; Chiang et al. 2009) From a kinetic point of view, stereoelectronic effects stabilize PPI or PPII by changing the activation energy of the PPII ↔ PPI interconversion.(Chiang et al. 2009) In fact, Chiang et al. (2009) state that electron-withdrawing groups placed at position 4R increase the PPII → PPI transition state barrier, thus favouring PPII whereas when they are placed in position 4S, they decrease the PPII → PPI transition state barrier, thus favouring the formation of PPI. Although high temperatures favour PPI conformation, inductive effects may undermine them and stabilize PPII even in a solution such as n-propanol (95% v/v), which actually would favour PPI if proline were not substituted by electron-withdrawing groups in position (4R) of proline.(Eberhardt et al. 1996; Horng and Raines 2006).
Another factor to consider is the role played by denaturants in PPII stability. The CD spectrum of AcO2A7O2NH2 showed that PPII is stabilized by increasing concentrations of the denaturant agent guanidine hydrochloride.(Liu et al. 2004b) From this observation, Liu et al. (2004b) suggested that the complete unfolded state of peptides in water at low temperature is PPII, an idea which was later opposed by Makowska et al. (2006) who rather suggested that PPII conformation was one in an ensemble of interconverting states of a denatured protein. In their experiment, Makowska et (al. 2006) observed that the PPII content of Ac-XX(A)7OO-NH2 (with X = diaminobutyric acid, O = ornithine and A = alanine) depended on the temperature, the pH as well as the types of ions present in the solution, PPII being favoured at low temperature. The increase in temperature favoured β-strands and statistical coil structures.
The puckering of the pyrrolidine ring has also been associated with the preference of proline residues for PPI or PPII conformation. DeTar and Luthra (1977) have found that only two parameters are necessary to define the conformational states of the pyrrolidine ring. The two puckered conformations of the pyrrolidine ring are referred to as ‘‘up-puckered conformation’’ and ‘‘down-puckered conformation’’.(Momany et al. 1975) Down-puckered conformation is the one for which Cγ and the carbonyl group (CO) are on the same side of the plane defined by the atoms Cα, N and Cδ of the prolyl ring whereas for up-puckered conformation, Cγ and the carbonyl group of proline residue are on the opposite sides.(Kang et al. 2006) PPII and PPI structures have different puckering conformations of proline residues; while PPII-like structures prefer all-down puckering in solutions, PPI ones have partially mixed puckering. However, as the polyproline chain grows longer, the up-puckered and the down-puckered conformations become equally probable in solutions.(Kang et al. 2006).
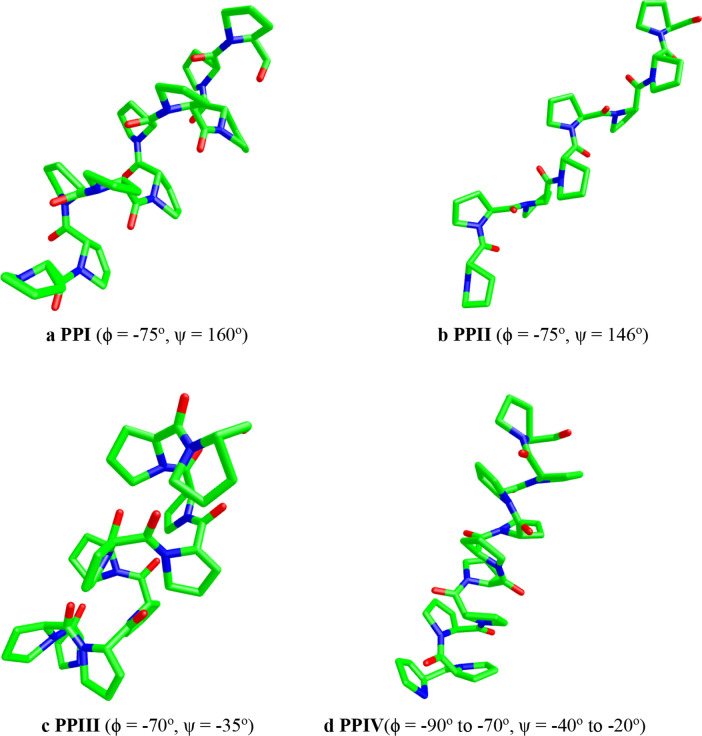
Apart from PPI (Fig. 1a) and PPII (Fig. 1b), there are other two forms of polyproline which have been identified by computational methods. These two forms seem to be intermediate between PPI and PPII and they are referred to as PPIII (Fig. 1c) and PPIV (Fig. 1d).
Fig. 1.
a PPI (ϕ = −75°, ψ = 160°), b PPII (ϕ = −75°, ψ = 146°), c PPIII (ϕ = -70°, ψ = −35°), d PPIV(ϕ = −90° to −70°, ψ = − 40° to −20°)
PPIII is right-handed and has trans amide bonds whereas PPIV is left-handed and has cis-amide bonds.(Hopfinger and Walton 1969; Zhong and Carlson 2006) PPIII has (ϕ, ψ) angles of approximately (−70°, −35°) while PPIV has ϕ angle ranging from −90° to −70° and ψ angle ranging from −40° to −20°.(Zhong and Carlson 2006) PPIV is not likely to be observed experimentally as opposed to PPIII and both PPIII and PPIV are higher in energy than PPI and PPII.(Zhong and Carlson 2006) Thus, it should be noted that the handedness of a polyproline structure does not only depend on the cis–trans conformation of amino acid residues but that it also depends on the torsional angles of the peptide bonds in the structure.(Zhong and Carlson 2006) Although PPIII and particularly PPIV cannot be isolated experimentally due to their high energy, the knowledge of their behaviour may help us understand the interconversion between PPII and PPI as well as help us understand the role played by polyprolines in protein folding.(Zhong and Carlson 2006).
When considering results obtained from simulations of PPII or other structures using molecular dynamics simulations which use classical molecular mechanics force fields, one has to remember that these results cannot accurately correspond to the experimental results due to the fact that the energy functions used which lead to limited mechanical descriptions cannot fully describe the quantum–mechanical properties of the molecules.(Mirkin and Krimm 2012).
The propensity of different amino acids to form the PPII structure
Not all the amino acids are prone to forming PPII with the same ease. Kelly et al. (2001) used a guest–host model to study the propensity of different amino acids to form PPII helix. Their model consisted in an N-acetylated seven-residue proline chain where the central proline residue was replaced by different amino acids and -glycine-tyrosine-NH2 residue was placed at the C-terminus. Acetylation and amidation served to remove charge interactions whereas -Gly-Tyr- residue was added for concentration determination purposes.(Kelly et al. 2001; Brandts and Kaplan 2002) Kelly et al. (2001) have found that while proline has the highest propensity to form PPII, glutamine, alanine and glycine are also very good at forming PPII. After realising that isoleucine and valine had lower PPII formation propensity when compared to methionine and leucine, despite each of the four amino acids being restricted to the β-region of (ϕ, ψ)-space by the following proline in the chain, Kelly et al. (2001) concluded that the low propensity of isoleucine and valine was not purely due to hydrophobicity. Noticing that glycine and leucine had similar propensity and that alanine was almost as good as proline at forming PPII, Kelly et al. (2001) suggested that the low propensity observed for isoleucine and valine may be due to the presence of a side chain at β-position. By molecular modelling, they could observe that when in PPII conformation, side-chains of valine and isoleucine prevented effective interaction with the solvent by occluding the backbone. They suggested that the high propensity of glutamine may partially be due to a probable side-chain to backbone hydrogen bond. However, in another recent study valine was predicted to have low propensity for PPII formation.(O’Brien et al. 2020) Furthermore, there is another study which previously suggested that apart from proline no other amino acid has an intrinsic propensity to form PPII helices.(Moradi et al. 2011) In spite of these two apparent contradictions, it would be better to simply mention that generally aromatic amino acids and short amino acids with polar side-chains have lower propensity for the formation of PPII.(Brown and Zondlo 2012) Obviously, the amino acid with the highest propensity to form the PPII structure remains proline whereas the amino acid with the lowest propensity to form the PPII helix is tryptophan.(Brown and Zondlo 2012).
Occurrence of the PPII structures in proteins and their importance
A survey of 274 non-homologous polypeptide chains from proteins of known structure showed that PPII helices appeared in regions that had a high proline content, that tended to contain glutamine and positively charged residues, that were highly solvent-exposed and that had hydrophobic residues stationed periodically at positions i and i + 3.(Stapley and Creamer 1999) Adzhubei and Sternberg (1993) had also previously analysed 80 globular proteins and noticed that proline was the preferred amino acid in PPII helices and that PPII helices tended to be on the protein surface and formed hydrogen bonds with water, which they thought to be the stabilizing force of PPII. In addition to their surface exposure, PPII helices have a higher number of free hydrogen bond donors and hydrogen bond acceptors when compared to α-helices and β-strands, which makes them suitable for protein–protein interactions.(Freund et al. 2008).
Proline, PPII and their analogues play a major role in the structural formation of proteins.(Arnott and Dover 1968; Boni et al. 1976; Silber et al. 2002) The structure of proteins can also be generated involving different configurations such as α- and β-helixes.(Arunkumar et al. 1997; Adzhubei et al. 2013) The amino acid proline is a vital component of protein synthesis and cell development, and various studies have showed that the body can make the later chemical substance on its own.(Adzhubei and Sternberg 1993) This biological compound is commonly known to promote the general function of cells.(Rath et al. 2005) Both L-proline and its analogues, such as 4-hydroxy-L-proline, serve as the basis for collagen, a protein found in connective tissues and which accounts for a third of all the proteins in the body.(Magdaleno et al. 2009) Studies have shown that PPII structures play a significant role in signal transduction, protein complex formation, and protein–protein and protein-nucleic acid interactions in addition to their structural function as binding sites.(Berisio et al. 2006) Notably, the binding regions of widely distributed SH3 domains frequently exhibit this structure.(Rath et al. 2005; Rojas et al. 2022) Adzhubei et al. (2013) reported that PII helices play a crucial structural role in amyloidogenic proteins and additionally play a role in gene expression, mobility of cells, self-assembly, and bacterial and viral pathogenicity.(Adzhubei et al. 2013).
One important role that is played by the distinctive PPII structure is its participation in peptide–protein and protein– protein interactions.(Bochicchio and Tamburro 2002) PPII in many cases has the ability to self-organize to produce a molecular mechanism which is said to be at the source of elasticity in elastomeric proteins, such as elastin, titin, and abductin.(Bochicchio and Tamburro 2002) As already stated above, polyproline II (PPII) structure has been shown to play a key role in biological mechanisms such as immune response, signal transduction and transcription.(Kelly et al. 2001) This role is explained by protein–protein interactions where the triple helix in arbitrate host–pathogen interactions, for example the collagen triple helix, is a widespread target for bacterial adhesions.(Berisio and Vitagliano 2012).
Proline-rich regions bind to a number of important proteins and regulate them. For example, they regulate src tyrosine kinase by binding to SH3 domain. SH3 domains have been identified as modular component of many eukaryote proteins and PxxP motif has been identified as a consensus binding sequence for these domains. However, the SH3-ligand bindings that show strong affinity are usually based on non-consensus motifs, some of which do not even adopt PPII conformation. Non-consensus motifs may bind to only one xP pocket of SH3 domains.(Stapley and Creamer 1999) Small peptidomimetics are good ligands. They are resistant to proteolysis and can serve as leads possessing structural and functional characteristics of bioactive peptides. Specificity in proline-rich motif recognition domains depends on the complementarity of charges between the residues surrounding proline-binding pocket and the ligands. Outside proline-binding pocket, additional selection is due to steric hindrance and charge repulsion between the residues neighbouring the binding pocket and the ligand’s side chains.(Freund et al. 2008) Some of the other proteins and protein domains that bind ligands in PPII conformations are profilin, WW domains, class II MHC proteins, Ena/VASP homology 1 domains, GYF domains and UEV domains.(Stapley and Creamer 1999; Kuehne et al. 2012; Meirson et al. 2020a, b) PPII structure is also believed to be involved in some diseases. For example, proline and glutamine-rich peptide (PQPQLPY) and analogues are believed to be involved in Celiac disease (or gluten-sensitive enteropathy). Understanding structural features involved in their affinity for HLA-DQ2 (the disease-specific class II major histocompatibility complex heterodimer) could provide new insights into pathogenicity of Celiac disease.(Parrot et al. 2002).
Understanding the unfolded state of a protein is essential to understanding its stability and its conformational changes during biological processes.(Kentsis et al. 2004) By means of Monte Carlo simulations, Kentsis et al. (2004) noticed that the difference in conformational entropies per residue for A7 polyalanine forming PPII helix was the same for different segment lengths, thus leading to the conclusion that PPII formation is non-cooperative. Chen et al. (2004) studied AcGG(A)nGGNH2 (n = 1–3) peptides and they also found PPII noncooperative, which led them to suggesting that the lack of cooperativity in PPII implies that hydration effects which are thought to play a great a role in PPII stability in water are highly localized. Monte Carlo simulations of A7 polyalanine, which is natively in the unfolded state, showed that the unfolded state of polyalanine is composed of segments of PPII helix connected with strands.(Kentsis et al. 2004) Although PPII helices with more than 9 residues can be found in proteins, a PPII helix has generally between 4 and 6 residues, and PPII helices tend to have maximum stability at 4–5 residues.(Adzhubei and Sternberg 1993; Stapley and Creamer 1999; Kentsis et al. 2004).
PPII is not only found in globular proteins. It is also found in fibrous proteins. PPII is present in great quantities in elastomeric proteins titin, abductin and elastin and this suggests that PPII may play an important role in their elasticity.(Bochicchio and Tamburro 2002) PPII conformation in elastin is due to the presence of the repeating sequence GGG while XYG motif (where X is usually proline or hydroxyproline) is the repeating motif which confers to each of the helices of the triple helical collagen its PPII conformation and is also thought to be the stabilising structure of the rigid collagen.(Pauling and Corey 1951; Cowan et al. 1955; Martino et al. 2000) In a few words, we can say that PPII structures, which are mainly found in disordered proteins, play a major role in protein–protein interactions, which makes them play a major role in different cellular functions and pathologies.(Kumar and Bansal 2016).
Proline, PPII and cell motility
Proline and its analogues have been proposed to participate in cell migration but little evidence is available to support this role. Proline-rich proteins are involved in the regulation of actin polymerization, cytoskeleton rearrangement, and contribute to the formation of membrane protrusions used for cell motility.(Holt and Koffer 2001; Comes et al. 2013) It was evidenced that a high amount of L-proline dislodges adherent embryonic stem cells (ESCs).(Comes et al. 2013) ESCs are converted into motile and metastatic stem cells that can even reach liver cells.(Comes et al. 2013) Furthermore, proline was found to be taken up by early stage melanoma cells and significantly enhanced their migration into the cell free area.(Mayr et al. 2019) Previously, it was reported that L-Pro-induced morphological and motility phenotypes contribute to the acquisition of cell plasticity.(Yang and Weinberg 2008) Therefore, L-Pro is thought to trigger an epithelial-mesenchymal transition (EMT)-like process in ESCs.(Comes et al. 2013) Proline is part of the Protein tyrosine phosphatase (PTP)-proline-, glutamate-, serine-, and threonine-rich sequence (PEST) which is necessary for both cell adhesion and migration in various cells/tissues.(Zheng and Lu 2013).
Profilin binds to poly-L-proline in PPII conformation for channelling actin monomers to membrane-associated sites of actin polymerization.(Holt and Koffer 2001) It has been reported that profilin-1 interacts both with actin and polyproline for efficient migration and invasion of vascular endothelial cells (VECs).(Ding et al. 2009) Proline-rich tyrosine kinase 2 (Pyk2) is suggested to increase the cell motility of hepatocellular carcinoma by regulating genes associated with epithelial to mesenchymal transition (EMT).(Sun et al. 2011) The two proline-rich motifs found in the C-terminal domain of Pyk2 are important for the binding of the SH3 domains of the signalling proteins such as p105Hef and p130Cas.(Astier et al. 1997; Lakkakorpi et al. 1999; Dikic 2002; Liu et al. 2004a) A recent study showed that the lack of Pyk2 significantly impaired random and directed fibroblast motility.(Lukic et al. 2021) Cell motility is a complex process regulated by various proteins including Pyk2, protein kinases, integrins, focal adhesion associated-kinase (FAK), guanine nucleotide exchange factors (GEFs), etc.(Zheng and Lu 2013).
The involvement of proline analogues (cis-4-hydroxy-L-proline, 3,4-dehydro-L-proline, and 2-azetidinecarboxylic acid) in enhancing the rate of cell migrations has been documented.(McAuslan et al. 1988).McAuslan et al. (1988) reported that migration of retinal capillary endothelial cells, corneal endothelial cells, aortal endothelial cells, aortal smooth muscle cells, and retinal pericytes was induced and increased by proline analogues at a concentration of 10–5 M. However, beyond this concentration, there was not induction of cell motility.(McAuslan et al. 1988) The mechanism behind this induction is related to the ability of proline analogues to reduce the production of extracellular collagen necessary for cell attachment, and thus decreasing the stability of cells.(Uitto and Prockop 1974; McAuslan et al. 1988) In short, proline and PPII are involved in the motility of different cells and designing their inhibitory analogues may help in the treatment of different pathologies which require cell motility.
Proline, PPII and signal transduction
The biological activities exhibited by proline are correlated with its hydrophobic behaviour(Kelly et al. 2001; Morgan and Rubenstein 2013) and its structural characteristics where it has one hydrogen atom attached to its nitrogen.(Morgan and Rubenstein 2013) As it is often found in binding sites, particularly those of widely spread SH3 domains, PPII helix has recently been found to play a major role in signal transduction and protein complex assembly.(Morgan and Rubenstein 2013) A typical example is triproline helices which may fold into right-handed or left-handed structures and which have been identified as participants in protein–protein signalling interactions.(Morgan and Rubenstein 2013).
Ma et al. (2014) have shown that proline analogues potentially affect the activity of human prolyl hydroxylase and the regulation of hypoxia-inducible factor (HIF) signal transduction pathway. The extracellular prolidase has been recently identified for inducing IGF-1R signalling pathways in an experimental model of wounded fibroblasts.(Baszanowska et al. 2021) The synthetic pathway of a series of [c]-fused bicyclic proline analogues have been reported by Calaza et al. (2015) who described their properties of acting as signal transducers and activators of the transcription process. The study conducted by Atkinson (1977) revealed that proline acts as an energy source during stress conditions and that therefore it could be a key signalling and regulatory molecule able to activate multiple responses that are part of the adaptation process. The important aspect of signalling events associated with proline accumulation in plants has been also demonstrated by Kishor et al. (2005). The biosynthesis of L-proline isomer performed under abiotic stress condition mitogen have been shown to activate protein kinase, which is the prime signal transduction pathway.(Meena et al. 2019) In addition to this, the synthesis and degradation of proline are considered as a model system for elucidating stress-related signal transduction.(Hare et al. 1999) In brief, proline and PPII helices take part in many different signalling pathways and scientists interested in targeting these pathways for drug discovery may focus on designing proline and PPII analogues with the desired properties such as rigidified structures locked in desired proline-like or PPII-like conformations.
Proline, PPII and transcription
The CTF/NF-I family of proteins is part of a large class of regulatory proteins which bind to specific DNA sequences in order to control its different transactions.(Mermod et al. 1989; Gresh 1996; Poppers et al. 2000, 2015) The CTF/NF-I family of proteins controls not only the DNA transcription but also its replication and is of particular interest because the CTF C-terminal domain has an unusual transcriptional activation domain which is 25% constituted of proline residues.(Dorn et al. 1987; Santoro et al. 1988; Mermod et al. 1989; Gresh 1996) This proline-rich domain indicates a new class of activators which are different from those with acidic or glutamine-rich activation domains.(Mermod et al. 1989) This shows that transcriptional stimulation is likely to be controlled via numerous pathways.(Mermod et al. 1989, 2015).
Makowska et al. (2007) used molecular modelling techniques to evaluate the degree to which PPII strands might be utilized for the formation of isohelical peptide-DNA interactions resulting in the preferential identification of the main pore of B-DNA. It has been confirmed that there is strong evidence that DNA-binding factors also control other processes such as the initiation of DNA transcription, DNA integration, DNA recombination and DNA transport.(Vlasov et al. 2005) Other reports focused on the selected DNA-binding polypeptides and have revealed that these binding agents could lead to the transcription process in eukaryotic organisms.(Silber et al. 2002).
A comparison of several DNA-binding transcription factors has revealed that different protein domains have been modified to mediate sequence-specific protein-DNA interactions.(Vlasov et al. 2005) The zinc finger motif, which has been found in numerous recognized and potential transcription factors, is a common structural framework for binding DNA.(Klug and Rhodes 1987).
The unfolded peptides and proteins include the PPII conformation in their structures and in addition to this, the protein–protein interactions and the protein-nucleic acid interactions depend heavily on PPII.(Rucker and Creamer 2002) According to numerous studies, the PPII structure is critical for the synthesis of proteins.(Duane D. et al. 1976; A. A. et al. 1994) According to host–guest studies of PPII, the tendencies of polar forms to generate a PPII helix are in part controlled by how their side chains interact with the solvent during structural and functional determination of proteins.(Duane D. et al. 1976) Different PPII-containing proteins were identified in poly(Glu–Ala), poly(Ala–Gly–Gly), poly(Pro–Ala–Gly), poly(Ala–Ala–Gly), and poly(Pro–Ser–Gly).(Kelly et al. 2001; Adzhubei et al. 2013) These proteins show protein–protein interactions, and the PPII helices promote a variety of processes such as signal transduction and DNA strands transcription.(Kelly et al. 2001) Hence, given that PPII helices are important for cellular DNA transcription, they are also important for cell growth and multiplication. Consequently, understanding their functionalities may also help understand the cellular functions especially those related to DNA transcription.
Proline, PPII and immune response
Insects found all over the globe come into contact with different microbes and have used antimicrobial peptides (AMPs) as a defence mechanism to illicit immune response necessary to fight bacterial infections.(Bulet et al. 1999) These AMPs contain proline-rich residues which are responsible for the antibacterial activity as well as immunomodulatory activity.(Thakur et al. 2022) Proline-rich peptides such as Bac5 exert their complex immunomodulatory activities by enhancing chemotaxis and differentiation of immune cells such as dendritic cells, activation of the adaptive immune response, suppression of the release of proinflammatory mediators through cytokines and toll-like receptors.(Mahlapuu et al. 2020).
Cathelicidins, which are proline-rich peptides, are part of the host defence peptides of innate immunity and act by recruiting inflammatory cells such as neutrophils, macrophages, monocytes, T-Lymphocytes and dendritic cells either directly or indirectly to the site of infection.(Scott et al. 2007) Human cathelicidin LL-37 can stimulate immune chemotaxic response indirectly through activation of chemokine production at low or moderate physiological conditions as well as direct chemo-attractant activity of immune response cells and promotion of wound healing through pro-angiogenic activity, promoting apoptosis in epithelial cells and degranulation of mast cells to enhance diapedesis.(Saugstad 1996; Hancock et al. 2016) Large cathelicidin peptides contain about 39–97 amino acid residues with repetitive proline motifs which form pyroproline-like structures.(Shinnar et al. 2003) The human cathelicidin LL-37 is expressed in various inflammatory cells such as epithelial, monocytes and keratinocytes and exerts its effects by binding to transactivating intracellular and extracellular receptors on various genes, activating the mitogen-activated protein kinase pathway (MAPK) and extracellular signal regulated kinase(ERK1) which induce a cascade of downstream signalling transduction pathways that activate cyclic AMP responsive binding protein 1(CREB-1), early growth response protein 1(EGR1), hypoxia inducible factor 1α (HIF1α), and activator protein 1 and 2(AP1,2).(Mookherjee et al. 2009; Hilchie et al. 2013) This complex action of cathelicidin LL-37 is thought to be responsible for all its immunomodulatory activities.(Hancock et al. 2016).
Studies of celiac disease have shown it to be a result of immune response to non-de-aminated gliadin peptide, 25‐mer P31‐55, which contains 36% of proline residues.(Mamone et al. 2007; Calvanese et al. 2019) These residues trigger an autoimmune response against transglutaminase-2 enzyme, leading to mucosal damage and loss of mucosal thickness.(Calvanese et al. 2019) Proline peptides also show an increased antibacterial activity against gram positive bacteria especially against Listeria monocytogenes.(Carvajal-Rondanelli et al. 2018) The positively-charged proline-rich (> 25% proline content) antimicrobial peptides (PrAMPs) and their analogues have become potential novel antimicrobial agents and they are well-tolerated in mice for daily doses of up to 320 mg/kg.(Lai et al. 2019; Knappe et al. 2019; Welch et al. 2020) However, one mouse model was able to illicit a weak immune response with low levels of IgG upon slow administration of intra-peritoneal injections of the proline-rich Api137 peptide.(Knappe et al. 2019).
In brief, proline-rich molecules such as AMPs and cathelicidins, which are found in different organisms including insects and humans, show good antimicrobial activity and could be developed into drugs against different microbial infections. This said, however, some of these compounds induce auto-immune diseases, which makes their development a challenge.
Proline, PPII and apoptosis
As mentioned earlier, proline is involved in a number of biochemical and physiological processes such as immune response, oxidative stress and cellular signalling which have been implicated in programmed cell death.(Tołoczko-Iwaniuk et al. 2020) During proline metabolism, the enzymes proline dehydrogenase and proline oxidase (PRODH/POX) convert proline to pyrroline-5-carboxylic acid (P5C), which leads to formation of reactive oxygen species (ROS) and ATP, important components in regulated apoptosis.(Phang and Liu 2012) P5C is synthesized from glutamate semi-aldehyde by pyrroline-5-carboxylate synthetase and then reduced to proline by P5C reductase.(Savouré et al. 1995) P5C is an interconversion substrate for three amino acids — proline, glutamate and ornithine and plays a critical role in survival of cancer cells because it acts as the main substrate in anaplerotic metabolism in place of glucose which needs oxygen for energy production.(Cairns et al. 2011) This ensures that there is a continuous supply of intermediates and fuel needed for the tricarboxylic acid cycle (TCA)or Krebs cycle in cancer cells without the need for glucose.(Pandhare et al. 2009) A study conducted in the yeast Metschnikowia citriensis subjected to oxidative stress showed an increase in superoxide dismutase (SOD) and catalase activity after administration of proline, followed by an improved tolerance to oxidative stress.(Liu et al. 2019) These enzymes act as scavengers of ROS and prevent ROS-induced apoptosis.(Liu et al. 2019) Similarly, plants under the stress of drought, high UV light and osmotic pressure have shown an increased accumulation of proline as a protective function.(Szabados and Savouré 2010).
Proline stabilises M4 lactate dehydrogenase at high temperatures and prevents protein aggregation, and in conditions of osmotic stress and heavy metal poisoning, protects nitrate reductase, the enzyme responsible for producing nitric oxide, the chemical species which acts as a scavenger for reactive oxygen species.(Rajendrakumar et al. 1994; Wei et al. 2020) Accumulation of arsenic and non-essential heavy metals in plants can lead to inhibition of cell growth by the binding of As3+ to protein sulphydryl groups, which causes cell membrane disruption and ultimately results in death.(Akter et al. 2005) However, plant studies in rice seedlings exposed to arsenic showed an impaired hydrolysis of RNA and DNA with an increased level of proline.(Mishra and Dubey 2006) This is believed to be due to the enzyme protective effect of proline by inhibiting proteases and ribonucleases that can lead to destruction of RNA and cellular organelles.(Mishra and Dubey 2006) Studies have also shown that inhibition of the enzyme prolyl hydroxylase, which metabolizes proline, leads to an increased secretion of free proline into inflammatory microenvironments in TGF-β-induced fibroblasts and stabilises the oxygen sensitive HIF-1α.(Vettore et al. 2021) Proline balances not only redox homeostasis but also proteostasis, and its restriction may lead to decreased proline-tRNA availability needed for protein synthesis and repair of cellular damage.(Loayza-Puch et al. 2016).
In short, proline is very important for the protection of organisms against stress conditions, and consequently protects against apoptosis. Furthermore, its derivative pyrroline-5-carboxylic acid (P5C), is an important substrate of cancer cells, which suggests that proline and P5C could be used as lead molecules for the synthesis of anticancer drugs.
Proline and PPII and their implications in different diseases
Some studies have shown some exciting clues about the recognition process. Current data prove that not only is a triple helix utilized by pathogens as a target in the pathogen-host interactions but also that it can be used as an attraction in these processes since the triple helix regions of the bacterial proteins have been revealed to interact with host proteins, and that this represents an early critical step in the process of infection.(Arora et al. 2021) The collagen-like adhesins Scl1 and Scl2 were found to be part of prokaryotic collagens and have been identified on the cell surface of Streptococcus pyogenes, which explains their role in promoting bacterial adhesion to the host.(Ellison et al. 2020) The adherence of extracellular pathogens, such as streptococci, staphylococci and enterococci to extracellular matrix is mediated by microbial surface components recognizing adhesive matrix molecules (MSCRAMMs) which belong to the adhesins protein family.(Foster and Höök 1998) Thus, Gram-positive bacteria’s proteins do not use the same sequence with integrins’ collagen binding domains and do not need metal ions to bind to collagen.(Berisio and Vitagliano 2012).
Although the secondary structure elements of PPII play an important function in the stabilization of protein folds, they can also be implicated in molecular recognition mechanism, such as protein-DNA, protein–protein, protein-sugars and protein-RNA, interactions.(Jones et al. 2001) The involvement of these elements in molecular recognition apparently depends on their position within the protein context.(Richardson 1981) A good example of this is shown by the capability of PPII motifs of the protein ActA of Listeria monocytogenes to attach to the EVH1 domain of the Mena mammalian protein, which is a key factor in cytoskeleton regulation.(Niebuhr et al. 1997) The interaction of the bacterial ActA with Mena EVH1 allows the bacterium to move and infect neighboring cells by using actin cytoskeleton.(Niebuhr et al. 1997) A lot of efforts are now being put in the development of peptides able to interfere with ActA/Mena interaction since the inhibition of this interaction may give an approach to prevent the motility of the pathogen and to stop its spread in the host organism.(Cornelia et al. 2006).
The role that PPII motifs play in host–pathogen recognition has also been shown for virus infections. HIV-1 Nef may be used as an example in this context, where a multifunctional protein is needed for full pathogenicity of the virus.(J et al. 2011) A PPII motif can also activate phosphatidylinositol 3-kinase (PI3K)/Akt signaling pathway by the influenza virus.(Benjamin G. et al. 2010) The activation of this pathway is advantageous for replication of the virus by stopping virus-induced apoptosis through phosphorylation of caspase-9.(Benjamin G. et al. 2010) Thus, PPII is involved in protein–protein interactions during infectious diseases and it can also help in host–pathogen interactions through other mechanisms since it may act as a structural spacer required for the correct positioning of the key elements required for infectivity and adhesion.(U and A 2013) A good example is the adhesion protein A3VP1, a fragment of the adhesin antigen I/II (AgI/II) of Streptococcus mutans, the causative agent of human dental caries.(Mr et al. 2010) It has been also suggested that PPII helices interactions with a number of proteins play a major role in cancer and neurodegenerative diseases.(Mompeán et al. 2021) Briefly, the uncontrolled protein–protein interactions may lead to unnecessary aggregation processes essential for some pathological states.(Js and Dc 2002).
Design and synthesis of proline mimetics and PPII mimetics
Given the importance of the PPII structure in biological processes, researchers have been trying to synthesize PPII mimetics which can serve as active pharmaceutical agents which target proline-rich motif binding domains.
The design of small molecule inhibitors of protein interactions has proved difficult. Most of the proteins which have been successfully targeted by small molecule inhibitors are enzymes whose natural substrates are themselves small organic molecules. In fact, while natural ligands of enzymes serve as templates for the design of inhibitors, it is difficult to design inhibitors of interactions which involve only proteins because of these reasons: firstly, small molecules binding at protein–protein interaction interface are not common, hence the lack of templates, and secondly, the binding sites are usually large and flat and essentially lack of characteristic sufficiently concave cavities that can be targeted by small molecules.(Arkin and Wells 2004; Freund et al. 2008) However, a number of proline derivatives are produced by living organisms and some of them have been used as probes for the design of ligands of some receptors. For example, members of the family of glutamic acid analogues have been widely used to design specific or highly selective ligands of EAA (excitatory amino acids) receptors.(Bräuner-Osborne et al. 2000) Other proline mimetics with the pyrrolidine ring substituted at different positions have been synthesized by different methods, and to cite some of them, those that lead to enantiomerically pure compounds, we can mention the methods of Flamant-Robin et al. (2002),(Sasaki et al. (1994), Sasaki et al. (1997), Zhang et al. (2003), Lygo et al. (2010), and more recently the method of Dias (2018).
PPII mimetics have also been successfully synthesized. Tremmel and Geyer (2002) synthesized a Ser-Pro dipeptide which mimicked the polyproline II helix conformation. The synthesis of this PPII mimetic took as few as only four steps and it could undergo further modifications for the generation of similar PPII mimetics.
Proline dipeptide mimetics invented by Kuehne et al. (2012) were found to adopt the PPII conformation and to bind to proline-rich motif binding domains, which gave them various applications as active pharmaceutical agents. According to the invention, these compounds can be used to treat diseases associated with modification of intracellular signal transduction processes mediated by PPII structures, including the use for the treatment of bacterial and viral infectious diseases, neurodegenerative diseases and tumours. The preferred neurodegenerative diseases are Alzheimer’s disease, Parkinson disease, Huntington’s disease and amyotrophic lateral sclerosis (ALS).
The CD 28 peptide mimetics are another example of the PPII mimetics so far synthesized. According to Kaumaya and Whitacre (2002), the CD28 peptide mimetics comprise the sequence MYPPPY or its retro-inverso isomer YPPPYM and are composed more preferably of 19 to 21 amino acids, although they may even contain 15 to 30 amino acids. They have been shown to be very useful for blocking or inhibiting the activity and proliferation of T cells, CD4+ in particular. These CD28 peptide mimetics adopt PPII conformation in water or buffer solution at physiological pH and 25 °C. CD28 peptide mimetics are very important since they have the potential to be used to treat individuals affected by T cell mediated auto-immune diseases or disorders.(Kaumaya and Whitacre 2002).
PPII peptidomimetic that have the potential to be used in the treatment of different types of cancer have been also stereoselectively synthesized.(Opitz et al. 2015; Reuter et al. 2015; Dohmen et al. 2020) These molecules (ProM-1 to Pro-M4) bind to Ena/VASP homology 1 (EVH1) domains of cancer cells and limit their migration, thus interfering with their invasiveness.(Opitz et al. 2015; Reuter et al. 2015; Dohmen et al. 2020) In other words, these EVH1 inhibitors reduce the tumour metastasis.(Opitz et al. 2015; Reuter et al. 2015; Dohmen et al. 2020) These inhibitors have the advantage that they are not easily hydrolysed and that they easily enter the cells to exert their activity.(Opitz et al. 2015; Reuter et al. 2015; Dohmen et al. 2020).
In the quest for PPII mimetics that can bind to the Ena/VASP EVH1 domain in order to use them as potential drugs against cancer, a tetracyclic scaffold ProM-19 was stereoselectively synthesized and it has been found to be locked in the PPII conformation.(Klein et al. 2022) Molecular modelling showed that the molecule binds to Ena/VASP EVH1 domains and that it could also have application as an anticancer drug.(Klein et al. 2022) This PPII mimetic imitates the xPP motif conformation.(Klein et al. 2022) However, its synthesis is challenging and it would not be easy to synthesize its analogues.(Klein et al. 2022).
An interesting feature of PPII ligands is the equal spacing of side-chains and backbone carbonyls when the helix is observed from either of the two possible N to C terminal orientations. This is believed to be the feature which allows proline-binding domains to bind to their PPII ligands in two orientations, which is a unique property among recognition modules.(Zarrinpar et al. 2003).
In summary, although there are not many developed PPII peptidomimetics, these molecules, when they can be easily synthesized, they have the advantage that they are not easily hydrolysable and that they can easily enter cells since they are small molecules as opposed to PPII peptides which are not resistant to hydrolysis and which do not easily enter cells due to their size. As a consequence, it is advisable to continue investing in the search for small PPII peptidomimetics, as these would help regulate many conditions related to protein–protein interactions whether the interactions are between the human cells and the pathogen or between only human cells.
Conclusion
Proline and PPII structure are biologically very important as they intervene in many biological processes such as energy production, signal transduction, stress tolerance, immune response, cellular DNA transcription and cell migration, etc. Synthesizing drugs that mimic these structures would be very important because there are not currently on the market drugs that are based on this structure. It is true that scientists have already done some work in terms of synthesizing proline and PPII mimetics but there is still much to do. Not only do these molecules need to be synthesized as pure enantiomers but also their synthetic methods need to be scaled up for further studies. Given that PPII structures bind to many important domains for protein–protein interactions as seen above, we encourage scientists to invest more in the development of PPII mimetics in the same idea that for all diseases, especially infectious ones, we need always to improve on our drugs by synthesizing novel scaffolds, which have new targets so that we improve on efficacy of our drugs and reduce resistance of microbes. The synthesis and test of proline mimetics is also encouraged as we have seen above that proline could have a role to play in stress reduction as well as in cancer cells’ resilience, which could be exploited to target them. Finally, we would like to mention that both proline and PPII structures should be greatly studied because they intervene in many cellular interactions, and the synthesis of their mimetics and the test of their biological activities are very promising in spite of being at an early stage.
Acknowledgements
We thank our respective institutions for allowing us to work on this manuscript.
Author contributions
Dr T. U. wrote the abstract and the introduction, wrote about the basis of PPII stability, different amino acids and their PPII formation propensity, occurrence and importance of PPII structures and the conclusion. He also compiled the document. -Dr N. G. reviewed the literature on proline, PPII, its analogues and cell motility. -Dr J. M. reviewed the literature on proline, PPII, its analogues and signal transduction. -Dr G. H. reviewed the literature on proline, PPII, its analogues and transcription. He also took part in the compilation of Table 1. —Mr J. K. reviewed the literature on proline, PPII, its analogues and immune response. He also reviewed the literature on proline, PPII, its analogues and apoptosis. -Mr A. R. reviewed the literature on proline, PPII, its analogues and their implications in different diseases. -Dr V. K. reviewed the whole paper and took part in the compilation of Table 1.
Funding
The authors received no funding to write this manuscript.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Adzhubei AA, Sternberg MJE (1993) Left-handed polyproline II helices commonly occur in globular proteins. J Mol Biol 229:472–493. 10.1006/jmbi.1993.1047 [DOI] [PubMed] [Google Scholar]
- Adzhubei AA, Sternberg MJE, Makarov AA (2013) Polyproline-II helix in proteins: structure and function. J Mol Biol 425:2100–2132. 10.1016/j.jmb.2013.03.018 [DOI] [PubMed] [Google Scholar]
- Akter KF, Owens G, Davey DE, Naidu R (2005) Arsenic speciation and toxicity in biological systems. Rev Environ Contam Toxicol 184:97–149. 10.1007/0-387-27565-7_3 [DOI] [PubMed] [Google Scholar]
- Arkin MR, Wells JA (2004) Small-molecule inhibitors of protein–protein interactions: progressing towards the dream. Nat Rev Drug Discov 3:301–317. 10.1038/nrd1343 [DOI] [PubMed] [Google Scholar]
- Arnott S, Dover SD (1968) The structure of poly-l-proline II. Acta Cryst B 24:599–601. 10.1107/S056774086800289X [DOI] [PubMed] [Google Scholar]
- Arora S, Gordon J, Hook M (2021) Collagen binding proteins of gram-positive pathogens. Front Microbiol 12:628798. 10.3389/fmicb.2021.628798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arunkumar AI, Kumar TK, Yu C (1997) Non-specific helix-induction in charged homopolypeptides by alcohols. Biochim Biophys Acta 1338:69–76. 10.1016/s0167-4838(96)00191-4 [DOI] [PubMed] [Google Scholar]
- Astier A, Manié SN, Avraham H et al (1997) The related adhesion focal tyrosine kinase differentially phosphorylates p130Cas and the CAS-like protein, p105HEF1*. J Biol Chem 272:19719–19724. 10.1074/jbc.272.32.19719 [DOI] [PubMed] [Google Scholar]
- Atkinson DE (1977) Cellular energy metabolism and its regulation. Academic Press, New York [Google Scholar]
- Baszanowska W, Misiura M, Oscilowska I et al (2021) Extracellular prolidase (PEPD) induces anabolic processes through EGFR, β1-integrin, and IGF-1R signaling pathways in an experimental model of wounded fibroblasts. Int J Mol Sci 22:942. 10.3390/ijms22020942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck DAC, Alonso DOV, Inoyama D, Daggett V (2008) The intrinsic conformational propensities of the 20 naturally occurring amino acids and reflection of these propensities in proteins. Proc Natl Acad Sci U S A 105:12259–12264. 10.1073/pnas.0706527105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin GH, Philip SK, David J et al (2010) Structural insights into phosphoinositide 3-kinase activation by the influenza a virus NS1 protein. Proceed Nat Acad Sci United States Am. 10.1073/pnas.0910715107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berisio R, Vitagliano L (2012) Polyproline and triple helix motifs in host-pathogen recognition. Curr Protein Pept Sci 13:855–865. 10.2174/138920312804871157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berisio R, Loguercio S, De Simone A et al (2006) Polyproline helices in protein structures: a statistical survey. Protein Pept Lett 13:847–854. 10.2174/092986606777841154 [DOI] [PubMed] [Google Scholar]
- Bliska J (1996) How pathogens exploit interactions mediated by SH3 domains. Chem Biol 3:7–11. 10.1016/S1074-5521(96)90076-9 [DOI] [PubMed] [Google Scholar]
- Bochicchio B, Tamburro AM (2002) Polyproline II structure in proteins: Identification by chiroptical spectroscopies, stability, and functions. Chirality 14:782–792. 10.1002/chir.10153 [DOI] [PubMed] [Google Scholar]
- Boni R, Di Blasi R, Farina A, Verdini AS (1976) Conformational properties of the sequential polyproline analogs poly(Pro-Aze-Pro) and poly(Aze-Pro-Aze). Biopolymers 15:1233–1241. 10.1002/bip.1976.360150702 [DOI] [PubMed] [Google Scholar]
- Brandts JF, Kaplan LJ (2002) Derivative spectroscopy applied to tyrosyl chromophores Ribonuclease., lima bean inhibitors, insulin, and pancreatic trypsin inhibitor. Biochemistry. 10.1021/bi00734a027 [DOI] [PubMed] [Google Scholar]
- Bräuner-Osborne H, Egebjerg J, Nielsen EØ et al (2000) Ligands for glutamate receptors: design and therapeutic prospects. J Med Chem 43:2609–2645. 10.1021/jm000007r [DOI] [PubMed] [Google Scholar]
- Bretscher LE, Jenkins CL, Taylor KM et al (2001) Conformational stability of collagen relies on a stereoelectronic effect. J Am Chem Soc 123:777–778. 10.1021/ja005542v [DOI] [PubMed] [Google Scholar]
- Brown AM, Zondlo NJ (2012) A propensity scale for type II polyproline helices (PPII): aromatic amino acids in proline-rich sequences strongly disfavor PPII due to proline-aromatic interactions. Biochemistry 51:5041–5051. 10.1021/bi3002924 [DOI] [PubMed] [Google Scholar]
- Bulet P, Hetru C, Dimarcq J-L, Hoffmann D (1999) Antimicrobial peptides in insects; structure and function. Dev Comp Immunol 23:329–344. 10.1016/S0145-305X(99)00015-4 [DOI] [PubMed] [Google Scholar]
- Cairns RA, Harris IS, Mak TW (2011) Regulation of cancer cell metabolism. Nat Rev Cancer 11:85–95. 10.1038/nrc2981 [DOI] [PubMed] [Google Scholar]
- Calaza MI, Sayago FJ, Laborda P, Cativiela C (2015) Synthesis of [c]-fused bicyclic proline analogues. Eur J Org Chem 2015:1633–1658. 10.1002/ejoc.201403121 [Google Scholar]
- Calvanese L, Nanayakkara M, Aitoro R et al (2019) Structural insights on P31–43, a gliadin peptide able to promote an innate but not an adaptive response in celiac disease. J Pept Sci 25:e3161. 10.1002/psc.3161 [DOI] [PubMed] [Google Scholar]
- Carvajal-Rondanelli P, Aróstica M, Álvarez CA et al (2018) Understanding the antimicrobial properties/activity of an 11-residue Lys homopeptide by alanine and proline scan. Amino Acids 50:557–568. 10.1007/s00726-018-2542-6 [DOI] [PubMed] [Google Scholar]
- Chen K, Liu Z, Kallenbach NR (2004) The polyproline II conformation in short alanine peptides is noncooperative. Proc Natl Acad Sci 101:15352–15357. 10.1073/pnas.0406657101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang Y-C, Lin Y-J, Horng J-C (2009) Stereoelectronic effects on the transition barrier of polyproline conformational interconversion. Protein Sci 18:1967–1977. 10.1002/pro.208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christgen SL, Becker DF (2019) Role of proline in pathogen and host interactions. Antioxid Redox Signal 30:683–709. 10.1089/ars.2017.7335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comes S, Gagliardi M, Laprano N et al (2013) L-Proline induces a mesenchymal-like invasive program in embryonic stem cells by remodeling H3K9 and H3K36 methylation. Stem Cell Reports 1:307–321. 10.1016/j.stemcr.2013.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelia H, Tatjana H, Jutta E (2006) Structure-based synthetic mimicry of discontinuous protein binding sites: inhibitors of the interaction of Mena EVH1 domain with proline-rich ligands. Chembiochem : a European J Chem Biol. 10.1002/cbic.200500465 [DOI] [PubMed] [Google Scholar]
- Cowan PM, McGavin S (1955) Structure of Poly-L-Proline. Nature 176:501–503. 10.1038/176501a0 [Google Scholar]
- Cowan PM, McGAVIN S, North ACT (1955) The Polypeptide chain configuration of collagen. Nature 176:1062–1064. 10.1038/1761062a0 [DOI] [PubMed] [Google Scholar]
- DeTar DF, Luthra NP (1977) Conformations of proline. J Am Chem Soc 99:1232–1244. 10.1021/ja00446a040 [DOI] [PubMed] [Google Scholar]
- Dias IES (2018) Enantioselective Synthesis and Biological Evaluation of PLG and GPE Proline Mimetics
- Dikic I (2002) Signal Transduction by Proline-Rich Tyrosine Kinase Pyk2
- Ding Z, Gau D, Deasy B et al (2009) Both actin and polyproline interactions of profilin-1 are required for migration, invasion and capillary morphogenesis of vascular endothelial cells. Exp Cell Res 315:2963–2973. 10.1016/j.yexcr.2009.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohmen S, Reiher M, Albat D et al (2020) Pd-catalyzed asymmetric n-allylation of amino acid esters with exceptional levels of catalyst control: stereo-divergent synthesis of ProM-15 and related bicyclic dipeptide mimetics. Chemistry 26:3049–3053. 10.1002/chem.202000307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorn A, Bollekens J, Staub A et al (1987) A multiplicity of CCAAT box-binding proteins. Cell 50:863–872. 10.1016/0092-8674(87)90513-7 [DOI] [PubMed] [Google Scholar]
- Drozdov AN, Grossfield A, Pappu RV (2004) Role of solvent in determining conformational preferences of alanine dipeptide in water. J Am Chem Soc 126:2574–2581. 10.1021/ja039051x [DOI] [PubMed] [Google Scholar]
- Duane DJ, Cindy S, W. Curtis J, (1976) Circular dichroism of collagen, gelatin, and poly(proline) II in the vacuum ultraviolet. Biopolymers. 10.1002/bip.1976.360150308 [DOI] [PubMed] [Google Scholar]
- Eberhardt ES, Panasik N, Raines RT (1996) Inductive effects on the energetics of prolyl peptide bond isomerization: implications for collagen folding and stability. J Am Chem Soc 118:12261–12266. 10.1021/ja9623119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Baba TJ, Fuller DR, Hales DA et al (2019) Solvent mediation of peptide conformations: polyproline structures in water, methanol, ethanol, and 1-propanol as determined by ion mobility spectrometry-mass spectrometry. J Am Soc Mass Spectrom 30:77–84. 10.1007/s13361-018-2034-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellison AJ, Dempwolff F, Kearns DB, Raines RT (2020) Role for cell-surface collagen of streptococcus pyogenes in infections. ACS Infect Dis 6:1836–1843. 10.1021/acsinfecdis.0c00073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanni S, János S, Nándor P et al (2023) Proline cis/trans isomerization in intrinsically disordered proteins and peptides. Front Biosci. 10.31083/j.fbl2806127 [Google Scholar]
- Flamant-Robin C, Wang Q, Chiaroni A, Sasaki NA (2002) An efficient method for the stereoselective synthesis of cis-3-substituted prolines: conformationally constrained α-amino acids. Tetrahedron 58:10475–10484. 10.1016/S0040-4020(02)01405-9 [Google Scholar]
- Foster TJ, Höök M (1998) Surface protein adhesins of staphylococcus aureus. Trends Microbiol 6:484–488. 10.1016/s0966-842x(98)01400-0 [DOI] [PubMed] [Google Scholar]
- Freund C, Schmalz H-G, Sticht J, Kühne R (2008) Proline-Rich Sequence Recognition Domains (PRD): Ligands, Function and Inhibition. In: Klussmann E, Scott J (eds) Protein-protein interactions as new drug targets. Springer, Berlin, Heidelberg, pp 407–429 [DOI] [PubMed] [Google Scholar]
- Gresh N (1996) Can a polyproline II helical motif be used in the context of sequence-selective major groove recognition of B-DNA? A molecular modelling investigation. J Biomol Struct Dyn 14:255–273. 10.1080/07391102.1996.10508117 [DOI] [PubMed] [Google Scholar]
- Gurung D, Danielson JA, Tasnim A et al (2023) Proline isomerization: from the chemistry and biology to therapeutic opportunities. Biology 12:1008. 10.3390/biology12071008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock REW, Haney EF, Gill EE (2016) The immunology of host defence peptides: beyond antimicrobial activity. Nat Rev Immunol 16:321–334. 10.1038/nri.2016.29 [DOI] [PubMed] [Google Scholar]
- Hare PD, Cress WA, van Staden J (1999) Proline synthesis and degradation: a model system for elucidating stress-related signal transduction. J Exp Bot 50:413–434. 10.1093/jxb/50.333.413 [Google Scholar]
- Hilchie AL, Wuerth K, Hancock REW (2013) Immune modulation by multifaceted cationic host defense (antimicrobial) peptides. Nat Chem Biol 9:761–768. 10.1038/nchembio.1393 [DOI] [PubMed] [Google Scholar]
- Holt MR, Koffer A (2001) Cell motility: proline-rich proteins promote protrusions. Trends Cell Biol 11:38–46. 10.1016/s0962-8924(00)01876-6 [DOI] [PubMed] [Google Scholar]
- Hopfinger AJ, Walton AG (1969) Theoretical conformation of proline oligomers. J Macromole Sci, Part B 3:171–193. 10.1080/00222346908217097 [Google Scholar]
- Horng J-C, Raines RT (2006) Stereoelectronic effects on polyproline conformation. Protein Sci 15:74–83. 10.1110/ps.051779806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- J V, G V, W W, B V (2011) The Nef-infectivity enigma: mechanisms of enhanced lentiviral infection. Current HIV Res. 10.2174/157016211798842099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S, Daley DTA, Luscombe NM et al (2001) Protein–RNA interactions: a structural analysis. Nucleic Acids Res 29:943–954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Js R, Dc R (2002) Natural beta-sheet proteins use negative design to avoid edge-to-edge aggregation. Proceed Nat Acad Sci United States Am. 10.1073/pnas.052706099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakinoki S, Hirano Y, Oka M (2005) On the stability of polyproline-I and II structures of proline oligopeptides. Polym Bull 53:109–115. 10.1007/s00289-004-0317-6 [Google Scholar]
- Kang YK, Jhon JS, Park HS (2006) Conformational preferences of proline oligopeptides. J Phys Chem B 110:17645–17655. 10.1021/jp0629792 [DOI] [PubMed] [Google Scholar]
- Kaumaya PTP, Whitacre CC (2002) Agents for blocking t cell mediated immune reactions
- Kelly MA, Chellgren BW, Rucker AL et al (2001) Host−guest study of left-handed polyproline II helix formation. Biochemistry 40:14376–14383. 10.1021/bi011043a [DOI] [PubMed] [Google Scholar]
- Kentsis A, Mezei M, Gindin T, Osman R (2004) Unfolded state of polyalanine is a segmented polyproline II helix. proteins: structure. Funct Bioinformat 55:493–501. 10.1002/prot.20051 [DOI] [PubMed] [Google Scholar]
- Kishor PBK, Sangam S, Amrutha RN et al (2005) Regulation of proline biosynthesis, degradation, uptake and transport in higher plants: its implications in plant growth and abiotic stress tolerance. Curr Sci 88:424–438 [Google Scholar]
- Klein MT, Krause BM, Neudörfl J-M et al (2022) Design and synthesis of a tetracyclic tripeptide mimetic frozen in a polyproline type II (PP2) helix conformation. Org Biomol Chem 20:9368–9377. 10.1039/D2OB01857H [DOI] [PubMed] [Google Scholar]
- Klug A, Rhodes D (1987) ‘Zinc fingers’: a novel protein motif for nucleic acid recognition. Trends Biochem Sci 12:464–469. 10.1016/0968-0004(87)90231-3 [Google Scholar]
- Knappe D, Schmidt R, Adermann K, Hoffmann R (2019) Continuous subcutaneous delivery of proline-rich antimicrobial peptide Api137 provides superior efficacy to intravenous administration in a mouse infection model. Front Microbiol 10:2283. 10.3389/fmicb.2019.02283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuehne R, Oschkinat H, Brockmann C, et al (2012) Structural mimetics of proline-rich peptides and the pharmaceutical use thereof.
- Kumar P, Bansal M (2016) Structural and functional analyses of polyproline-II helices in globular proteins. J Struct Biol 196:414–425. 10.1016/j.jsb.2016.09.006 [DOI] [PubMed] [Google Scholar]
- Lai P-K, Tresnak DT, Hackel BJ (2019) Identification and elucidation of proline-rich antimicrobial peptides with enhanced potency and delivery. Biotechnol Bioeng 116:2439–2450. 10.1002/bit.27092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakkakorpi PT, Nakamura I, Nagy RM et al (1999) Stable association of PYK2 and p130Cas in osteoclasts and their co-localization in the sealing zone. J Biol Chem 274:4900–4907. 10.1074/jbc.274.8.4900 [DOI] [PubMed] [Google Scholar]
- Law PB, Daggett V (2010) The relationship between water bridges and the polyproline II conformation: a large-scale analysis of molecular dynamics simulations and crystal structures. Protein Eng Des Sel 23:27–33. 10.1093/protein/gzp069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Liao Z, Camden J et al (2004a) Src homology 3 binding sites in the P2Y2 nucleotide receptor interact with Src and regulate activities of Src, proline-rich tyrosine kinase 2, and growth factor receptors. J Biol Chem 279:8212–8218. 10.1074/jbc.M312230200 [DOI] [PubMed] [Google Scholar]
- Liu Z, Chen K, Ng A et al (2004b) Solvent dependence of pii conformation in model alanine peptides. J Am Chem Soc 126:15141–15150. 10.1021/ja047594g [DOI] [PubMed] [Google Scholar]
- Liu Y, Yi L, Ruan C et al (2019) Proline increases pigment production to improve oxidative stress tolerance and biocontrol ability of metschnikowia citriensis. Front Microbiol 10:1273. 10.3389/fmicb.2019.01273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loayza-Puch F, Rooijers K, Buil LCM et al (2016) Tumour-specific proline vulnerability uncovered by differential ribosome codon reading. Nature 530:490–494. 10.1038/nature16982 [DOI] [PubMed] [Google Scholar]
- Lukic N, Lapetina S, Grobe H et al (2021) Pyk2 regulates cell-edge protrusion dynamics by interacting with Crk. Mol Biol Cell 32:17. 10.1091/mbc.E20-10-0640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lygo B, Beynon C, McLeod MC et al (2010) Application of asymmetric phase-transfer catalysis in the enantioselective synthesis of cis-5-substituted proline esters. Tetrahedron 66:8832–8836. 10.1016/j.tet.2010.09.075 [Google Scholar]
- M AA, A IA, P II et al (1994) Melting of the left-handed helical conformation of charged poly-L-lysine. Biopolymers. 10.1002/bip.360340816 [DOI] [PubMed] [Google Scholar]
- Ma X, Wang X, Cao J et al (2014) Effect of proline analogues on activity of human prolyl hydroxylase and the regulation of HIF signal transduction pathway. PLoS ONE 9:e95692. 10.1371/journal.pone.0095692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magdaleno A, Ahn I-Y, Paes LS, Silber AM (2009) Actions of a proline analogue, L-thiazolidine-4-carboxylic acid (T4C), on trypanosoma cruzi. PLoS ONE 4:e4534. 10.1371/journal.pone.0004534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahlapuu M, Björn C, Ekblom J (2020) Antimicrobial peptides as therapeutic agents: opportunities and challenges. Crit Rev Biotechnol 40:978–992. 10.1080/07388551.2020.1796576 [DOI] [PubMed] [Google Scholar]
- Makowska J, Rodziewicz-Motowidło S, Bagińska K et al (2006) Polyproline II conformation is one of many local conformational states and is not an overall conformation of unfolded peptides and proteins. Proc Natl Acad Sci 103:1744–1749. 10.1073/pnas.0510549103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makowska J, Rodziewicz-Motowidło S, Baginska K et al (2007) Further evidence for the absence of polyproline II stretch in the XAO peptide. Biophys J 92:2904–2917. 10.1529/biophysj.106.097550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malešević M, Schumann M, Jahreis G et al (2012) Design of cyclic peptides featuring proline predominantly in the cis conformation under physiological conditions. ChemBioChem 13:2122–2127. 10.1002/cbic.201200366 [DOI] [PubMed] [Google Scholar]
- Mamone G, Ferranti P, Rossi M et al (2007) Identification of a peptide from α-gliadin resistant to digestive enzymes: Implications for celiac disease. J Chromatogr B 855:236–241. 10.1016/j.jchromb.2007.05.009 [DOI] [PubMed] [Google Scholar]
- Martino M, Bavoso A, Guantieri V et al (2000) On the occurrence of polyproline II structure in elastin. J Mol Struct 519:173–189. 10.1016/S0022-2860(99)00299-9 [Google Scholar]
- Mayr K, Wasinger C, Mayer S, Hohenegger M (2019) The nonessential amino acid proline enhances migration and proliferation of human melanoma cells. Eur J Cancer 110:S10–S11. 10.1016/j.ejca.2019.01.046 [Google Scholar]
- McAuslan BR, Reilly W, Hannan GN et al (1988) Induction of endothelial cell migration by proline analogs and its relevance to angiogenesis. Exp Cell Res 176:248–257. 10.1016/0014-4827(88)90328-x [DOI] [PubMed] [Google Scholar]
- Meena M, Divyanshu K, Kumar S et al (2019) Regulation of L-proline biosynthesis, signal transduction, transport, accumulation and its vital role in plants during variable environmental conditions. Heliyon 5:e02952. 10.1016/j.heliyon.2019.e02952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meirson T, Bomze D, Kahlon L et al (2020a) A helical lock and key model of polyproline II conformation with SH3. Bioinformatics 36:154–159. 10.1093/bioinformatics/btz527 [DOI] [PubMed] [Google Scholar]
- Meirson T, Bomze D, Markel G, Samson AO (2020b) κ-helix and the helical lock and key model: a pivotal way of looking at polyproline II. Bioinformatics 36:3726–3732. 10.1093/bioinformatics/btaa186 [DOI] [PubMed] [Google Scholar]
- Mermod N, O’Neill EA, Kelly TJ, Tjian R (1989) The proline-rich transcriptional activator of CTF/NF-I is distinct from the replication and DNA binding domain. Cell 58:741–753. 10.1016/0092-8674(89)90108-6 [DOI] [PubMed] [Google Scholar]
- Mezei M, Fleming PJ, Srinivasan R, Rose GD (2004) Polyproline II helix is the preferred conformation for unfolded polyalanine in water. proteins: structure. Funct Bioinformat 55:502–507. 10.1002/prot.20050 [DOI] [PubMed] [Google Scholar]
- Milorey B, Schwalbe H, O’Neill N, Schweitzer-Stenner R (2021) Repeating aspartic acid residues prefer turn-like conformations in the unfolded state: implications for early protein folding. J Phys Chem B 125:11392–11407. 10.1021/acs.jpcb.1c06472 [DOI] [PubMed] [Google Scholar]
- Mirkin NG, Krimm S (2012) Water interaction differences determine the relative energetic stability of the polyproline II conformation of the alanine dipeptide in aqueous environments. Biopolymers 97:789–794. 10.1002/bip.22064 [DOI] [PubMed] [Google Scholar]
- Mishra S, Dubey RS (2006) Inhibition of ribonuclease and protease activities in arsenic exposed rice seedlings: role of proline as enzyme protectant. J Plant Physiol 163:927–936. 10.1016/j.jplph.2005.08.003 [DOI] [PubMed] [Google Scholar]
- Momany FA, McGuire RF, Burgess AW, Scheraga HA (1975) Energy parameters in polypeptides. VII. geometric parameters, partial atomic charges, nonbonded interactions, hydrogen bond interactions, and intrinsic torsional potentials for the naturally occurring amino acids. J Phys Chem 79:2361–2381. 10.1021/j100589a006 [Google Scholar]
- Mompeán M, Oroz J, Laurents DV (2021) Do polyproline II helix associations modulate biomolecular condensates? FEBS Open Bio 11:2390–2399. 10.1002/2211-5463.13163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mookherjee N, Hamill P, Gardy J et al (2009) Systems biology evaluation of immune responses induced by human host defence peptide LL-37 in mononuclear cells. Mol Biosyst 5:483–496. 10.1039/b813787k [DOI] [PubMed] [Google Scholar]
- Moradi M, Babin V, Roland C et al (2009) Conformations and free energy landscapes of polyproline peptides. Proc Natl Acad Sci 106:20746–20751. 10.1073/pnas.0906500106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moradi M, Babin V, Sagui C, Roland C (2011) A statistical analysis of the PPII propensity of amino acid guests in proline-rich peptides. Biophys J 100:1083–1093. 10.1016/j.bpj.2010.12.3742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan AA, Rubenstein E (2013) Proline: the distribution, frequency, positioning, and common functional roles of proline and polyproline sequences in the human proteome. PLoS ONE 8:e53785. 10.1371/journal.pone.0053785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mr L, Kr R, Mh P et al (2010) Elongated fibrillar structure of a streptococcal adhesin assembled by the high-affinity association of alpha- and PPII-helices. Proceed Nat Acad Sci United States Am. 10.1073/pnas.0912293107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musacchio A, Wilmanns M, Saraster M (1994) Structure and function of the SH3 domain. Prog Biophys Mol Biol 61:283–297. 10.1016/0079-6107(94)90003-5 [DOI] [PubMed] [Google Scholar]
- Niebuhr K, Ebel F, Frank R et al (1997) A novel proline-rich motif present in ActA of Listeria monocytogenes and cytoskeletal proteins is the ligand for the EVH1 domain, a protein module present in the Ena/VASP family. EMBO J 16:5433–5444. 10.1093/emboj/16.17.5433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien KT, Mooney C, Lopez C et al (2020) Prediction of polyproline II secondary structure propensity in proteins. Royal Soc Open Sci 7:191239. 10.1098/rsos.191239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opitz R, Müller M, Reuter C et al (2015) A modular toolkit to inhibit proline-rich motif–mediated protein–protein interactions. Proc Natl Acad Sci 112:5011–5016. 10.1073/pnas.1422054112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandhare J, Donald SP, Cooper SK, Phang JM (2009) Regulation and function of proline oxidase under nutrient stress. J Cell Biochem 107:759–768. 10.1002/jcb.22174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappu RV, Rose GD (2002) A simple model for polyproline II structure in unfolded states of alanine-based peptides. Protein Sci 11:2437–2455. 10.1110/ps.0217402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrot I, Huang PC, Khosla C (2002) Circular dichroism and nuclear magnetic resonance spectroscopic analysis of immunogenic gluten peptides and their analogs. J Biol Chem 277:45572–45578. 10.1074/jbc.M207606200 [DOI] [PubMed] [Google Scholar]
- Pauling L, Corey RB (1951) The structure of fibrous proteins of the collagen-gelatin group. Proc Natl Acad Sci U S A 37:272–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phang JM, Liu W (2012) Proline metabolism cancer. Front Biosci 17:1835–1845. 10.2741/4022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollastrini M, Pasquinelli L, Górecki M et al (2022) A Unique and stable polyproline I helix sorted out from conformational equilibrium by solvent polarity. J Org Chem 87:13715–13725. 10.1021/acs.joc.2c01377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poppers J, Mulvey M, Khoo D, Mohr I (2000) Inhibition of PKR activation by the proline-rich RNA binding domain of the herpes simplex virus type 1 Us11 protein. J Virol 74:11215–11221. 10.1128/jvi.74.23.11215-11221.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajendrakumar CS, Reddy BV, Reddy AR (1994) Proline-protein interactions: protection of structural and functional integrity of M4 lactate dehydrogenase. Biochem Biophys Res Commun 201:957–963. 10.1006/bbrc.1994.1795 [DOI] [PubMed] [Google Scholar]
- Rajewski BH, Wright MM, Gerrein TA, Del Valle JR (2023) N-aminoglycine and its derivatives stabilize ppii secondary structure. Org Lett 25:4366–4370. 10.1021/acs.orglett.3c01502 [DOI] [PubMed] [Google Scholar]
- Rath A, Davidson AR, Deber CM (2005) The structure of “unstructured” regions in peptides and proteins: role of the polyproline II helix in protein folding and recognition. Biopolymers 80:179–185. 10.1002/bip.20227 [DOI] [PubMed] [Google Scholar]
- Ravi Chandra B, Gowthaman R, Raj Akhouri R et al (2004) Distribution of proline-rich (PxxP) motifs in distinct proteomes: functional and therapeutic implications for malaria and tuberculosis. Protein Eng Des Sel 17:175–182. 10.1093/protein/gzh024 [DOI] [PubMed] [Google Scholar]
- Reuter C, Opitz R, Soicke A et al (2015) Design and stereoselective synthesis of ProM-2: a spirocyclic diproline mimetic with polyproline type II (PPII) helix conformation. Chemistry 21:8464–8470. 10.1002/chem.201406493 [DOI] [PubMed] [Google Scholar]
- Richardson JS (1981) The Anatomy and Taxonomy of Protein Structure. In: Anfinsen CB, Edsall JT, Richards FM (eds) Advances in protein chemistry. Academic Press, pp 167–339 [DOI] [PubMed] [Google Scholar]
- Rojas R, Aróstica M, Carvajal-Rondanelli P et al (2022) Relationship between type II polyproline helix secondary structure and thermal hysteresis activity of short homopeptides. Electron J Biotechnol 59:62–73. 10.1016/j.ejbt.2022.08.003 [Google Scholar]
- Rucker AL, Creamer TP (2002) Polyproline II helical structure in protein unfolded states: lysine peptides revisited. Protein Sci 11:980–985. 10.1110/ps.4550102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saibi W, Feki K, Yacoubi I, Brini F (2015) Bridging between proline structure, functions, metabolism, and involvement in organism physiology. Appl Biochem Biotechnol 176:2107–2119. 10.1007/s12010-015-1713-0 [DOI] [PubMed] [Google Scholar]
- Saksela K, Permi P (2012) SH3 domain ligand binding: what’s the consensus and where’s the specificity? FEBS Lett 586:2609–2614. 10.1016/j.febslet.2012.04.042 [DOI] [PubMed] [Google Scholar]
- Santoro C, Mermod N, Andrews PC, Tjian R (1988) A family of human CCAAT-box-binding proteins active in transcription and DNA replication: cloning and expression of multiple cDNAs. Nature 334:218–224. 10.1038/334218a0 [DOI] [PubMed] [Google Scholar]
- Sasaki NA, Pauly R, Fontaine C et al (1994) Enantioselective synthesis of (2S,3S)- and (2R,3R)-pyrrolidine-2,3-dicarboxylic acids: conformationally constrained (S)- and (R)-aspartic acid analogues. Tetrahedron Lett 35:241–244. 10.1016/S0040-4039(00)76521-X [Google Scholar]
- Sasaki NA, Dockner M, Chiaroni A et al (1997) A novel stereodivergent synthesis of optically pure cis- and trans-3-substituted proline derivatives. J Org Chem 62:765–770. 10.1021/jo961790r [DOI] [PubMed] [Google Scholar]
- Saugstad OD (1996) Mechanisms of tissue injury by oxygen radicals: implications for neonatal disease. Acta Paediatr 85:1–4. 10.1111/j.1651-2227.1996.tb13880.x [DOI] [PubMed] [Google Scholar]
- Savouré A, Jaoua S, Hua XJ et al (1995) Isolation, characterization, and chromosomal location of a gene encoding the delta 1-pyrroline-5-carboxylate synthetase in arabidopsis thaliana. FEBS Lett 372:13–19. 10.1016/0014-5793(95)00935-3 [DOI] [PubMed] [Google Scholar]
- Scott MG, Dullaghan E, Mookherjee N et al (2007) An anti-infective peptide that selectively modulates the innate immune response. Nat Biotechnol 25:465–472. 10.1038/nbt1288 [DOI] [PubMed] [Google Scholar]
- Shi L, Holliday AE, Bohrer BC et al (2016) “Wet” versus “Dry” folding of polyproline. J Am Soc Mass Spectrom 27:1037–1047. 10.1007/s13361-016-1372-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinnar AE, Butler KL, Park HJ (2003) Cathelicidin family of antimicrobial peptides: proteolytic processing and protease resistance. Bioorg Chem 31:425–436. 10.1016/s0045-2068(03)00080-4 [DOI] [PubMed] [Google Scholar]
- Silber AM, Tonelli RR, Martinelli M et al (2002) Active transport of L-proline in trypanosoma cruzi. J Eukaryot Microbiol 49:441–446. 10.1111/j.1550-7408.2002.tb00225.x [DOI] [PubMed] [Google Scholar]
- Sreerama N, Woody RW (1999) Molecular dynamics simulations of polypeptide conformations in water: a comparison of α, β, and poly(pro)II conformations. Proteins: Structure. Funct Bioinformat 36:400–406 [PubMed] [Google Scholar]
- Stapley BJ, Creamer TP (1999) A survey of left-handed polyproline II helices. Protein Sci 8:587–595. 10.1110/ps.8.3.587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun CK, Ng KT, Lim ZX et al (2011) Proline-rich tyrosine kinase 2 (Pyk2) promotes cell motility of hepatocellular carcinoma through induction of epithelial to mesenchymal transition. PLoS ONE 6:e18878. 10.1371/journal.pone.0018878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabados L, Savouré A (2010) Proline: a multifunctional amino acid. Trends Plant Sci 15:89–97. 10.1016/j.tplants.2009.11.009 [DOI] [PubMed] [Google Scholar]
- Thakur A, Sharma A, Alajangi HK et al (2022) In pursuit of next-generation therapeutics: antimicrobial peptides against superbugs, their sources, mechanism of action, nanotechnology-based delivery, and clinical applications. Int J Biol Macromol 218:135–156. 10.1016/j.ijbiomac.2022.07.103 [DOI] [PubMed] [Google Scholar]
- Tołoczko-Iwaniuk N, Dziemiańczyk-Pakieła D, Celińska-Janowicz K et al (2020) Proline-dependent induction of apoptosis in oral squamous cell carcinoma (OSCC)-the effect of celecoxib. Cancers (basel) 12:136. 10.3390/cancers12010136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub W, Shmueli U (1963) Structure of poly-L-proline I. Nature 198:1165–1166 [Google Scholar]
- Tremmel P, Geyer A (2002) An oligomeric ser-pro dipeptide mimetic assuming the polyproline II helix conformation. J Am Chem Soc 124:8548–8549. 10.1021/ja026098u [DOI] [PubMed] [Google Scholar]
- U K, A E (2013) Protein-protein interaction networks: probing disease mechanisms using model systems. Genome Med. 10.1186/gm441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uitto J, Prockop DJ (1974) Incorporation of proline analogues into collagen polypeptides effects on the production of extracellular procollagen and on the stability of the triple helical structure of the molecule. Biochimica Et Biophysica Acta BBA Protein Struct 336:234–251. 10.1016/0005-2795(74)90401-2 [Google Scholar]
- Vettore LA, Westbrook RL, Tennant DA (2021) Proline metabolism and redox; maintaining a balance in health and disease. Amino Acids 53:1779–1788. 10.1007/s00726-021-03051-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlasov PK, Vlasova AV, Tumanyan VG, Esipova NG (2005) A tetrapeptide-based method for polyproline II-type secondary structure prediction. Proteins 61:763–768. 10.1002/prot.20670 [DOI] [PubMed] [Google Scholar]
- Wedemeyer WJ, Welker E, Scheraga HA (2002) Proline Cis−trans isomerization and protein folding. ACS Publications. 10.1021/bi020574b [DOI] [PubMed] [Google Scholar]
- Wei L, Zhang M, Wei S et al (2020) Roles of nitric oxide in heavy metal stress in plants: cross-talk with phytohormones and protein S-nitrosylation. Environ Pollut 259:113943. 10.1016/j.envpol.2020.113943 [DOI] [PubMed] [Google Scholar]
- Welch NG, Li W, Hossain MA et al (2020) (Re)Defining the proline-rich antimicrobial peptide family and the identification of putative new members. Front Chem. 10.3389/fchem.2020.607769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Weinberg RA (2008) Epithelial-mesenchymal transition: at the crossroads of development and tumor metastasis. Dev Cell 14:818–829. 10.1016/j.devcel.2008.05.009 [DOI] [PubMed] [Google Scholar]
- Zafra-Ruano A, Luque I (2012) Interfacial water molecules in SH3 interactions: getting the full picture on polyproline recognition by protein–protein interaction domains. FEBS Lett 586:2619–2630. 10.1016/j.febslet.2012.04.057 [DOI] [PubMed] [Google Scholar]
- Zanna N, Milli L, Del Secco B, Tomasini C (2016) Factors affecting the stabilization of polyproline ii helices in a hydrophobic environment. Org Lett 18:1662–1665. 10.1021/acs.orglett.6b00532 [DOI] [PubMed] [Google Scholar]
- Zarrinpar A, Bhattacharyya RP, Lim WA (2003) The Structure and function of proline recognition domains. Science’s STKE 2003:8–8. 10.1126/stke.2003.179.re8 [DOI] [PubMed] [Google Scholar]
- Zhang J, Wang W, Xiong C, Hruby VJ (2003) Efficient and stereoselective synthesis of novel cis-4-substituted proline analogues. Tetrahedron Lett 44:1413–1415. 10.1016/S0040-4039(02)02865-4 [Google Scholar]
- Zheng Y, Lu Z (2013) Regulation of tumor cell migration by protein tyrosine phosphatase (PTP)-proline-, glutamate-, serine-, and threonine-rich sequence (PEST). Chin J Cancer 32:75–83. 10.5732/cjc.012.10084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong H, Carlson HA (2006) Conformational Studies of Polyprolines. J Chem Theory Comput 2:342–353. 10.1021/ct050182t [DOI] [PubMed] [Google Scholar]
- Zondlo NJ (2022) Solvation stabilizes intercarbonyl n→π* interactions and polyproline II helix. Phys Chem Chem Phys 24:13571–13586. 10.1039/D2CP00857B [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.