Abstract
Rapid advances in biological knowledge and technological innovation have greatly advanced the fields of stem cell and gene therapies to combat a broad spectrum of neurologic disorders. Researchers are currently exploring a variety of stem cell types (e.g., embryonic, progenitor, induced pluripotent) and various transplantation strategies, each with its own advantages and drawbacks. Similarly, various gene modification techniques (zinc finger, TALENs, CRISPR-Cas9) are employed with various delivery vectors to modify underlying genetic contributors to neurologic disorders. While these two individual fields continue to blaze new trails, it is the combination of these technologies which enables genetically engineered stem cells and vastly increases investigational and therapeutic opportunities. The capability to culture and expand stem cells outside the body, along with their potential to correct genetic abnormalities in patient-derived cells or enhance cells with extra gene products, unleashes the full biological potential for innovative, multifaceted approaches to treat complex neurological disorders. In this review, we provide an overview of stem cell and gene therapies in the context of neurologic disorders, highlighting recent advances and current shortcomings, and discuss prospects for future therapies in clinical settings.
Keywords: Gene therapy, Genetically modified cell therapy, Genome editing, Stem cells, Stem cell therapy, Transplantation
Introduction
The explosion of biotechnology and genetics advances in recent decades has led to increased understanding of the underlying contributors to a multitude of human diseases, including neurologic disorders. The resulting insight into neurological disorder pathogenesis, which ranges from single gene defects to complicated multidimensional processes, has likewise fueled the development of new, innovative treatment strategies based on stem cell therapeutics [1,2] or gene therapy [3,4]. Stem cell-based therapies offer a comprehensive, multifaceted approach toward complex disease mechanisms, while gene therapy offers a means to impact precise genetic targets. Each therapeutic strategy encompasses varying options with inherent benefits and challenges that inform their utility, and we are just beginning to appreciate the true capacity of the available approaches and emerging innovations. While a complete catalog of stem cell-based strategies for neurologic diseases would be impossible in a single review, this article highlights the categories of various therapeutics explored and the characteristics that make them amenable to certain therapeutic applications. We aim to provide a broad conceptual overview and facilitate an entry for the reader toward the referenced literature for more in depth study. Stem cell classes and their properties are reviewed, followed by a special discussion on issues related to cell transplantation and maintenance. Current gene therapy methodologies are also reviewed, highlighting methods utilized in preclinical and clinical settings. Additionally, we discuss the potential intersection of the two strategies using gene modified cell therapies, an approach that is enhancing applicability for certain human diseases, including neurologic disorders.
Stem Cell Therapeutics
Therapeutic approaches leveraging stem cells have evolved significantly since the inception of bone marrow transplants over fifty years ago [5]. Today, stem cells hold immense promise for treating neurologic disorders, offering a unique and transformative approach to address the complexities of conditions affecting the nervous system. The development and application of therapies for neurological disorders encounter significant hurdles due to the distinct characteristics of the nervous system. Mature neurons in the adult nervous system are post-mitotic, which poses a challenge in replenishing damaged neurons. Moreover, the blood-brain barrier (BBB) is a formidable obstacle, impeding the delivery of pharmaceuticals and biologics into the central nervous system (CNS). Furthermore, many neurological diseases invoke multiple pathologic mechanisms that cannot all be effectively addressed by single pharmacologic agents. However, stem cell therapies can potentially overcome these limitations comprehensively.
One of the primary benefits of stem cells comes from their remarkable ability to differentiate into various cell types, including neurons and glial cells, crucial for repairing and replacing damaged neural tissue [6,7]. Once introduced into the target tissue, stem cells can act as biological factories, providing long-term benefits, even in organs with highly regulated access like the brain's BBB. Additionally, unlike traditional treatments that may only alleviate symptoms or target a single pathway, stem cell therapies can potentially engage multiple mechanisms to support diseased neurons, such as secretion of macromolecules, immune modulation, toxin scavenging, etc. In this way, stem cells may even target the root causes of neurologic disorders, promoting regeneration and functional recovery of native cells [8]. Additionally, the potential for stem cells to form neurons and new synapses also allows them to integrate into existing neural networks, restoring and augmenting affected neural circuits [9]. Current research efforts focus on a variety of stem cell classes that vary by source, availability, and capacity to differentiate, migrate, and proliferate, thus further enhancing the ability to tailor treatment to individual patient needs. Understanding the characteristics of different stem cell types, as well as available means to secure and deliver cellular therapies, is important for appreciating their potential for clinical use for neurologic disorders.
Stem cell classifications and therapeutic applications
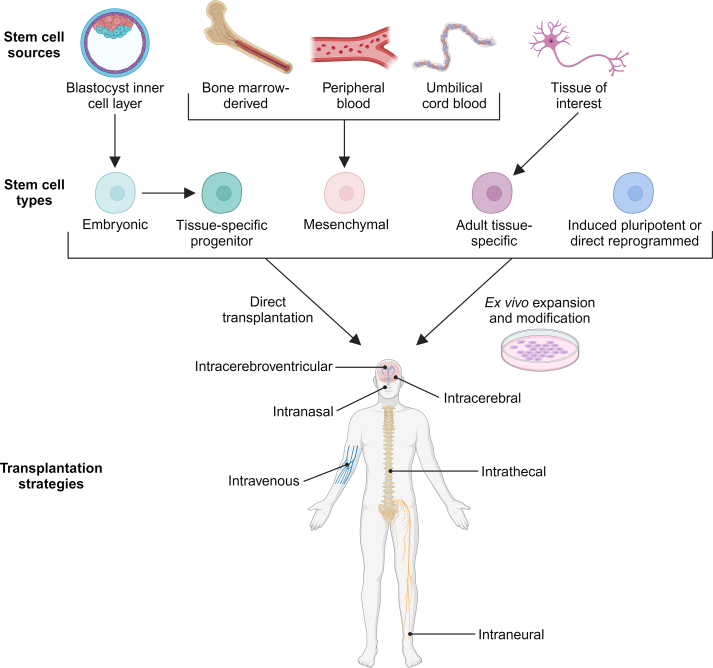
Stem cells are defined by the ability to both self-renew and differentiate into multiple cell types; however, the inherent properties of each stem cell class offer unique advantages and opportunities (Fig. 1) [10].
Fig. 1.
Stem cell classifications and delivery strategies for therapeutic applications. Stem cell therapies encompass a wide array of cell sources and types for addressing neurologic diseases. After isolation, these stem cells can be immediately transplanted or undergo ex vivo expansion and modification before implantation. Implantation strategies include intravenous, intranasal, intracerebroventricular, intracerebral, intrathecal and intraneural routes of delivery. Image created using BioRender.com.
Embryonic stem cells
Embryonic stem cells (ESCs) possess remarkable potential, exhibiting either totipotency, with the theoretical capacity to transform into any cell type, or pluripotency, maintaining the ability to differentiate into cells representing all three developmental germ cell layers. Since they can be cultured in an undifferentiated state, ESCs hold promise in their capability to develop into a diverse array of terminal cell or tissue types upon exposure to appropriate morphogenic signals. This versatility extends to the realm of neurological and developmental diseases, where ESCs serve as invaluable models for studying neuronal and glial tissue. ESCs also do not express high levels of major histocompatibility complex (MHC) proteins, and thus could have less risk for rejection in transplantation paradigms [11,12].
Despite their broad developmental potential, ESCs may be of limited use in treating neurologic disease as the ability to form mesenchymal or endodermal tissues is unlikely to be useful in the context of the nervous system. Furthermore, the immature, undifferentiated state of ESCs carries a concern for uncontrolled cell proliferation and tumor or teratoma formation upon implantation into a host [13,14]. Therefore, the transplantation of truly naïve undifferentiated ESCs has seen little clinical application for neurologic disorders.
Another consideration is that, while current ESC lines can be propagated in culture, new ESCs would need to be sourced from the blastocyst inner cell mass of a developing human embryo. This derivation of new lines is highly regulated and the use of ESCs is constrained by strict ethical and regulatory guidelines [15], thus impacting their use for research and medical applications. Fortunately, there are several intermediate cell types preceding the formation of terminally differentiated neural tissue that maintain the advantages of stem cell therapy while avoiding the risks associated with ESCs. These cell types will be discussed in the following sections.
Neural stem cells and central nervous system progenitor cells
There are many stages and levels along the developmental pathway between the earliest totipotent ESCs and the final terminal cell type of a given organ. These intermediate populations are often broadly termed progenitor cells or precursor cells. Progenitor cells have the benefit of being closer to terminal differentiation, with less concern for tumor formation and more control of final cell phenotype [16]. In the CNS, early stage progenitor cells are termed neural stem cells (NSCs), capable of forming both neurons and glia [17]. Cell types that are further along in the differentiation pathway include neuronal stem cells that only form neurons (or only specific subtypes of neurons, such as dopaminergic or γ-aminobutyric acid (GABA)-ergic neuron precursors) or glial precursor cells (which may be restricted only to astrocytes or oligodendrocytes). The unique transitional properties of progenitor cell populations in the nervous system make them a promising therapeutic tool.
NSCs are multipotent stem cells found in the nervous system that have a remarkable ability to self-renew and differentiate into various cell types of the nervous system, including neurons, astrocytes, and oligodendrocytes [17]. During embryonic development, NSCs arise from the neural tube, an ectodermal structure that gives rise to the entire CNS. In adult rodents and other mammals, NSCs persist in specific regions of the brain known as neurogenic niches, where they continue to generate new neurons and glial cells through an individual's lifetime in response to various stimuli, such as injury or disease. Two primary neurogenic niches in the adult brain are the subventricular zone lining the lateral ventricles and the subgranular zone of the hippocampal dentate gyrus. The relevance of these populations in primates and humans is debated, but cell replacement from these niches is likely to be less relevant [18].
Although harnessing inherent neurogenesis from native NSC niches does not seem practical in humans currently, NSCs can still be propagated as cell lines and serve a therapeutic role as allograft transplants. A significant advantage of NSCs lies in their ability to integrate into existing neural circuits, provide trophic support to damaged cells, promote functional recovery, and modulate the local environment. Challenges include potential tumorigenicity (to less of a degree than ESCs), sometimes challenging in vitro proliferation, as well as their limited differentiation capabilities. Additionally, since NSC harvest and autologous transplant is not feasible in humans at this time, immune rejection of allogenic transplants remains a concern, as discussed in later sections.
Preclinical studies successfully demonstrate the therapeutic potential of NSCs in multiple models of neurologic disorders. In a multiple sclerosis mouse model, intraspinal transplant of human NSCs increases regulator T cell levels, promotes remyelination, and decreases neuroinflammation [19]. Human NSCs infused into the fimbria fornix of the hippocampus of an Alzheimer's disease mouse model improve spatial memory and normalize genes dysregulated by Alzheimer's disease [20]. Additionally, transplantation of NSCs into the lateral ventricle of a superoxide dismutase 1 mouse model of ALS delays time to disease onset, prevents motor neuron loss, and promotes proliferation in neurogenic regions of the brain [21].
Perhaps the neurological disease with the longest history of stem cell-based therapy is Parkinson's disease. Given the defined desire to restore dopaminergic cells in motor systems, NSCs make sense as a restorative strategy [22]. Earliest attempts utilized tissue obtained from fetal ventral mesencephalon [23,24], or other fetal tissues to lesser effect [25], and survival and engraftment of donor cells were established. In one study (N = 34, prospective randomized double-blind study over 24-months including a sham surgery arm), the trial failed to meet the primary endpoint (significant improvement in scores of the Unified Parkinson's Disease Rating Scale, UPDRS), and over 50% of patients developed intractable “runaway” dyskinesias that were not related to medication side effect [26]. In another similar study (N = 40, prospective randomized double-blind study over 1 year including sham surgery arm), improvement was most robust in younger patients (<60 years old), although 5 of these younger patients developed worsening dystonia and dyskinesia over time [27]. This pioneering work led to plans for another European trial (TRANSNEURO) using fetal-derived ventral mesencephalic neuronal precursors [28], although final outcomes have yet to be reported. Despite these trailblazing studies, sourcing NSCs from fetal donor tissue brings significant barriers, including uniformity/characterization of cell product, obtaining sufficient viable cell numbers, problems with rejection/immune suppression (see below), as well as ethical quandaries. With modern well-established stem cell culture techniques, stem cell therapies derived from established NSC lines circumvents these issues. Indeed, many of the same investigators associated with TRANSNEURO are also performing a trial evaluating a dopamine neuron precursor line (STEM-PD, NCT05635409) [29,30], and a similar dopaminergic precursor product is in early stages of evaluation (MSK-DA01, bemdaneprocel, NCT04802733) [31].
Given the lessons learned from Parkinson's disease and positive preclinical results in other areas, recent clinical studies are exploring the safety and potential efficacy of NSC treatment in patients with other neurologic diseases. In a Phase I clinical trial for individuals with progressive multiple sclerosis (N = 15), intracerebroventricular transplantation of allogeneic neural stem cells (hNSC 03/14b) was investigated in escalating doses (3 subjects each at 5 million, 10 million, and 16 million cells and 6 subjects receiving 24 million cells). While not designed to assess efficacy and lacking a placebo control group, the trial found no detrimental effects on disability or disease progression, and no effect, either beneficial or harmful, was seen in multiple sclerosis-related lesions on imaging. No deaths or serious adverse events were reported related to the stem cell therapy, lending support at least for the safety of this approach [32]. Likewise, intraspinal transplantation of NSCs in individuals with ALS has recently been studied. A Phase I and Phase IIa study investigating a human NSC line (NSI-566RSC) utilized a design of escalating risk (from 10 to 40 spinal injections, beginning with lumbar targets and escalating to cervical targets) in addition to escalating cell dose (from 2 million to 16 million cells) in 24 subjects [33]. Two serious adverse events were described, but neither were clearly related to the cellular therapy or method of transplantation. Given this was not a placebo-controlled trial, a long-term post hoc analysis compared results with placebo-treated cohorts from prior large ALS clinical trials. This suggested improvement in clinical measures and survival compared to historical controls [34]. A similar study in ALS evaluated intraspinal human NSC transplantation in 18 subjects with no serious adverse events [35]. While additional research is still needed, the use of NSCs for treating neurologic disorders represents a promising avenue that could revolutionize the field of regenerative neurology.
NSCs give rise to both neuronal and glial progenitor cells. Neuronal progenitor cells are committed to forming neurons while glial progenitor cells are committed to glial cell lineages. As described above, neuronal progenitors or specific neuron precursor cell populations arise from NSCs during development. For glial precursor cells, endogenous sources of rodent glial precursor cells include the spinal cord [36,37], optic nerve [38], and cerebral cortex [39]. In humans, endogenous glial precursor cells have been isolated and purified from white matter [40] and the spinal cord [41].
Current approaches aim to use neuronal progenitor cells capable of differentiating into various neuronal cell types to replace damaged or degenerated neural tissue in conditions like spinal cord injuries [42], stroke [43], and Parkinson's disease [44]. For example, in a mouse model of drug-resistant mesial temporal lobe epilepsy, single-dose intrahippocampal delivery of pallial GABAergic interneurons derived from medial ganglionic eminence progenitor cells suppresses seizures and increases lifespan [45]. These promising results led to a Phase 1/2 clinical trial (NCT05135091) focused on drug-resistant mesial temporal lobe epilepsy that is currently ongoing. Additionally, a clinical case series reported that transplantation of human neuronal progenitor cells into the dorsal putamina of Parkinson's disease patients enhances midbrain dopaminergic activity and improves motor function [46]. Here, we can see that for neurologic conditions in which particular neuronal subtypes are affected in very specified neural circuits, a strategy using a more defined and differentiated cellular product shows great potential.
Venturing down the other major branch of differentiation, glia, chiefly astrocytes and oligodendrocytes, provide supportive functions for neuronal cells in the nervous system. As such, dysregulation of glial function contributes significantly to the pathology of neurological diseases, with implications for neuroinflammation, demyelination, and neuronal damage [47]. The ability to generate robust populations of these cells from glial progenitor cells or astrocyte/oligodendrocyte progenitor cells offers promising avenues for regenerative stem cell-based therapies. Currently, use of glial progenitor cells as a therapeutic treatment option has been investigated preclinically for ALS, multiple sclerosis, and spinal cord injury [48]. Trials assessing glial-restricted precursors cells are recruiting for treatment of transverse myelitis (NCT03887273) or in the planning phases for ALS (NCT02478450). The safety and tolerability of a single intrathecal injection of astrocyte-restricted progenitors was reported in 10 ALS subjects [49], testing doses of 100 million and 250 million cells. No deaths or serious adverse events were noted, and transient improvement in the slope of disease progression was noted in the 3 months following injection.
Oligodendrocyte progenitors, on the other hand, could be used to restore axon myelination in demyelinating conditions and some early animal models show promise in this realm [50]. In autoimmune demyelinating diseases, such as multiple sclerosis, an additional challenge for oligodendrocyte progenitor cells are the hostile local environments that make transplant survival impossible [48]. Still, use of oligodendrocyte progenitor cells has been explored for ALS and spinal cord injury [51]; a Phase 1 trial exploring ESC-derived glial/oligodendrocyte progenitor cells was established for participants with spinal cord injuries, but was later closed without publication of study results (NCT01217008). While additional research is still needed, the use of neuronal and glial progenitor cells for treating neurologic disorders represents a promising avenue that could revolutionize the field of regenerative neurology.
Mesenchymal stem cells
Mesenchymal stem cells (MSCs) are derived from mesodermal-derived tissue, which includes adult tissues such as bone marrow, adipose tissue, and peripheral blood, as well as neonatal tissues like placenta, amnion, umbilical cord, and umbilical cord blood [52]. MSCs are multipotent and naturally produce mesodermal lineages, although recent studies report the ability to induce cross-lineage differentiation to generate less directly related cell types, e.g., neurons [53]. The accessibility and availability of MSCs are greater compared to ESCs. MSCs are also thought to be less immunogenic, with evidence supporting lower risk of rejection even with allogenic transplantation compared to other progenitor cell types. Additionally, in contrast to NSCs, they offer a source of stem cells that can be used for autologous cellular therapy development, thereby decreasing the risk of rejection and avoiding the need for immunosuppressive agents [54]. However, by using an autologous transplant strategy, the pathology leading to the underlying neurologic illness may also impact the behavior of patient-derived MSCs. For example, bone marrow-derived MSCs from ALS patients demonstrate deficits in migration and growth factor secretion compared to those from healthy controls [[55], [56], [57], [58], [59]].
Preclinical studies investigating the use of MSCs in neurodegenerative diseases, such as Parkinson's disease [60] and Alzheimer's diseases [61], indicate encouraging outcomes. These studies reveal that MSC transplantation promotes neuronal survival, modulates microglial activation, and enhances the release of neurotrophic factors, collectively contributing to neuroprotection. Targeted activation of MSCs or specific subpopulations have also shown promise in ALS preclinical studies [62,63].
Delivery strategies of MSCs, also described in more detail below, should be considered in light of their purported mechanisms of action. Delivery via the vasculature (intravenous or intra-arterial) is attractive due to the non-invasive (and potentially autologous) nature of this approach. MSCs do appear to cross the blood-brain barrier (BBB) from the vasculature, perhaps facilitated by compromise of tight junctions in disease or in settings of inflammation [64,65]. However, a large proportion of cells appears to remain entrapped in small vessels [[66], [67], [68]]. Thus, a therapeutic mechanism invoking predominantly secreted factors (macromolecules or extracellular vesicles) or interactions with cells outside of the CNS (e.g., immune interactions) may take a primary role [69]. Interfaces directly with diseased neurons or strategies aimed at cell replacement may require injection into the spinal fluid or directly into brain/spinal cord tissue [52]. Current clinical trials are currently exploring the application of MSCs to treat individuals with stroke [70], Alzheimer's disease (Phase IIa, NCT02833792), Parkinson's disease (Phase II, NCT04995081), and ALS (Phase II, NCT03268603), among many other neurological diseases, underscoring the translation of preclinical success to potential therapeutic applications.
Induced pluripotent stem cells
Finally, recent advances also support the development of pluripotent cells from adult somatic tissue [71]. Induced pluripotent stem cells (iPSCs) are created by genetically reprogramming patient-derived cells, usually fibroblasts obtained from skin punch biopsy, by transducing them with a select combination of transcription factors. These factors – the Yamanaka factors Oct3/4, Sox2, Klf4, and c-Myc – de-differentiate the cells to an ESC-like state [72]. Although this is a relatively new technology and long-term characterization of these cells is still in progress, iPSCs offer a means to establish patient-specific disease models, as well as a potential resource for autologous cellular therapy development. A chief advantage lies in the theorized ability to embark upon personalized or precision medicine, with ex vivo modification of underlying genetic or epigenetic drivers of pathology (see following sections) [73]. Upon expansion and differentiation, the modified cells can be transplanted in an autologous fashion with decreased concern for immune rejection. The flip side of this approach is that many undefined genetic polymorphisms or epigenetic changes will be carried into the final cell product, imparting a degree of variance in achieving targeted effects. Also, to create an iPSC line for every patient could prove labor intensive. Use of a single “off the shelf” iPSC line in an allograft paradigm would be more expedient but may sacrifice the unique “personalized” aspect of therapy and require immune suppression to prevent host rejection.
iPSC technology has thus thrown open a floodgate for human-relevant investigation and holds immense promise for revolutionizing the treatment landscape of neurologic disorders. These cells offer an unprecedented opportunity to create patient-specific, functional neural cells for disease modelling and transplantation. As iPSCs can be differentiated into various neural cell types, including neurons and glial cells, they provide a platform for understanding the underlying mechanisms of neurological disorders and drug screening [74,75]. Furthermore, iPSCs can not only model neurological disease, but can serve as a potential therapeutic tool. Preclinical work has been performed applying iPSC-based therapy in stroke, spinal cord injury, Alzheimer's disease, Parkinson's disease, ALS, and more [76]. The first report of iPSCs applied in human subjects was in 2020 [77]. Subsequently, a clinical trial (NCT06145711) was initiated in October 2023 to investigate the safety of an autologous iPSC-based therapy in three Parkinson's disease participants. A similar trial using iPSCs in Parkinson's disease, albeit as an allogeneic transplant and not in an autologous fashion, is underway in Japan [78]. While challenges remain, such as the ensuring the safety and efficacy of iPSC-derived therapies, ongoing preclinical research and clinical trials are advancing the field, signaling a hopeful future for harnessing the regenerative power of iPSCs to study and treat diverse neurologic disorders.
As can be seen by this brief overview, the attributes of the varying stem cell types, including their source of derivation, their differentiation potential, and their availability, influence their prospective utility for research and clinical translation.
Clinical considerations for stem cell therapy
In addition to selecting an appropriate stem cell type, establishing an effective stem cell therapy also means ensuring cells are successfully delivered to the proper tissue and promoting the survival and functionality of the cells once they reach their intended site.
Delivery of stem cell therapies
The desired outcome of stem cell therapy, whether it be cell replacement, paracrine effects within the microenvironment, or delivery of therapeutic factors to specific anatomical regions, requires careful selection of an appropriate delivery strategy. Approaches for stem cell delivery have utilized low invasive intravenous or intraarterial injection, methods to tap into cerebrospinal fluid flow such as intrathecal or intracerebroventricular injection, or more targeted approaches of direct intracerebral injection into brain parenchyma or intraneural injection into peripheral nerves (Fig. 1; Table 1). Additionally, recent experimental approaches have explored the delivery of stem cells through nasal passages [79].
Table 1.
Comparison of stem cell transplantation strategies.
| Transplantation Route | Strengths | Limitations |
|---|---|---|
| Intravenous | Rapid systemic distribution Non-invasive Easily administered in clinical settings Reduced risk of tissue damage Potential for repeated dosing |
Dilution in circulation Clearance by filtering organs Limited homing to target tissues Immune system clearance Potential off-target effects |
| Intrathecal | Direct access to CSF and fluid dynamics Enhanced targeting Reduced systemic exposure Less invasive than intracerebral |
Risk of complications Immune response in the CNS Challenges in cell retention Potential off-target effects |
| Intracerebroventricular | Direct access to CSF and fluid dynamics Enhanced targeting Reduced systemic exposure Less invasive than intracerebral |
Risk of complications Immune response in the CNS Challenges in cell retention Potential off-target effects |
| Intracerebral | Precise targeting Enhanced integration Minimized dilution and clearance Potential for local effects Long-term retention |
Risk of neurological complications Immune response in the brain Invasive procedure Limited distribution Challenges in precise targeting |
| Intraneural | Precise targeting Enhanced integration Minimized dilution and clearance Potential for local effects |
Risk of nerve injury Invasive procedure Limited distribution Immune response in the nerve Challenges in precise targeting |
| Intranasal | Non-invasive Direct access to the brain Rapid absorption Minimized systemic exposure Enhanced patient compliance |
Variable absorption Clearance mechanisms Risk of inflammation Lack of consistency in protocols |
CSF, cerebral spinal fluid.
Systemic administration via intravenous injection facilitates broad distribution of cells which allows cells to either promote global effects or home to specific regions. Some preclinical studies suggest that in certain cases, such as ischemic stroke, intravenous stem cell infusion may be as effective as direct implantation due to the potential paracrine action of stem cells and the subsequent impact on brain regeneration [80,81]. While less invasive than direct brain injections, systemic administration faces challenges, such as the limited ability of stem cells to target specific areas within the brain or spinal cord. Cells must navigate through the complex circulatory system, potentially getting trapped enroute, for instance in the lungs due to their large size [82], and then cross the BBB to reach the affected sites. Additional research is examining methods to open the BBB to permit the passage of infused cells [83] and enhance the homing and engraftment capabilities of stem cells, thus, optimizing their therapeutic use for neurologic disorders of the CNS. Approaches to increase homing and engraftment include the addition of cytokines during in vitro expansion to stimulate expression of homing molecules, pre-treatment with various growth factors, such as granulocyte colony stimulating-factor, and surface modifications with natural or synthetic polymers [84]. Additionally, a peripheral approach may be ideal when targeting effects on diseases of the peripheral nervous system. Widespread geographic regions can be impacted in polyneuropathies or multiplex mononeuropathies without a need to overcome the BBB of the CNS [85], although human trials using this approach to target peripheral nerves has not yet been described.
Alternatively, direct transplantation delivers a high concentration of cells to specific sites of injury or pathology. Intrathecal and intracerebroventricular injection deliver stem cells directly into the cerebrospinal fluid (CSF), bypassing the BBB. Specifically, intrathecal injection introduces stem cells into the space surrounding the spinal cord, while intracerebroventricular injection targets CSF-filled cavities within the brain's ventricular system. Access to the lumbar CSF cistern is a common, relatively low-risk procedure and is therefore a strategy that can be implemented widely and even repeatedly, if desired. This approach has been applied in ALS [[86], [87], [88], [89], [90]], multiple sclerosis [91], spinal cord injury [[92], [93], [94]], multiple system atrophy [95], and others [96]. Intracerebroventricular injection may also make use of an implanted catheter with subcutaneous reservoir, permitting repeated injections over time with only one pass through the brain parenchyma. This approach has been used, for example, as a strategy to treat ALS [97]. By leveraging the natural fluid dynamics of the CSF, these methods facilitate the distribution of stem cells throughout the CNS and to affected areas, thus enhancing their therapeutic potential for regeneration.
For peripheral nerve injuries, transplantation frequently involves intraneural transplantation of stem cells with bioengineered conduits, which are artificial scaffolds that provide a physical framework to support and guide integration into the injured nerve tissue [98]. Additionally, bioengineered conduits can release growth factors or other bioactive molecules that promote stem cell survival, proliferation, and differentiation, thus further enhancing therapeutic potential.
Many studies that have progressed to early clinical trials have utilized intracerebral injection to directly deliver stem cells into specific regions of the CNS affected by injury or disease. Direct transplantation of stem cells is particularly relevant in traumatic or degenerative conditions where the localized nature of the pathology requires a targeted therapeutic intervention. Additionally, direct administration can facilitate integration of transplanted cells into existing neural circuits, promoting functional recovery. Despite its advantages, direct transplantation does come with considerations regarding invasiveness and the need for sophisticated delivery techniques. Typically, intracerebral injection requires stereotactic surgical techniques to precisely and accurately guide the insertion of a needle or catheter directly into specific targets [99]. An analogous approach for diseases of the spinal cord has required the development of similar techniques to target motor pathways [100]. This has enabled a Phase I and Phase IIa trial of NSCs in ALS [33,34,101]. Overall, intracerebral and intraspinal injection has the advantage of being the most targeted method, capable of introducing millions of cells into specific anatomic structures, but is also a more invasive approach with attendant procedural risks.
Intranasal delivery of stem cells leverages the olfactory pathway to bypass the BBB and transport cells directly to the brain [102]. This transplantation method has several advantages, including minimal invasiveness, reduced risk of complications, and rapid delivery to the CNS. Recent preclinical studies in rodent models of Alzheimer's and Parkinson's disease report encouraging results, as intranasally administered stem cells survive, proliferate, and promote therapeutic effects [79], and an interventional clinical trial (NCT02795052) that will isolate and intranasally deliver autologous bone marrow derived stem cells to patients with neurologic conditions is currently recruiting. However, limitations of intranasal delivery include variation in biodistribution across different brain regions, potential inefficiencies in cell delivery, and a lack of consistent data on the optimal concentration of stem cells required to achieve therapeutic effects [79].
With recent FDA approval of focused ultrasound for treatment of movement disorders [103], there is greater momentum in applying this modality toward BBB permeabilization in conjunction with gene and stem cell therapy [104,105]. In this arena, it is low intensity, pulsed focused ultrasound (typically with use of intravascular microbubbles) [106,107] which mediates BBB opening and permits passage of macromolecules, including nucleotides, exosomes, and even cells. Early preclinical efforts have demonstrated stem cells successfully enter brain parenchyma after focused ultrasound opening of BBB followed by intra-arterial cell injection [108]. Of the 2 × 106 cells injected, about 33 cells/mm2 were found 24 h after injection. Retention of cells could theoretically be augmented by preloading stem cells with superparamagnetic iron oxide nanoparticles and applying a magnetic field to the brain after administration in conjunction with focused ultrasound BBB opening [109]. Focused ultrasound has also been applied in animal models in conjunction with MSC therapy [110], with nearly twice the engraftment with intravenous MSC administration into target regions, possibly mediated by focused ultrasound-induced upregulation of cell adhesion molecules. Endogenous stem cell populations are seen to be enriched with focused ultrasound in rats [111], but as above, the significance of adult neural stem cell niches has yet to be established in humans.
Immune suppression and modulation
Stem cell therapy success also depends on preventing adverse complications due to transplantation and ensuring cell survival following delivery. While the brain was previously, and controversially, thought to lack a strong immune response to transplanted stem cells due to the presence of few professional antigen-presenting cells, limited lymphatic drainage, and a protective BBB, current perspectives raise concerns regarding the potential rejection of stem cells [112]. Thus, immune suppression and modulation regimens are needed to prevent stem cell rejection by the host tissue during transplantation. Drawing from the experience of bone marrow transplant and solid organ transplant, immunosuppressive approaches in CNS stem cell transplants often involve the administration of immunosuppressive drugs, such as steroids, inhibitors of calcineurin, inosine monophosphate dehydrogenase, and interleukins [113]. Unfortunately, the use of immunosuppressive drugs is associated with several limitations, including varying and inconsistent protocols, toxic side effects, and inability to sufficiently overcome host immune responses. More recently, a number of approaches using monoclonal antibody-based immune suppression demonstrates superior cell survival and decreased toxicity [114,115]. Although aspects such as immunosuppressive regimen and optimal duration of immunosuppression for stem cell transplants in the CNS remains under study, rejection of cells currently remains a mandatory consideration for all allogenic transplant paradigms.
The harsh microenvironment of the diseased or injured nervous system, coupled with challenges such as inflammation and oxidative stress, also poses significant hurdles to the long-term viability of transplanted cells. We have previously mentioned this as a significant challenge in autoimmune disease such as multiple sclerosis. This appears also relevant in neurodegenerative diseases such as ALS. The spinal cord microenvironment in ALS is characterized by oxidative stress, excitotoxicity, inflammation, and a paucity of neurotrophic support, which can all be detrimental to stem cell survival. Supplemental therapies to condition the “soil” into which the stem cell “seed” is placed may become important in maximize the effectiveness of cellular therapy [116]. Regardless, as previously referenced, trials of intraspinal neural progenitor cell transplants have progressed for ALS [117,118]. Other innovations to address this issue includes the incorporation of biomaterial scaffolds to provide structural support and create a conducive niche for cell survival and integration [119].
Nucleotide-Based Therapeutics
Like stem cell therapy, treatment strategies for neurologic disorders targeting genes and their products have expanded considerably over the past three decades since the first gene therapy application in 1990 [3,4]. This stems from the growing number of mutations associated with neurological disorders, the increasing availability of genetic testing, and the rapid pace of technological advances in gene editing and other gene-targeting technologies. In one strategy, the objective of gene therapy is to deliver genetic material and/or genome editing tools to silence, add, replace, or repair a gene. A related strategy utilizes a defined nucleotide sequence as a way to interfere with a target gene's expression, using RNA-interference (RNAi) or antisense oligonucleotides (ASOs). These approaches have shown promise in early clinical trials. Herein, again, we will be unable to review the details of every trial, but aim to provide a framework for general understanding and touch upon some exemplary cases [[120], [121], [122]].
Gene therapy
Gene therapy approaches involve using a nucleotide-based therapeutic, either DNA or RNA, and introducing these sequences into the cell for long-term alteration of gene expression [123,124]. One strategy is to take a gene replacement approach, in which a defective or missing genetic sequence is replaced or augmented by introduction of a corrected DNA sequence. Alternatively, a strategy of pathological gene inhibition may be employed, whereby the gene therapeutic agent may encode a sequence that blocks the expression of a defective gene at the transcriptional, translational, or post-translational level. The complete nucleic acid payload also includes promoters, enhancers, 5′- and 3′-untranslated regions, or other sequences that can influence transgene expression levels and even cellular specificity, including neuronal cells [125]. To deliver the nucleic acid material for gene therapy, a vehicle of non-viral or viral vectors is often utilized. Discussion of delivery strategies will be undertaken in a subsequent section, but here we will first focus on the overall conceptual approaches taken by various gene therapy products in the neurological arena.
Gene therapy is particularly appealing for neurological diseases that involve well-characterized loss-of-function mutations or truncations in critical proteins. As such, success has been seen with the FDA approval of Onasemnogene abeparvovec, a self-complimentary construct to express intact SMN protein in SMA patients [126]. Additionally, the AAV-based gene therapy delandistrogene - moxeparvovec-rokl increases transgenic micro-dystrophin protein expression, and was approved by the FDA in June 2023 as the first gene therapy treatment for Duchenne muscular dystrophy [127], a disease often treated by neuromuscular Neurology specialists. Consequently, investigation into gene therapy is underway for several neurologic disorders with underlying genetic causes, including clinical trials for muscular dystrophies (NCT03333590, NCT03368742, NCT05096221), lysosomal storage disorders [128], Huntington's disease [129], epilepsy [3,130], and even brain tumors [125].
Gene therapy may also still hold promise for neurologic disorders that are less well defined as monogenic in causality, such as neurodegenerative diseases [121,131]. In Alzheimer's disease, one approach is to provide trophic support for neurons in an environment of multifaceted and incompletely understood pathophysiology. Efforts include supplementing nerve growth factor (NGF) using an AAV2 vector; however, in a Phase II study (N = 49 randomized to sham injection versus 2.0 × 1011 vector genomes of AAV2-NGF by intracerebral injection into the nucleus basalis of Meynert), there was no significant difference detected in cognitive outcomes [132], and limited spread of the viral vector or inaccurate injections have been suspected [133]. Building from this experience, a new trial evaluating a vector for delivering brain derived neurotrophic factor (AAV2-BDNF) is underway (NCT05040217). In a different approach, augmented expression of apolipoprotein E2 (AAVrh.10hAPOE2, or LX1001) by intrathecal injection is being applied in Alzheimer's disease patients who are homozygotes for the high-risk gene apoliporotein E4 (NCT03634007).
In Parkinson's disease, an initial strategy utilized AAV2 to overexpress glutamic acid decarboxylase (AAV2-GAD) within the subthalamic nucleus in an effort to augment inhibitory signal in this nucleus and restore subcortical motor network function. This seemed well-tolerated [134], with a small but statistically significant improvement in motor function during a double-blind, sham-controlled Phase II trial (N = 37) [135]. Another neurotransmitter-modulating strategy used aromatic L-amino acid decarboxylase (AAV2-AADC) injected into the putamen to promote greater conversion of levodopa to dopamine [136]. In an early trial, 10 subjects were enrolled and received either low-dose (n = 5, 9 × 1010 vector genomes) or high-dose (n = 5, 3 × 1011 vector genomes) injections, with all patients demonstrating improvement in motor scores [137] (with 4 subjects going on to receive deep brain stimulation therapy, and one patient with symptomatic hemorrhage from the surgical procedure [138]). This approach has been revived using intraoperative-MRI guided delivery (VY-AADC01), with no serious adverse events related to the surgical procedure or the gene therapy product. In this Phase I study (N = 15 subjects across 3 doses, open-label without sham control), motor outcomes either remained stable or improved slightly, with again no serious adverse events related to the procedure or the gene therapy product [139]. Additionally, another study which reported the first use of a lentiviral vector in humans (ProSavin, carrying AADC as well as genes for tyrosine hydroxylase and cyclohydrolase 1), enrolled 15 subjects in escalating doses by intracerebral injection into the putamen [140]. Significant improvements in motor scores were seen across all subjects, with a suggestion of a dose-response curve, and no serious adverse events related to the surgery or the viral vector.
An alternative strategy of providing trophic support rather than direct manipulation of neurotransmitter levels has also been tried. Previously, trials were performed aimed at increasing levels of Neurturin (AAV2-NTN), targeting striatum and substantia nigra by intracerebral injection [141]. However, subsequent studies failed to show a benefit in motor scores when compared to sham injection [142,143]. This includes delivery of glial-derived neurotrophic factor (AAV2-GDNF, NCT01621581).
Nucleic acid therapeutics
Aside from the strategy of gene therapy, which involves modification to the host genome, nucleic acid sequences can also be directly used to alter the process of mRNA maturation/splicing, mRNA trafficking, or the eventual translation to protein products. In the current era, these therapies mostly take the form of ASOs. ASO approaches involve the use of synthetic oligonucleotides specifically designed to bind to target RNAs in cells through complementary base pairing, thereby impacting expression of the target genes [124] (Fig. 2).
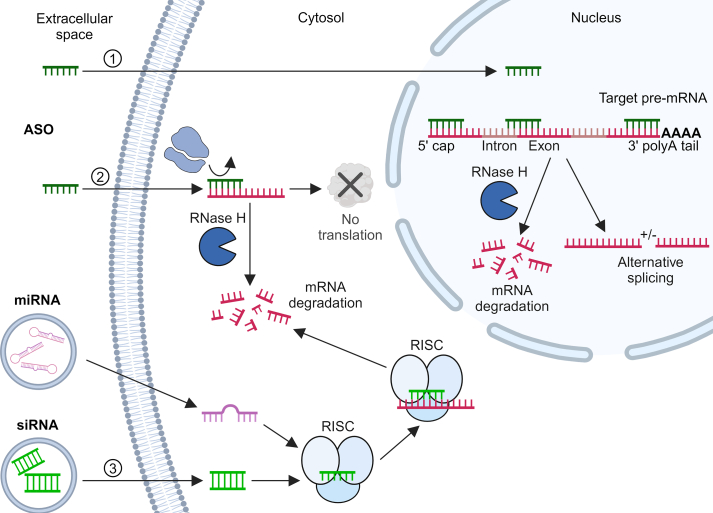
Fig. 2.
ASO and siRNA mechanisms of action. (1) Antisense oligonucleotides (ASO) present in the extracellular space cross both the cellular and nuclear membranes to access the nucleus. Within the nucleus, ASOs can bind to various regions of target pre-mRNA: at the 5′ cap to disrupt formation of the cap structure essential for mRNA maturation, at the poly A tail to interfere with mRNA stability and processing, and at splicing sites to disrupt the splicing process and cause aberrant mRNA formation. In each case, ASO binding creates a substrate for ribonuclease H (RNaseH), which recognizes the ASO-RNA duplex and cleaves the RNA strand, ultimately resulting in degradation of the target pre-mRNA. ASOs may also bind to regions that promote alternative splicing of pre-mRNA, producing transcripts with truncations or even additional favorable exons. Alternatively, (2) the ASO may cross the outer cell membrane and enter the cytosol, where it binds to the target mRNA, hindering ribosome attachment and blocking protein translation. ASO-RNA duplex formation within the cytosol is also susceptible to RNase H degradation. (3) Small interfering RNA (siRNA) and microRNA (miRNA) cross the outer cell membrane into the cytosol, typically using viral, lipid, nanoparticle, or other delivery systems. For siRNA, once inside the cell, the siRNA strands separate, and one strand incorporates into the RNA-induced silencing complex (RISC). This complex then uses the siRNA as a guide to recognize complementary sequences on the target mRNA. Upon binding, RISC cleaves the mRNA, leading to its degradation and preventing protein synthesis. Similarly, miRNAs are processed and eventually the mature miRNA integrated into the RISC, serving as a template for targeted mRNA degradation. Image created using BioRender.com.
ASOs are short, single-stranded deoxyribonucleic acids that typically range from ∼10 to 30 nucleotides in length [144]. ASOs must be highly specific to their target RNA to prevent off-target effects, bind target RNAs with high efficiency, be highly bioavailable, and remain stable until they reach their target tissue. They can be designed to promote target RNA degradation by binding to pre-mRNAs in the nucleus or mRNAs in the cytosol, thus invoking RNase H-mediated degradation. ASOs can also directly inhibit translation by binding to mRNA and preventing action of translational enzymatic processes. Additionally, ASOs can target exon splice sites near disease mutations, resulting in alternatively spliced or truncated protein products that, while missing exon(s), may still retain some function and can reduce disease severity. Conversely, ASOs may block splicing enhancer regions in pre-mRNA, and promote the formation of mRNA containing full length regions or exons that would not otherwise be included. This appears to be the mechanism of action for nusinersen, FDA approved for treatment of SMA, which promotes inclusion of exon 7 in SMN2 gene and thus compensates for loss of the typically predominantly functional SMN1 gene [145,146]. Additional ASO therapies are approved by the FDA for polyneuropathy associated with transthyretin amyloidosis [147] and Duchenne muscular dystrophy [[148], [149], [150]]. An even wider range of ASOs are being evaluated in preclinical studies and clinical trials for neurodegenerative diseases. In Alzheimer's disease, for example, an ASO (BIIB080) directly targeting the accumulation of tau is being explored. Phase I data (N = 46 in 4 cohorts receiving escalating doses of drug by serial intrathecal injection) showed no serious adverse events along with detectable reduction in tau [151,152], and is currently enrolling in a Phase II trial (NCT05399888).
However, it is important to note that several ASOs have been abandoned as treatment strategies due to challenges achieving beneficial outcomes. For example, in Huntington's disease, two ASO clinical trials were halted due to inability to demonstrate reduction in mutant huntingtin protein in one trial [153], and even worsened progression in another Phase III trial [154]. Additionally, trials of ASOs for ALS (tofersen, targeting SOD1, and BIIB078, targeting C9orf72) failed to meet primary endpoints in a Phase III [155] and Phase I trial (NCT03626012), respectively. Nevertheless, hints at efficacy in certain outcome measures has spurred additional future studies (e.g., NCT04768972 and NCT04494256).
A similar yet distinct approach, RNAi-based therapies, utilize double-stranded small interfering RNAs (siRNAs), short hairpin RNAs, or microRNAs (miRNA) to interface with and prevent translation of target mRNAs, reducing the expression of toxic or mutant proteins [156]. This approach leverages natural cellular mechanisms that control gene activity. RNAi-based therapies function in the cytoplasm by promoting the formation of a mature RNA-induced silencing complex (RISC), which includes enzymes (an endonuclease) and an antisense siRNA strand to induce degradation of the target mRNA. Like ASOs, RNAi-based therapies require high specificity, stability, and bioavailability, but challenges in this latter category have necessitated the need for siRNA delivery using lipid, polymer, or viral carrier vectors (see below).
Preclinical studies demonstrate the feasibility of targeting specific genes associated with neurological diseases, including ALS [157], Alzheimer's disease [158,159] and Huntington's diseases [160]. For example, superoxide dismutase 1 (SOD1) mutations are implicated in a subset of ALS patients [161]. Administering anti-SOD1 miRNA therapy using an AAV vector was recently trialed in two patients. SOD1 protein transiently decreased at week 8 in the cerebrospinal fluid in one subject and did not show any change in the second subject. Interestingly, despite the spinal fluid levels, the first subject showed highly diminished SOD1 protein expression and enzymatic activity in postmortem analysis when compared to other ALS patients and healthy controls [162]. Following from this, a clinical trial is currently underway to assess safety, tolerability, and efficacy of a single intrathecal dose of AMT-162, an AAVrh10 vector carrying a miRNA targeting SOD1 mRNA, in ALS participants (NCT06100276). Additionally, the development of the first FDA-approved RNAi therapeutic patisiran marks a significant milestone in the clinical application of RNAi therapy [163]. Patisiran targets and degrades the underlying cause of hereditary transthyretin amyloidosis (hATTR), the transthyretin (TTR) protein, thereby reducing the accumulation of amyloid deposits in various tissues. Patisiran's success paved the way for the development of vutrisiran, another RNAi-based therapy targeting TTR which was deemed efficacious and well-tolerated for treatment of hATTR in a recent Phase III clinical trial [164]. The progress made thus far underscores the promising future of RNAi-based interventions in the treatment of neurologic disorders.
Human genome editing tools
Recent technological advances in genome editing tools have similarly facilitated the alteration of DNA in a targeted, permanent, and specified manner, providing a whole new realm of gene therapy opportunities [124,165,166]. The premise relies on introducing a nuclease to cut genomic DNA at a specified target site, and then harnessing natural DNA repair mechanisms to repair the break in the double-stranded DNA. Innate repair mechanisms of double-stranded DNA breaks include non-homologous end joining or homology-directed repair. Non-homologous end joining may introduce errors at the repair site, and these small insertions or deletions (indels) may be utilized to knockdown a gene or sometimes restoring a wild-type sequence. Homology-directed repair carries a higher chance of gene correction or gene insertion, by providing a donor DNA template to influence the process of double-strand break repair [167].
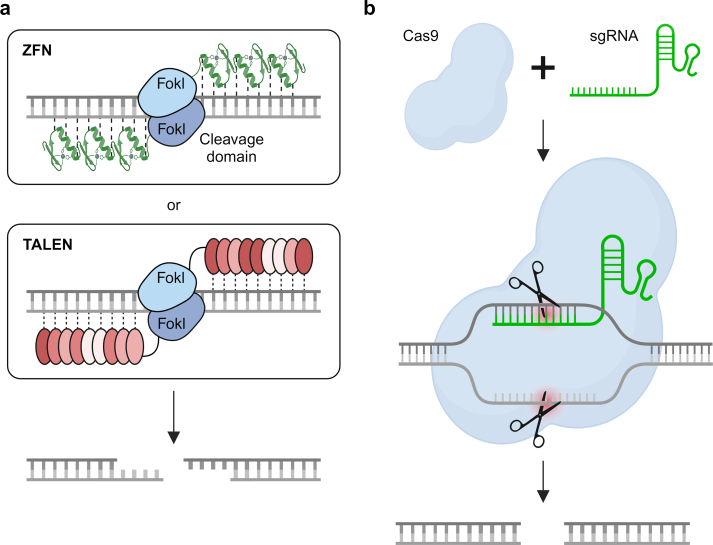
Nuclease-based targeted genome editing utilizes protein and RNA guides to direct the nuclease to a precise genomic site (Fig. 3) [124]. Protein-guided approaches include zinc finger nucleases (ZFNs) and transcription activator-like effector (TALE) nucleases (TALENs). ZFNs use zinc finger DNA recognition motifs which bind with 3 base pairs in DNA. Since the characterization of early, naturally occurring zinc finger sequences, a wide array of engineered sequences combined with the modular assembly of multiple zinc finger motifs in a single polypeptide have enabled the selective recognition of unique sequences (about 9–18 base pairs) in the genome [[168], [169], [170]]. These customized zinc finger domains can be coupled with the FokI restriction endonuclease to generate double stranded breaks in DNA (two FokI proteins functioning as a dimer are required, and therefore must be guided by two separate zinc finger binding peptides in non-palindromic sequences, see Fig. 3) [166]. Similarly, TALENS use TALEs to guide the nuclease FokI to the target site and generate a staggered double-stranded break. TALEs are 33–35 amino acid domains that can recognize a specific, single base pair, depending on which two amino acids are present at positions 12 and 13 in the polypeptide chain. Specific TALEs can then be linked together to target a specific genomic sequence [171,172], which can then be linked to FokI or other effector domains to implement DNA breaks in a highly targeted fashion [173]. The highly customizable advantage of TALENs is offset by the large peptide sequences (and therefore parental nucleic acid sequences) required, which may hamper delivery in in vivo settings [166].
Fig. 3.
Genome editing tools. The two major classes of genome editing tools can be either protein- (a) or RNA-guided (b). (a) Top. Each zinc finger nuclease (ZFN) consists of a DNA-binding domains composed of zinc finger motifs, which can be engineered to recognize a desired DNA sequence, and a nuclease domain derived from the Fokl endonuclease. When a pair of ZFNs bind to adjacent target sequences within the genome, the Fokl nuclease domains dimerize, forming an active site that cleaves the DNA, creating a double-stranded break. Bottom. Transcription activator-like effector nucleases (TALENs) consist of a customizable DNA-binding domain derived from transcription activator-like effectors (TALEs) fused to a nuclease domain, often derived from the Fokl endonuclease. The DNA-binding domain can be engineered to recognize specific DNA sequences. When a pair of TALENs binds to adjacent target sites within the genome, the Fokl nuclease domains dimerize, leading to the formation of a double-stranded break in the DNA. (b) Cas9 is an endonuclease enzyme that, guided by a short RNA molecule known as single guide RNA (sgRNA), targets specific DNA sequences complementary to the sgRNA. When the Cas9-sgRNA complex binds to its target DNA, Cas9 introduces a double-stranded break in the DNA. Image created using BioRender.com.
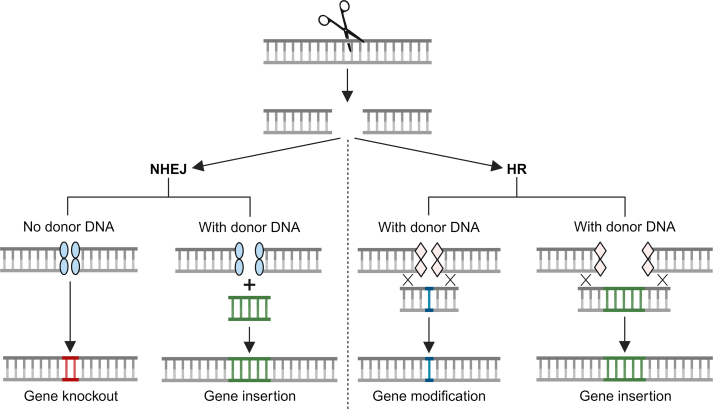
More recently, the RNA-guided approach using the CRISPR-Cas9 (clustered regularly interspaced short palindromic repeats-CRISPR associated protein 9) system has gained widespread popularity, where a single guide RNA that contains the complementary nucleotide sequence for the target directs the Cas9 nuclease, which creates a bunt-ended double-strand DNA break [174]. The double-stranded DNA breaks are then repaired by either non-homologous end joining or homologous directed repair, which can introduced desired genomic manipulations as outlined above (Fig. 4) [124].
Fig. 4.
DNA repair mechanisms during genome editing. The genome editing nuclease (scissor) cleaves the target DNA, resulting in a double-stranded DNA (dsDNA) break. Subsequently, DNA repair occurs through either nonhomologous end joining (NHEJ) or by homologous recombination (HR), provided a homologous strand is available. In NHEJ, host repair proteins (blue ovals) are recruited to the dsDNA break, often resulting in imperfect repair and the introduction of insertions or deletions (indels). These indels cause frameshift mutations and premature stop codons, leading to the production of truncated and inactive proteins, effectively knocking out the gene. In the presence of donor DNA, gene insertion can also occur. Alternatively, if a complementary homologous strand (indicated by ‘X’ symbols) is present, host HR repair proteins (pink rhombuses) are recruited to the dsDNA break. With a donor DNA template, DNA from the homologous template strand, such as a gene of interest, can integrate into the host DNA, facilitating gene modification or insertion. Image created using BioRender.com.(For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article).
Interest in genome editing approaches is continually expanding, and there are several applications for neurologic disorders currently under investigation using the strategies introduced above [175]. In preclinical studies, ZFNs and TALENs demonstrate efficacy in modifying genes associated with neurological disorders, such as tau for Alzheimer's disease and huntingtin for Huntington's disease, providing proof-of-concept for their potential therapeutic applications [[176], [177], [178]]. Following its discovery, CRISPR has essentially supplanted ZFN and TALEN methods due to its simplicity, efficiency, and versatility [174]. Ababneh et al. [179] recently demonstrated that deleting the C9orf72 repeat region using CRISPR-Cas9 in an iPSC line derived from an ALS patient improves pathological phenotypes in iPSC-derived motor neurons. In a groundbreaking development, the FDA granted approval for the first CRISPR-based therapy for sickle cell disease in 2023, marking a historic milestone in the field of genetic medicine and offering patients new hope [180]. However, the application of genome editing in the clinical setting, especially for neurological disorders, is still limited and evolving, and most approaches apply genome editing tools ex vivo to model disease or modify a cell therapy prior to transplantation (discussed further in the following section). Enhanced technologies, such as base or prime editing, which employs a catalytically impaired Cas9 protein, a reverse transcriptase, and a prime editing guide RNA to facilitate targeted deletions, insertions, or repair without the need for double-strand DNA breaks, are also being investigated [181,182]. The base and prime editing techniques are relatively new to the field, but the potential applications for this or related approaches for precision gene therapy are enticing.
Delivery of nucleotide-based therapies
The majority of nucleotide-based approaches rely on delivery of nucleic acids or genome editing tools via viral or non-viral delivery systems [183]. Strategies employing a viral vector are most common in gene therapy trials for neurologic disorders and include adenovirus, adeno-associated virus (AAV), and lentivirus-based methods [184]. Importantly, the loading capacity, transduction efficiency, immunogenicity, stability of resulting transgene expression, and safety record of the various systems inform their clinical utility [183]. Non-viral methods have included various methods of protecting and introducing a genetic payload to the nucleus, including via nanoparticles, lipid complexes, or cationic polymers [185,186]. However, to date, these methods have not shown wide success in persistent integration and transgene expression in clinical settings involving neurological disorders.
As described in more detail above, nucleotide-based therapies also face the same challenges in crossing the blood brain barrier as stem cells, with the addition of host immune mechanisms designed to clear viral vectors. If a wider effect is desired (e.g., ASOs or RNA-based therapy), often intrathecal administration is chosen. Intracerebral or intraspinal injection is utilized when neuroanatomic specificity is required. Focused ultrasound for BBB opening has also shown promise, at least in animal models, in enabling delivery of DNA, RNA, or viral vectors [[187], [188], [189], [190], [191], [192], [193]], and has even been applied to spinal cord [194].
Viral-based vectors
Adenoviruses are a family of DNA viruses that offer several advantages as viral vectors for gene therapy, including their ability to efficiently transduce a wide range of cell types, both dividing and non-dividing, making them versatile delivery vehicles [184]. They can also accommodate large transgenes and have relatively high transduction efficiency, making them effective for delivering therapeutic genes. Additionally, adenoviral vectors do not integrate into the host genome, reducing the risk of insertional mutagenesis. However, this transient expression may necessitate repeated administrations for sustained therapeutic effects. In practice, this can be a significant challenge, since adenoviruses can induce significant immune responses in the host, limiting duration of gene expression, and potentially triggering adverse reactions [195]. As such, the pro-inflammatory nature of adenovirus has found utility in vaccine and cancer-related applications, but has made less progress in application for neurological disorders [196].
In contrast, AAVs are highly studied, small viruses that carry single-stranded DNA and depend on co-infection with a helper virus for the machinery needed to replicate and express its own genes [128]. Shortcomings of AAVs include their limited loading capacity given their small genomes [128]. However, AAVs carry many advantages over the larger adenovirus; as such, they have become a favored vector in the gene therapy/nucleotide therapy space [120,128,197]. The native AAV genome carries replication genes (REP), capsid genes (CAP), and the assembly-activating protein (AAP). Their ability to integrate into host genomes (facilitated by REP proteins) has been exploited in in vitro and preclinical studies for sustained expression of transgenes. In this context, AAVs typically integrate in the AAVS1 locus which is considered a “safe harbor,” as it does not disrupt normal cellular function [198,199]. However, AAV does integrate into other sites in the genome, including functional genes (particularly studied in mouse models) [200], and there is some concern of a link between wild-type AAV (specifically AAV2) and hepatocellular carcinoma [201]. To date, the preponderance of data and clinical experience in humans does not suggest an association between AAV gene insertion and oncogenic events [202]. With these properties in mind, the first application of an AAV vector for gene therapy in a neurological disease was the transfer of the intact aspartoacylase gene using AAV2 in Canavan disease via intraparenchymal injection [203,204].
Most modern AAV vectors remove the REP- and CAP-encoding genes in the process of inserting transgene cargo, rendering the virus replication deficient [197]. These recombinant AAV then express their genetic cargo episomally, reducing the risk of genomic disruption. A potential drawback with this approach is dilution/clearance of episomal genetic material in dividing cells, or the risk of eventual attenuation of gene expression in non-dividing cells. However, the lack of genome integration and ability to limit viral replication (and ability to insert larger genetic payloads) has led to many investigative trials focusing on recombinant AAV vectors. Additionally, some consideration may be given to the particular AAV serotype that is employed, as this can make an impact on cellular tropism, intracellular behavior, and even host immune response. For example, AAV9 is able to cross the BBB and has a propensity for neurons [205], and the AAV9 capsid is utilized to carry the self-complementary sequence used in onasemnogene abeparvovec [206], mentioned above, as well as a predominance of current gene therapy trials [207]. Other serotypes being explored include AAV1 in frontotemporal dementia and progranulin mutations (NCT04747431), AAVrh10 in Alzheimer's disease (NCT03634007), or AAVhu68 in Krabbe disease [208] and GM1 gangliosidosis (NCT04713475).
Lentiviruses are a type of retrovirus that have high tropism for both dividing and non-dividing cells and can carry relatively large amounts of DNA, thus enabling multi-gene expression [209]. They integrate into the genome, supporting long-lasting expression, albeit with a concomitant risk for insertional mutagenesis. However, lentiviruses have a proven safety record, especially among newer-generation vectors, and their application includes restoration of local dopamine production in Parkinson's disease patients through expression of the dopamine synthesis enzymes tyrosine hydroxylase, aromatic L-amino acid decarboxylase, and cyclohydrolase 1 as observed in a recent Phase I/II clinical trial [140,210].
Additional viral vectors include those derived from herpes simplex virus (HSV), predominantly finding applications as oncolytic viral treatment of brain tumors which is outside the scope of this review, but has found some early application for chronic pain [211].
Non-viral approaches
The constraints associated with viral-based gene therapy tools, such as limited cargo capacity, immunogenicity, and potential safety concerns, spurred extensive research into the development of non-viral gene therapy vehicles, including polymer-, lipid-, and peptide-based delivery methods [212]. Advantages of these non-viral delivery strategies are their relatively low immunogenicity and cytotoxicity and a high loading capacity. However, these approaches are limited by low transfection efficiency, low specificity, and transient transgene expression, particularly within intricate neural networks. Direct nucleotide delivery requires an approach to introduce the material past the cell membrane, e.g., by injection or photoporation [213], while polymer- and lipid-based strategies typically employ endocytosis [214]. Polymer-based approaches complex negatively charged genetic material with a cationic polymer to transport the material into the target cell [183]. Non-biodegradable polymers are available but exhibit low specificity and can induce cytotoxicity [215]. Alternatively, synthetic or natural biodegradable polymers are more versatile and less cytotoxic, supporting repeat administration, but require attention to product quality and control [215]. Lipid-based delivery vectors offer another alternative whereby negatively charged nucleic acids bind to the positively charged lipid head groups and assemble into liposomes or other lipid-based carrier structures [216]. Lipid-based vectors are biodegradable, exhibit low toxicity, and can complex with hydrophobic or hydrophilic compounds. Finally, peptide nucleic acid conjugates and polypeptide-based complexes, including those using poly(L-lysine) and poly(L-glutamine), are biodegradable, relatively stable, and biocompatible, facilitating transfer of large DNA molecules [217]. While some iterations suffer from low transfection capacity, newer versions employing different functional peptides enhance targeting efficiency and transport and show preclinical promise. Collectively, these strategies, and hybrid combination strategies using the above approaches, support the delivery of the necessary genetic materials or tools for gene therapy development.
CNS delivery
One of the foremost challenges in gene therapy for neurological disorders remains the same as for stem cell therapy (discussed above), and this lies in overcoming the BBB. While direct administration, such as intracerebral injection, offers a localized approach, it poses risks of tissue damage and infection. Therefore, several strategies can be employed to ensure transport across the BBB and targeted delivery to neural tissues following systemic administration. Surface modifications with ligands that exhibit affinity for specific receptors on neuronal cells or the endothelial cells of the BBB, such as transferrin or LRP-1, enhance receptor-mediated neuronal uptake or transcytosis [218,219]. For example, Bender et al. [220] recently developed a liposome-siRNA-peptide complex that bypasses the BBB in mice and targets nicotinic acetylcholine receptor-expression neuronal cells using a modified peptide derived from rabies virus glycoprotein. Additionally, incorporating cell-penetrating peptides into liposomes facilitates direct penetration through the BBB, enabling effective gene delivery to neural cells [221]. Further, the incorporation of stimuli-responsive materials, such as pH-sensitive polymers or environmental-responsive nanoparticles, enables controlled release of gene payloads within the brain microenvironment [214,222]. Lastly, the exploitation of physical approaches like focused ultrasound, magnetic targeting, or microbubble-assisted delivery transiently disrupt the BBB, enhancing the penetration of non-viral vectors and improving gene delivery efficiency [[223], [224], [225]]. The combination of these mechanisms in non-viral delivery systems demonstrates a multifaceted approach to effectively navigate the complexities of the BBB.
Genetically Modified Cell Therapies
As discussed above, harnessing stem cells holds immense potential for regenerating damaged tissue and leveraging the complete biological repertoire of cells to address pathology, including protein secretion, toxin endocytosis, and establishment of crucial cell-cell contacts, among other essential biological processes. Applying cutting-edge advances in genetic manipulation to stem cell products provides an added layer of benefit. Inherent mutations or polymorphisms in patient-derived stem cells (e.g., MSCs, adult stem cells, iPSCs) can be corrected ex vivo using the above editing tools, prior to autologous transplantation. Alternatively, established stem cell lines can be augmented using genetic manipulation to produce “off the shelf” cell lines that combat pathology or rescue native cells.
Early success has already been seen in combining cell-based therapy with genetic modification. Elivaldogene autotemcel [226,227] is a therapy for cerebral adrenoleukodystrophy, a demyelinating manifestation of adrenoleukodystrophy caused by mutations in the ABCD1 gene, and can lead to inflammatory demyelination. It is based on modification of autologous CD34+ stem cells obtained by apheresis, then genetically modified ex vivo using a Lentiviral vector carrying wild-type ABCD1 cDNA. The modified cells are re-infused intravenously after myeloablative conditioning, with the genetically corrected cells engrafting into host bone marrow and producing cells carrying a functioning gene. A similar approach is used with atidarsagene autotemcel, in which autologous hematopoietic stem cells are modified to express normal arylsulfatase A for the treatment of metachromatic leukodystrophy [228]. Similar approaches are being studied for Hurler syndrome [229] and Sanfilippo syndrome [230], mucopolysaccharidoses which have significant neurologic manifestations.
As demonstrated by the pioneering therapies above, genetically modified stem cells may serve as “factories” that continually produce enzymes, nucleic acids, extracellular vesicles, or other macromolecules that may be difficult to administer peripherally (e.g., past a blood-organ barrier), cannot be easily stored, or require steady exposure over prolonged periods. In preclinical models, researchers have successfully modified stem cells to secrete a variety of trophic, anti-inflammatory, and survival factors (e.g., insulin-like growth factor 1 [IGF-1], brain derived neurotrophic factor, and vascular endothelial growth factor) [16]. The modified cells, once introduced into the affected regions of the nervous system, continuously produce and release these neurotrophic factors, creating a conducive microenvironment for nerve regeneration and protection [1]. McGinley et al. [231] successfully established an IGF-1-expressing human cortex-derived NSC population and demonstrated that transplantation of these cells into the fimbria fornix of the hippocampus of Alzheimer's disease mice restores spatial memory [232]. In Parkinson's disease, accumulation of advanced glycation end products-albumin upregulates the receptor for AGE, triggering dopamine neuron apoptosis [233]. However, this detrimental process is mitigated by the soluble receptor to AGE, which umbilical cord-derived MSCs can be engineered to secrete using CRISPR-Cas9 technology [234]. Transplantation of these modified cells into the corpus striatum of a Parkinson's disease mouse model reduces neuronal cell death and also improves movement, highlighting the therapeutic potential of this innovative approach in ameliorating neurodegeneration in Parkinson's disease.
Trials using modified cell therapies have taken early steps in the clinical realm. For example, in Alzheimer's disease, fibroblasts obtained from 8 subjects were modified ex-vivo to express NGF using a Moloney leukemia viral vector, then transplanted in an autologous fashion via intracerebral injection targeting the nucleus basalis of Meynert [235]. Two subjects had severe adverse events related to the surgical procedure, but none related to the NGF-delivering cells directly. In the realm of ALS, a trial of neural progenitor cells modified using a lentiviral vector to express glial-derived neurotrophic factor (CNS10-NPC-GDNF) was conducted in 18 subjects by intraspinal injection (n = 9 in a low dose 2 × 106 cells group, and n = 9 in a high dose 5 × 106 cells groups, without sham control) [236]. There was a trend toward improved motor function in the limb ipsilateral to cell treatment, with some limitation due to suspicion that virus may not have penetrated fully into the ventral horn as well as one participant with strong immune response. A trial of the same cells directed toward motor cortex is ongoing (NCT05306457).
By utilizing inducible gene expression systems, researchers have also engineered stem cells that respond to external stimuli, allowing for temporal and spatial control of their activities. This can then be used to prevent incomplete/excessive therapeutic outcomes and potential side effects. For example, a concern regarding the transplantation of dopamine-producing stem cells into the brains of individuals with Parkinson's disease is the potential for dopamine overproduction, leading to a phenomenon known as dyskinesias or increased, involuntary movements [27,237]. Human pluripotent stem cells expressing both inhibitory and excitatory DREADD (Designer Receptors Exclusively Activated by Designer Drugs) systems, a molecular tool that uses modified G protein-coupled receptors to selectively and temporally control neural activity in response to specific synthetic ligands, were created using CRISPR-Cas9 technology [238]. These DREADD-expressing human pluripotent stem cells were then differentiated into midbrain dopaminergic neurons and transplanted into a mouse model of Parkinson's disease. Researchers discovered that transplanted cells effectively rescued motor defects and graft function, including motor behaviors, which was modifiable under DREADD control. This innovative approach could provide targeted and adaptive therapies, minimizing off-target effects and enhancing treatment efficacy.
Additionally, the success of stem cell transplantation is often hindered by the risk of rejection mediated by human leukocyte antigen (HLA) complexes. Given the diversity of HLA complexes among individuals, transplanted stem cells may be recognized as foreign by the recipient's immune system, leading to graft rejection [239]. Using CRISPR-Cas9 gene editing tools, researchers are modifying HLA complexes in donor stem cells to render them immunocompatible with the recipient, thereby promoting successful integration and minimizing the immunological barriers associated with stem cell transplantation [240].
Finally, the holy grail of stem cell and regenerative therapy is to reconstitute a damaged cell population, and genetic modification ex vivo is likely required to achieve this. For instance, in diseases like Huntington's, where specific genetic mutations contribute to the pathology, modifying stem cells ex vivo can address the underlying genetic aberrations before transplantation, potentially slowing or halting disease progression. While there has been much success for this in bone marrow stem cell transplant, hematologic diseases, and cancers, the landscape for stem cell therapeutics for neurologic disorders remains fertile for discovery and therapeutic opportunity.
The exploration of stem cells and gene therapy, both independently and in synergy, represents a profound frontier in the quest to address the complexities of neurologic disorders. Superficially outlined above, cutting edge concepts in stem cell biology and nucleotide based therapies are now reaching the clinic for a variety of neurological diseases. Notably, only a handful of Phase III placebo-controlled trials have demonstrated success. Given the considerations given to route of administration (with some that are more invasive and difficult to blind, or ethical dilemmas when designing trials integrating sham surgery), the placebo effect must be considered and temper enthusiasm when evaluating early phase trials [241,242]. For example, in a number of Parkinson's disease stem cell trials, sham surgical groups appeared to have equivalent improvements when compared to treatment groups [243,244]. In one study, the subjects' perception of treatment was correlated with improved quality of life and even influenced medical staff objective measures, regardless of actual stem cell transplant versus sham surgery [245]. There is even discussion of whether the neurobiological basis of the placebo effect itself can mediate therapeutic benefit, when taken in the context of disorders of neural circuit and neurotransmitters [246]. Ultimately, while the ethics of placebo-controlled trials in neurological diseases remains debated and alternatives are explored especially for rare diseases and vulnerable populations, the randomized, double-blinded, placebo-controlled Phase III trial remains the gold standard when evaluating the efficacy of a clinical intervention.
As the collective knowledge in stem cell biology and gene editing continues to expand, so does the potential for innovative therapeutic interventions. The capacity of stem cells to propagate and differentiate, coupled with the precision of genetic manipulation, offers a versatile toolkit for reshaping cellular physiology and impacting the course of neurologic disorders. From restoring damaged neural circuits to addressing the underlying genetic anomalies, these advancements hold promise for transforming the landscape of neurology. The intersection of these technologies holds immense potential to deepen our understanding of neurologic disorders as well as pave the way for novel interventions that restore human neurological health.
Author Contributions
All authors contributed to the conception and design of the manuscript. KSC, SAS, and EJK contributed to acquisition of data, writing of the manuscript, and preparation of figures and tables. All authors contributed to reviewing/editing and final approval of the manuscript.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Eva L. Feldman reports financial support was provided by National Institutes of Health. Kevin S. Chen reports financial support was provided by Alzheimer's Association. Kevin S. Chen reports financial support was provided by Frances and Kenneth Eisenberg Emerging Scholar Fund. Eva L. Feldman reports financial support was provided by Robert and Katherine Jacobs Environmental Health Initiative. Eva L. Feldman reports financial support was provided by Andrea and Lawrence A. Wolfe Brain Health Initiative. Eva L. Feldman reports financial support was provided by Richard and Jane Manoogian Foundation. Eva L. Feldman reports financial support was provided by Frank L. and Helen Gofrank Foundation Research Program in Alzheimer's Disease and Brain Health. Eva L. Feldman reports financial support was provided by Kiriluk Family Fund for Brain Health Research. Eva L. Feldman reports financial support was provided by Taubman Foundation. Eva L. Feldman reports financial support was provided by NeuroNetwork for Emerging Therapies. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors received funding support from the National Institutes of Health (U01AG057562 to ELF), the Alzheimer's Association (AACSF-22-970586 to KSC), the Frances and Kenneth Eisenberg Emerging Scholar Fund (to KSC), the Robert and Katherine Jacobs Environmental Health Initiative, the Andrea and Lawrence A. Wolfe Brain Health Initiative, Richard and Jane Manoogian Foundation, Frank L. and Helen Gofrank Foundation Research Program in Alzheimer's Disease and Brain Health, Kiriluk Family Fund for Brain Health Research, the Taubman Foundation, and the NeuroNetwork for Emerging Therapies.
References
- 1.Puranik N., Arukha A.P., Yadav S.K., Yadav D., Jin J.O. Exploring the role of stem cell therapy in treating neurodegenerative diseases: challenges and current perspectives. Curr Stem Cell Res Ther. 2022;17(2):113–125. doi: 10.2174/1574888X16666210810103838. [DOI] [PubMed] [Google Scholar]
- 2.Rahman M.M., Islam M.R., Islam M.T., Harun-Or-Rashid M., Islam M., Abdullah S., et al. Stem cell transplantation therapy and neurological disorders: current status and future perspectives. Biology. 2022;11(1) doi: 10.3390/biology11010147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morris G., Schorge S. Gene therapy for neurological disease: state of the art and opportunities for next-generation approaches. Neuroscience. 2022;490:309–314. doi: 10.1016/j.neuroscience.2022.03.010. [DOI] [PubMed] [Google Scholar]
- 4.Xie Y.X., Lv W.Q., Chen Y.K., Hong S., Yao X.P., Chen W.J., et al. Advances in gene therapy for neurogenetic diseases: a brief review. J Mol Med (Berl) 2022;100(3):385–394. doi: 10.1007/s00109-021-02167-y. [DOI] [PubMed] [Google Scholar]
- 5.Hoang D.M., Pham P.T., Bach T.Q., Ngo A.T.L., Nguyen Q.T., Phan T.T.K., et al. Stem cell-based therapy for human diseases. Signal Transduct Targeted Ther. 2022;7(1):272. doi: 10.1038/s41392-022-01134-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mohebichamkhorami F., Fattahi R., Niknam Z., Aliashrafi M., Khakpour Naeimi S., Gilanchi S., et al. Periodontal ligament stem cells as a promising therapeutic target for neural damage. Stem Cell Res Ther. 2022;13(1):273. doi: 10.1186/s13287-022-02942-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jovanovic V.M., Weber C., Slamecka J., Ryu S., Chu P.H., Sen C., et al. A defined roadmap of radial glia and astrocyte differentiation from human pluripotent stem cells. Stem Cell Rep. 2023;18(8):1701–1720. doi: 10.1016/j.stemcr.2023.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Assis A.C.C., Reis A.L.S., Nunes L.V., Ferreira L.F.R., Bilal M., Iqbal H.M.N., et al. Stem cells and tissue engineering-based therapeutic interventions: promising strategies to improve peripheral nerve regeneration. Cell Mol Neurobiol. 2023;43(2):433–454. doi: 10.1007/s10571-022-01199-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li J., Luo W., Xiao C., Zhao J., Xiang C., Liu W., et al. Recent advances in endogenous neural stem/progenitor cell manipulation for spinal cord injury repair. Theranostics. 2023;13(12):3966–3987. doi: 10.7150/thno.84133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen K.S., Feldman E.L. In: Molecular and cellular therapies for motor neuron diseases. Boulis N., O'Connor D., Donsante A., editors. Academic Press; 2017. Stem cell therapy for amyotrophic lateral sclerosis; pp. 207–231. [Google Scholar]
- 11.Menendez P., Bueno C., Wang L., Bhatia M. Human embryonic stem cells: potential tool for achieving immunotolerance? Stem Cell Rev. 2005;1(2):151–158. doi: 10.1385/SCR:1:2:151. [DOI] [PubMed] [Google Scholar]
- 12.Drukker M., Katz G., Urbach A., Schuldiner M., Markel G., Itskovitz-Eldor J., et al. Characterization of the expression of MHC proteins in human embryonic stem cells. Proc Natl Acad Sci U S A. 2002;99(15):9864–9869. doi: 10.1073/pnas.142298299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ben-David U., Benvenisty N. The tumorigenicity of human embryonic and induced pluripotent stem cells. Nat Rev Cancer. 2011;11(4):268–277. doi: 10.1038/nrc3034. [DOI] [PubMed] [Google Scholar]
- 14.Keene C.D., Chang R.C., Leverenz J.B., Kopyov O., Perlman S., Hevner R.F., et al. A patient with Huntington's disease and long-surviving fetal neural transplants that developed mass lesions. Acta Neuropathol. 2009;117(3):329–338. doi: 10.1007/s00401-008-0465-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murugan V. Embryonic stem cell research: a decade of debate from Bush to Obama. Yale J Biol Med. 2009;82(3):101–103. [PMC free article] [PubMed] [Google Scholar]
- 16.Lunn J.S., Sakowski S.A., Hur J., Feldman E.L. Stem cell technology for neurodegenerative diseases. Ann Neurol. 2011;70(3):353–361. doi: 10.1002/ana.22487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ballini A., Cantore S., Scacco S., Coletti D., Tatullo M. Mesenchymal stem cells as promoters, enhancers, and playmakers of the translational regenerative medicine 2018. Stem Cell Int. 2018;2018 doi: 10.1155/2018/6927401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bonfanti L., La Rosa C., Ghibaudi M., Sherwood C.C. Adult neurogenesis and “immature” neurons in mammals: an evolutionary trade-off in plasticity? Brain Struct Funct. 2023 doi: 10.1007/s00429-023-02717-9. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McIntyre L.L., Greilach S.A., Othy S., Sears-Kraxberger I., Wi B., Ayala-Angulo J., et al. Regulatory T cells promote remyelination in the murine experimental autoimmune encephalomyelitis model of multiple sclerosis following human neural stem cell transplant. Neurobiol Dis. 2020;140 doi: 10.1016/j.nbd.2020.104868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen K.S., Noureldein M.H., McGinley L.M., Hayes J.M., Rigan D.M., Kwentus J.F., et al. bioRxiv.; 2023. Human neural stem cells restore spatial memory in a transgenic Alzheimer's disease mouse model by an immunomodulating mechanism. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhong S.J., Gong Y.H., Lin Y.C. Combined intranasal nerve growth factor and ventricle neural stem cell grafts prolong survival and improve disease outcome in amyotrophic lateral sclerosis transgenic mice. Neurosci Lett. 2017;656:1–8. doi: 10.1016/j.neulet.2017.07.005. [DOI] [PubMed] [Google Scholar]
- 22.Piccini P., Brooks D.J., Bjorklund A., Gunn R.N., Grasby P.M., Rimoldi O., et al. Dopamine release from nigral transplants visualized in vivo in a Parkinson's patient. Nat Neurosci. 1999;2(12):1137–1140. doi: 10.1038/16060. [DOI] [PubMed] [Google Scholar]
- 23.Freed C.R., Breeze R.E., Rosenberg N.L., Schneck S.A., Kriek E., Qi J.X., et al. Survival of implanted fetal dopamine cells and neurologic improvement 12 to 46 months after transplantation for Parkinson's disease. N Engl J Med. 1992;327(22):1549–1555. doi: 10.1056/NEJM199211263272202. [DOI] [PubMed] [Google Scholar]
- 24.Lindvall O., Rehncrona S., Brundin P., Gustavii B., Astedt B., Widner H., et al. Human fetal dopamine neurons grafted into the striatum in two patients with severe Parkinson's disease. A detailed account of methodology and a 6-month follow-up. Arch Neurol. 1989;46(6):615–631. doi: 10.1001/archneur.1989.00520420033021. [DOI] [PubMed] [Google Scholar]
- 25.Parmar M., Grealish S., Henchcliffe C. The future of stem cell therapies for Parkinson disease. Nat Rev Neurosci. 2020;21(2):103–115. doi: 10.1038/s41583-019-0257-7. [DOI] [PubMed] [Google Scholar]
- 26.Olanow C.W., Goetz C.G., Kordower J.H., Stoessl A.J., Sossi V., Brin M.F., et al. A double-blind controlled trial of bilateral fetal nigral transplantation in Parkinson's disease. Ann Neurol. 2003;54(3):403–414. doi: 10.1002/ana.10720. [DOI] [PubMed] [Google Scholar]
- 27.Freed C.R., Greene P.E., Breeze R.E., Tsai W.Y., DuMouchel W., Kao R., et al. Transplantation of embryonic dopamine neurons for severe Parkinson's disease. N Engl J Med. 2001;344(10):710–719. doi: 10.1056/NEJM200103083441002. [DOI] [PubMed] [Google Scholar]
- 28.Barker R.A., consortium T. Designing stem-cell-based dopamine cell replacement trials for Parkinson's disease. Nat Med. 2019;25(7):1045–1053. doi: 10.1038/s41591-019-0507-2. [DOI] [PubMed] [Google Scholar]
- 29.Kirkeby A., Nelander J., Hoban D.B., Rogelius N., Bjartmarz H., Novo Nordisk Cell Therapy R., et al. Preclinical quality, safety, and efficacy of a human embryonic stem cell-derived product for the treatment of Parkinson's disease. STEM-PD. Cell Stem Cell. 2023;30(10):1299–12314 e9. doi: 10.1016/j.stem.2023.08.014. [DOI] [PubMed] [Google Scholar]
- 30.Nolbrant S., Heuer A., Parmar M., Kirkeby A. Generation of high-purity human ventral midbrain dopaminergic progenitors for in vitro maturation and intracerebral transplantation. Nat Protoc. 2017;12(9):1962–1979. doi: 10.1038/nprot.2017.078. [DOI] [PubMed] [Google Scholar]
- 31.Piao J., Zabierowski S., Dubose B.N., Hill E.J., Navare M., Claros N., et al. Preclinical efficacy and safety of a human embryonic stem cell-derived midbrain dopamine progenitor product, MSK-DA01. Cell Stem Cell. 2021;28(2):217–229 e7. doi: 10.1016/j.stem.2021.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leone M.A., Gelati M., Profico D.C., Gobbi C., Pravata E., Copetti M., et al. Phase I clinical trial of intracerebroventricular transplantation of allogeneic neural stem cells in people with progressive multiple sclerosis. Cell Stem Cell. 2023;30(12):1597–15609 e8. doi: 10.1016/j.stem.2023.11.001. [DOI] [PubMed] [Google Scholar]
- 33.Glass J.D., Hertzberg V.S., Boulis N.M., Riley J., Federici T., Polak M., et al. Transplantation of spinal cord-derived neural stem cells for ALS: analysis of phase 1 and 2 trials. Neurology. 2016;87(4):392–400. doi: 10.1212/WNL.0000000000002889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goutman S.A., Brown M.B., Glass J.D., Boulis N.M., Johe K., Hazel T., et al. Long-term Phase 1/2 intraspinal stem cell transplantation outcomes in ALS. Ann Clin Transl Neurol. 2018;5(6):730–740. doi: 10.1002/acn3.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mazzini L., Gelati M., Profico D.C., Sgaravizzi G., Projetti Pensi M., Muzi G., et al. Human neural stem cell transplantation in ALS: initial results from a phase I trial. J Transl Med. 2015;13:17. doi: 10.1186/s12967-014-0371-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rao M.S., Noble M., Mayer-Proschel M. A tripotential glial precursor cell is present in the developing spinal cord. Proc Natl Acad Sci U S A. 1998;95(7):3996–4001. doi: 10.1073/pnas.95.7.3996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fok-Seang J., Miller R.H. Astrocyte precursors in neonatal rat spinal cord cultures. J Neurosci. 1992;12(7):2751–2764. doi: 10.1523/JNEUROSCI.12-07-02751.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ffrench-Constant C., Raff M.C. Proliferating bipotential glial progenitor cells in adult rat optic nerve. Nature. 1986;319(6053):499–502. doi: 10.1038/319499a0. [DOI] [PubMed] [Google Scholar]
- 39.Wu B., Guo S., Jiang T., Ren X. In vitro culture and characterization of oligodendrocyte precursor cells derived from neonatal rats. Neurol Res. 2011;33(6):593–599. doi: 10.1179/1743132810Y.0000000024. [DOI] [PubMed] [Google Scholar]
- 40.Windrem M.S., Roy N.S., Wang J., Nunes M., Benraiss A., Goodman R., et al. Progenitor cells derived from the adult human subcortical white matter disperse and differentiate as oligodendrocytes within demyelinated lesions of the rat brain. J Neurosci Res. 2002;69(6):966–975. doi: 10.1002/jnr.10397. [DOI] [PubMed] [Google Scholar]
- 41.Dromard C., Guillon H., Rigau V., Ripoll C., Sabourin J.C., Perrin F.E., et al. Adult human spinal cord harbors neural precursor cells that generate neurons and glial cells in vitro. J Neurosci Res. 2008;86(9):1916–1926. doi: 10.1002/jnr.21646. [DOI] [PubMed] [Google Scholar]
- 42.Fischer I., Dulin J.N., Lane M.A. Transplanting neural progenitor cells to restore connectivity after spinal cord injury. Nat Rev Neurosci. 2020;21(7):366–383. doi: 10.1038/s41583-020-0314-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McCrary M.R., Jesson K., Wei Z.Z., Logun M., Lenear C., Tan S., et al. Cortical transplantation of brain-mimetic glycosaminoglycan scaffolds and neural progenitor cells promotes vascular regeneration and functional recovery after ischemic stroke in mice. Adv Healthcare Mater. 2020;9(5) doi: 10.1002/adhm.201900285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guo Y., Wang Y.Y., Sun T.T., Xu J.J., Yang P., Ma C.Y., et al. Neural progenitor cells derived from fibroblasts induced by small molecule compounds under hypoxia for treatment of Parkinson's disease in rats. Neural Regen Res. 2023;18(5):1090–1098. doi: 10.4103/1673-5374.355820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bershteyn M., Broer S., Parekh M., Maury Y., Havlicek S., Kriks S., et al. Human pallial MGE-type GABAergic interneuron cell therapy for chronic focal epilepsy. Cell Stem Cell. 2023;30(10):1331–1350 e11. doi: 10.1016/j.stem.2023.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Madrazo I., Kopyov O., Avila-Rodriguez M.A., Ostrosky F., Carrasco H., Kopyov A., et al. Transplantation of human neural progenitor cells (NPC) into putamina of parkinsonian patients: a case series study, safety and efficacy four years after surgery. Cell Transplant. 2019;28(3):269–285. doi: 10.1177/0963689718820271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Quincozes-Santos A., Santos C.L., de Souza Almeida R.R., da Silva A., Thomaz N.K., Costa N.L.F., et al. Gliotoxicity and glioprotection: the dual role of glial cells. Mol Neurobiol. 2021;58(12):6577–6592. doi: 10.1007/s12035-021-02574-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Martins-Macedo J., Lepore A.C., Domingues H.S., Salgado A.J., Gomes E.D., Pinto L. Glial restricted precursor cells in central nervous system disorders: current applications and future perspectives. Glia. 2021;69(3):513–531. doi: 10.1002/glia.23922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gotkine M., Caraco Y., Lerner Y., Blotnick S., Wanounou M., Slutsky S.G., et al. Safety and efficacy of first-in-man intrathecal injection of human astrocytes (AstroRx(R)) in ALS patients: phase I/IIa clinical trial results. J Transl Med. 2023;21(1):122. doi: 10.1186/s12967-023-03903-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Walczak P., All A.H., Rumpal N., Gorelik M., Kim H., Maybhate A., et al. Human glial-restricted progenitors survive, proliferate, and preserve electrophysiological function in rats with focal inflammatory spinal cord demyelination. Glia. 2011;59(3):499–510. doi: 10.1002/glia.21119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Goldman S.A., Nedergaard M., Windrem M.S. Glial progenitor cell-based treatment and modeling of neurological disease. Science. 2012;338(6106):491–495. doi: 10.1126/science.1218071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Andrzejewska A., Dabrowska S., Lukomska B., Janowski M. Mesenchymal stem cells for neurological disorders. Adv Sci. 2021;8(7) doi: 10.1002/advs.202002944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Urrutia D.N., Caviedes P., Mardones R., Minguell J.J., Vega-Letter A.M., Jofre C.M. Comparative study of the neural differentiation capacity of mesenchymal stromal cells from different tissue sources: an approach for their use in neural regeneration therapies. PLoS One. 2019;14(3) doi: 10.1371/journal.pone.0213032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Watanabe Y., Tsuchiya A., Terai S. The development of mesenchymal stem cell therapy in the present, and the perspective of cell-free therapy in the future. Clin Mol Hepatol. 2021;27(1):70–80. doi: 10.3350/cmh.2020.0194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Koh S.H., Huh Y.M., Noh M.Y., Kim H.Y., Kim K.S., Lee E.S., et al. beta-PIX is critical for transplanted mesenchymal stromal cell migration. Stem Cell Dev. 2012;21(11):1989–1999. doi: 10.1089/scd.2011.0430. [DOI] [PubMed] [Google Scholar]
- 56.Cho G.W., Noh M.Y., Kim H.Y., Koh S.H., Kim K.S., Kim S.H. Bone marrow-derived stromal cells from amyotrophic lateral sclerosis patients have diminished stem cell capacity. Stem Cell Dev. 2010;19(7):1035–1042. doi: 10.1089/scd.2009.0453. [DOI] [PubMed] [Google Scholar]
- 57.Corti S., Locatelli F., Donadoni C., Guglieri M., Papadimitriou D., Strazzer S., et al. Wild-type bone marrow cells ameliorate the phenotype of SOD1-G93A ALS mice and contribute to CNS, heart and skeletal muscle tissues. Brain. 2004;127(Pt 11):2518–2532. doi: 10.1093/brain/awh273. [DOI] [PubMed] [Google Scholar]
- 58.Ohnishi S., Ito H., Suzuki Y., Adachi Y., Wate R., Zhang J., et al. Intra-bone marrow-bone marrow transplantation slows disease progression and prolongs survival in G93A mutant SOD1 transgenic mice, an animal model mouse for amyotrophic lateral sclerosis. Brain Res. 2009;1296:216–224. doi: 10.1016/j.brainres.2009.08.012. [DOI] [PubMed] [Google Scholar]
- 59.Martin L.J., Liu Z. Adult olfactory bulb neural precursor cell grafts provide temporary protection from motor neuron degeneration, improve motor function, and extend survival in amyotrophic lateral sclerosis mice. J Neuropathol Exp Neurol. 2007;66(11):1002–1018. doi: 10.1097/nen.0b013e318158822b. [DOI] [PubMed] [Google Scholar]
- 60.Heris R.M., Shirvaliloo M., Abbaspour-Aghdam S., Hazrati A., Shariati A., Youshanlouei H.R., et al. The potential use of mesenchymal stem cells and their exosomes in Parkinson's disease treatment. Stem Cell Res Ther. 2022;13(1):371. doi: 10.1186/s13287-022-03050-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Regmi S., Liu D.D., Shen M., Kevadiya B.D., Ganguly A., Primavera R., et al. Mesenchymal stromal cells for the treatment of Alzheimer's disease: strategies and limitations. Front Mol Neurosci. 2022;15 doi: 10.3389/fnmol.2022.1011225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Terashima T., Kojima H., Urabe H., Yamakawa I., Ogawa N., Kawai H., et al. Stem cell factor-activated bone marrow ameliorates amyotrophic lateral sclerosis by promoting protective microglial migration. J Neurosci Res. 2014;92(7):856–869. doi: 10.1002/jnr.23368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Corti S., Nizzardo M., Nardini M., Donadoni C., Salani S., Simone C., et al. Systemic transplantation of c-kit+ cells exerts a therapeutic effect in a model of amyotrophic lateral sclerosis. Hum Mol Genet. 2010;19(19):3782–3796. doi: 10.1093/hmg/ddq293. [DOI] [PubMed] [Google Scholar]
- 64.Sherman L.S., Romagano M.P., Williams S.F., Rameshwar P. Mesenchymal stem cell therapies in brain disease. Semin Cell Dev Biol. 2019;95:111–119. doi: 10.1016/j.semcdb.2019.03.003. [DOI] [PubMed] [Google Scholar]
- 65.Walczak P., Wojtkiewicz J., Nowakowski A., Habich A., Holak P., Xu J., et al. Real-time MRI for precise and predictable intra-arterial stem cell delivery to the central nervous system. J Cerebr Blood Flow Metabol. 2017;37(7):2346–2358. doi: 10.1177/0271678X16665853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.von Bahr L., Batsis I., Moll G., Hagg M., Szakos A., Sundberg B., et al. Analysis of tissues following mesenchymal stromal cell therapy in humans indicates limited long-term engraftment and no ectopic tissue formation. Stem Cell. 2012;30(7):1575–1578. doi: 10.1002/stem.1118. [DOI] [PubMed] [Google Scholar]
- 67.Koc O.N., Gerson S.L., Cooper B.W., Dyhouse S.M., Haynesworth S.E., Caplan A.I., et al. Rapid hematopoietic recovery after coinfusion of autologous-blood stem cells and culture-expanded marrow mesenchymal stem cells in advanced breast cancer patients receiving high-dose chemotherapy. J Clin Oncol. 2000;18(2):307–316. doi: 10.1200/JCO.2000.18.2.307. [DOI] [PubMed] [Google Scholar]
- 68.Pendharkar A.V., Chua J.Y., Andres R.H., Wang N., Gaeta X., Wang H., et al. Biodistribution of neural stem cells after intravascular therapy for hypoxic-ischemia. Stroke. 2010;41(9):2064–2070. doi: 10.1161/STROKEAHA.109.575993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Oh S.H., Choi C., Chang D.J., Shin D.A., Lee N., Jeon I., et al. Early neuroprotective effect with lack of long-term cell replacement effect on experimental stroke after intra-arterial transplantation of adipose-derived mesenchymal stromal cells. Cytotherapy. 2015;17(8):1090–1103. doi: 10.1016/j.jcyt.2015.04.007. [DOI] [PubMed] [Google Scholar]
- 70.Lee J.S., Hong J.M., Moon G.J., Lee P.H., Ahn Y.H., Bang O.Y., et al. A long-term follow-up study of intravenous autologous mesenchymal stem cell transplantation in patients with ischemic stroke. Stem Cell. 2010;28(6):1099–1106. doi: 10.1002/stem.430. [DOI] [PubMed] [Google Scholar]
- 71.Rowe R.G., Daley G.Q. Induced pluripotent stem cells in disease modelling and drug discovery. Nat Rev Genet. 2019;20(7):377–388. doi: 10.1038/s41576-019-0100-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Takahashi K., Tanabe K., Ohnuki M., Narita M., Ichisaka T., Tomoda K., et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 73.Hamazaki T., El Rouby N., Fredette N.C., Santostefano K.E., Terada N. Concise review: induced pluripotent stem cell research in the era of precision medicine. Stem Cell. 2017;35(3):545–550. doi: 10.1002/stem.2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang X., Hu D., Shang Y., Qi X. Using induced pluripotent stem cell neuronal models to study neurodegenerative diseases. Biochim Biophys Acta, Mol Basis Dis. 2020;1866(4) doi: 10.1016/j.bbadis.2019.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Birger A., Ben-Dor I., Ottolenghi M., Turetsky T., Gil Y., Sweetat S., et al. Human iPSC-derived astrocytes from ALS patients with mutated C9ORF72 show increased oxidative stress and neurotoxicity. EBioMedicine. 2019;50:274–289. doi: 10.1016/j.ebiom.2019.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cefalo M.G., Carai A., Miele E., Po A., Ferretti E., Mastronuzzi A., et al. Human iPSC for therapeutic approaches to the nervous system: present and future applications. Stem Cell Int. 2016;2016 doi: 10.1155/2016/4869071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Schweitzer J.S., Song B., Herrington T.M., Park T.Y., Lee N., Ko S., et al. Personalized iPSC-derived dopamine progenitor cells for Parkinson's disease. N Engl J Med. 2020;382(20):1926–1932. doi: 10.1056/NEJMoa1915872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Takahashi J. iPS cell-based therapy for Parkinson's disease: a Kyoto trial. Regen Ther. 2020;13:18–22. doi: 10.1016/j.reth.2020.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhang Y.T., He K.J., Zhang J.B., Ma Q.H., Wang F., Liu C.F. Advances in intranasal application of stem cells in the treatment of central nervous system diseases. Stem Cell Res Ther. 2021;12(1):210. doi: 10.1186/s13287-021-02274-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rodriguez-Frutos B., Otero-Ortega L., Gutierrez-Fernandez M., Fuentes B., Ramos-Cejudo J., Diez-Tejedor E. Stem cell therapy and administration routes after stroke. Transl Stroke Res. 2016;7(5):378–387. doi: 10.1007/s12975-016-0482-6. [DOI] [PubMed] [Google Scholar]
- 81.Gutierrez-Fernandez M., Rodriguez-Frutos B., Alvarez-Grech J., Vallejo-Cremades M.T., Exposito-Alcaide M., Merino J., et al. Functional recovery after hematic administration of allogenic mesenchymal stem cells in acute ischemic stroke in rats. Neuroscience. 2011;175:394–405. doi: 10.1016/j.neuroscience.2010.11.054. [DOI] [PubMed] [Google Scholar]
- 82.Schrepfer S., Deuse T., Reichenspurner H., Fischbein M.P., Robbins R.C., Pelletier M.P. Stem cell transplantation: the lung barrier. Transplant Proc. 2007;39(2):573–576. doi: 10.1016/j.transproceed.2006.12.019. [DOI] [PubMed] [Google Scholar]
- 83.Tajiri N., Lee J.Y., Acosta S., Sanberg P.R., Borlongan C.V. Breaking the blood-brain barrier with mannitol to aid stem cell therapeutics in the chronic stroke brain. Cell Transplant. 2016;25(8):1453–1460. doi: 10.3727/096368916X690971. [DOI] [PubMed] [Google Scholar]
- 84.Wong J.K.U., Mehta A., Vu T.T., Yeo G.C. Cellular modifications and biomaterial design to improve mesenchymal stem cell transplantation. Biomater Sci. 2023;11(14):4752–4773. doi: 10.1039/d3bm00376k. [DOI] [PubMed] [Google Scholar]
- 85.Klass M., Gavrikov V., Drury D., Stewart B., Hunter S., Denson D.D., et al. Intravenous mononuclear marrow cells reverse neuropathic pain from experimental mononeuropathy. Anesth Analg. 2007;104(4):944–948. doi: 10.1213/01.ane.0000258021.03211.d0. [DOI] [PubMed] [Google Scholar]
- 86.Karussis D., Karageorgiou C., Vaknin-Dembinsky A., Gowda-Kurkalli B., Gomori J.M., Kassis I., et al. Safety and immunological effects of mesenchymal stem cell transplantation in patients with multiple sclerosis and amyotrophic lateral sclerosis. Arch Neurol. 2010;67(10):1187–1194. doi: 10.1001/archneurol.2010.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Barczewska M., Grudniak M., Maksymowicz S., Siwek T., Oldak T., Jezierska-Wozniak K., et al. Safety of intrathecal injection of Wharton's jelly-derived mesenchymal stem cells in amyotrophic lateral sclerosis therapy. Neural Regen Res. 2019;14(2):313–318. doi: 10.4103/1673-5374.243723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Petrou P., Kassis I., Yaghmour N.E., Ginzberg A., Karussis D. A phase II clinical trial with repeated intrathecal injections of autologous mesenchymal stem cells in patients with amyotrophic lateral sclerosis. Front Biosci. 2021;26(10):693–706. doi: 10.52586/4980. [DOI] [PubMed] [Google Scholar]
- 89.Oh K.W., Moon C., Kim H.Y., Oh S.I., Park J., Lee J.H., et al. Phase I trial of repeated intrathecal autologous bone marrow-derived mesenchymal stromal cells in amyotrophic lateral sclerosis. Stem Cells Transl Med. 2015;4(6):590–597. doi: 10.5966/sctm.2014-0212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cudkowicz M.E., Lindborg S.R., Goyal N.A., Miller R.G., Burford M.J., Berry J.D., et al. A randomized placebo-controlled phase 3 study of mesenchymal stem cells induced to secrete high levels of neurotrophic factors in amyotrophic lateral sclerosis. Muscle Nerve. 2022;65(3):291–302. doi: 10.1002/mus.27472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sahraian M.A., Mohyeddin Bonab M., Baghbanian S.M., Owji M., Naser Moghadasi A. Therapeutic use of intrathecal mesenchymal stem cells in patients with multiple sclerosis: a pilot study with booster injection. Immunol Invest. 2019;48(2):160–168. doi: 10.1080/08820139.2018.1504301. [DOI] [PubMed] [Google Scholar]
- 92.Hur J.W., Cho T.H., Park D.H., Lee J.B., Park J.Y., Chung Y.G. Intrathecal transplantation of autologous adipose-derived mesenchymal stem cells for treating spinal cord injury: a human trial. J Spinal Cord Med. 2016;39(6):655–664. doi: 10.1179/2045772315Y.0000000048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bydon M., Qu W., Moinuddin F.M., Hunt C.L., Garlanger K.L., Reeves R.K., et al. Intrathecal delivery of adipose-derived mesenchymal stem cells in traumatic spinal cord injury: phase I trial. Nat Commun. 2024;15(1):2201. doi: 10.1038/s41467-024-46259-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cao T.T., Chen H., Pang M., Xu S.S., Wen H.Q., Liu B., et al. Dose optimization of intrathecal administration of human umbilical cord mesenchymal stem cells for the treatment of subacute incomplete spinal cord injury. Neural Regen Res. 2022;17(8):1785–1794. doi: 10.4103/1673-5374.332151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Singer W., Dietz A.B., Zeller A.D., Gehrking T.L., Schmelzer J.D., Schmeichel A.M., et al. Intrathecal administration of autologous mesenchymal stem cells in multiple system atrophy. Neurology. 2019;93(1):e77–e87. doi: 10.1212/WNL.0000000000007720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mesa Bedoya LE., Camacho Barbosa J.C., Lopez Quiceno L., Barrios Arroyave F., Halpert K., Espana Pena J.A., et al. The safety profile of mesenchymal stem cell therapy administered through intrathecal injections for treating neurological disorders: a systematic review and meta-analysis of randomised controlled trials. Stem Cell Res Ther. 2024;15(1):146. doi: 10.1186/s13287-024-03748-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Baek W., Kim Y.S., Koh S.H., Lim S.W., Kim H.Y., Yi H.J., et al. Stem cell transplantation into the intraventricular space via an Ommaya reservoir in a patient with amyotrophic lateral sclerosis. J Neurosurg Sci. 2012;56(3):261–263. [PubMed] [Google Scholar]
- 98.Tohill M., Terenghi G. Stem-cell plasticity and therapy for injuries of the peripheral nervous system. Biotechnol Appl Biochem. 2004;40(Pt 1):17–24. doi: 10.1042/BA20030173. [DOI] [PubMed] [Google Scholar]
- 99.Kim H.J., Seo S.W., Chang J.W., Lee J.I., Kim C.H., Chin J., et al. Stereotactic brain injection of human umbilical cord blood mesenchymal stem cells in patients with Alzheimer's disease dementia: a phase 1 clinical trial. Alzheimers Dement (N Y) 2015;1(2):95–102. doi: 10.1016/j.trci.2015.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Riley J., Glass J., Feldman E.L., Polak M., Bordeau J., Federici T., et al. Intraspinal stem cell transplantation in amyotrophic lateral sclerosis: a phase I trial, cervical microinjection, and final surgical safety outcomes. Neurosurgery. 2014;74(1):77–87. doi: 10.1227/NEU.0000000000000156. [DOI] [PubMed] [Google Scholar]
- 101.Feldman E.L., Boulis N.M., Hur J., Johe K., Rutkove S.B., Federici T., et al. Intraspinal neural stem cell transplantation in amyotrophic lateral sclerosis: phase 1 trial outcomes. Ann Neurol. 2014;75(3):363–373. doi: 10.1002/ana.24113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Li Y.H., Feng L., Zhang G.X., Ma C.G. Intranasal delivery of stem cells as therapy for central nervous system disease. Exp Mol Pathol. 2015;98(2):145–151. doi: 10.1016/j.yexmp.2015.01.016. [DOI] [PubMed] [Google Scholar]
- 103.Krishna V., Sammartino F., Rezai A. A review of the current therapies, challenges, and future directions of transcranial focused ultrasound technology: advances in diagnosis and treatment. JAMA Neurol. 2018;75(2):246–254. doi: 10.1001/jamaneurol.2017.3129. [DOI] [PubMed] [Google Scholar]
- 104.Fishman P.S., Fischell J.M. Focused ultrasound mediated opening of the blood-brain barrier for neurodegenerative diseases. Front Neurol. 2021;12 doi: 10.3389/fneur.2021.749047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cammalleri A., Croce P., Lee W., Yoon K., Yoo S.S. Therapeutic potentials of localized blood-brain barrier disruption by noninvasive transcranial focused ultrasound: a technical review. J Clin Neurophysiol. 2020;37(2):104–117. doi: 10.1097/WNP.0000000000000488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hynynen K., McDannold N., Vykhodtseva N., Jolesz F.A. Noninvasive MR imaging-guided focal opening of the blood-brain barrier in rabbits. Radiology. 2001;220(3):640–646. doi: 10.1148/radiol.2202001804. [DOI] [PubMed] [Google Scholar]
- 107.Ahmed N., Gandhi D., Melhem E.R., Frenkel V. MRI guided focused ultrasound-mediated delivery of therapeutic cells to the brain: a review of the state-of-the-art methodology and future applications. Front Neurol. 2021;12 doi: 10.3389/fneur.2021.669449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Burgess A., Ayala-Grosso C.A., Ganguly M., Jordao J.F., Aubert I., Hynynen K. Targeted delivery of neural stem cells to the brain using MRI-guided focused ultrasound to disrupt the blood-brain barrier. PLoS One. 2011;6(11) doi: 10.1371/journal.pone.0027877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Shen W.B., Anastasiadis P., Nguyen B., Yarnell D., Yarowsky P.J., Frenkel V., et al. Magnetic enhancement of stem cell-targeted delivery into the brain following MR-guided focused ultrasound for opening the blood-brain barrier. Cell Transplant. 2017;26(7):1235–1246. doi: 10.1177/0963689717715824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cui H., Zhu Q., Xie Q., Liu Z., Gao Y., He Y., et al. Low intensity ultrasound targeted microbubble destruction assists MSCs delivery and improves neural function in brain ischaemic rats. J Drug Target. 2020;28(3):320–329. doi: 10.1080/1061186X.2019.1656724. [DOI] [PubMed] [Google Scholar]
- 111.Seo Y., Han S., Song B.W., Chang J.W., Na Y.C., Chang W.S. Endogenous neural stem cell activation after low-intensity focused ultrasound-induced blood-brain barrier modulation. Int J Mol Sci. 2023;24(6) doi: 10.3390/ijms24065712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Krystkowiak P., Gaura V., Labalette M., Rialland A., Remy P., Peschanski M., et al. Alloimmunisation to donor antigens and immune rejection following foetal neural grafts to the brain in patients with Huntington's disease. PLoS One. 2007;2(1):e166. doi: 10.1371/journal.pone.0000166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Michniacki T.F., Choi S.W., Peltier D.C. Immune suppression in allogeneic hematopoietic stem cell transplantation. Handb Exp Pharmacol. 2022;272:209–243. doi: 10.1007/164_2021_544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.McGinley L.M., Chen K.S., Mason S.N., Rigan D.M., Kwentus J.F., Hayes J.M., et al. Monoclonal antibody-mediated immunosuppression enables long-term survival of transplanted human neural stem cells in mouse brain. Clin Transl Med. 2022;12(9) doi: 10.1002/ctm2.1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Busca A. The use of monoclonal antibodies for the treatment of graft-versus-host disease following allogeneic stem cell transplantation. Expet Opin Biol Ther. 2011;11(6):687–697. doi: 10.1517/14712598.2011.566852. [DOI] [PubMed] [Google Scholar]
- 116.Sakowski S.A., Chen K.S. Stem cell therapy for central nervous system disorders: metabolic interactions between transplanted cells and local microenvironments. Neurobiol Dis. 2022;173 doi: 10.1016/j.nbd.2022.105842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chen K.S., Sakowski S.A., Feldman E.L. Intraspinal stem cell transplantation for amyotrophic lateral sclerosis. Ann Neurol. 2016;79(3):342–353. doi: 10.1002/ana.24584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Goutman S.A., Savelieff M.G., Sakowski S.A., Feldman E.L. Stem cell treatments for amyotrophic lateral sclerosis: a critical overview of early phase trials. Expet Opin Invest Drugs. 2019;28(6):525–543. doi: 10.1080/13543784.2019.1627324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kharbikar B.N., Mohindra P., Desai T.A. Biomaterials to enhance stem cell transplantation. Cell Stem Cell. 2022;29(5):692–721. doi: 10.1016/j.stem.2022.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Deverman B.E., Ravina B.M., Bankiewicz K.S., Paul S.M., Sah D.W.Y. Gene therapy for neurological disorders: progress and prospects. Nat Rev Drug Discov. 2018;17(10):767. doi: 10.1038/nrd.2018.158. [DOI] [PubMed] [Google Scholar]
- 121.Sudhakar V., Richardson R.M. Gene therapy for neurodegenerative diseases. Neurotherapeutics. 2019;16(1):166–175. doi: 10.1007/s13311-018-00694-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Choong C.J., Baba K., Mochizuki H. Gene therapy for neurological disorders. Expet Opin Biol Ther. 2016;16(2):143–159. doi: 10.1517/14712598.2016.1114096. [DOI] [PubMed] [Google Scholar]
- 123.Kulkarni J.A., Witzigmann D., Thomson S.B., Chen S., Leavitt B.R., Cullis P.R., et al. The current landscape of nucleic acid therapeutics. Nat Nanotechnol. 2021;16(6):630–643. doi: 10.1038/s41565-021-00898-0. [DOI] [PubMed] [Google Scholar]
- 124.Savelieff M.G., Stino A.M. In: Atlas of neuromuscular diseases. 3 ed. Feldman E.L., Russell J.W., Loscher W.N., Grisold W., Meng S., editors. Springer; Cham: 2021. New neuromuscular therapies; pp. 35–43. [Google Scholar]
- 125.Simonato M., Bennett J., Boulis N.M., Castro M.G., Fink D.J., Goins W.F., et al. Progress in gene therapy for neurological disorders. Nat Rev Neurol. 2013;9(5):277–291. doi: 10.1038/nrneurol.2013.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Day J.W., Finkel R.S., Chiriboga C.A., Connolly A.M., Crawford T.O., Darras B.T., et al. Onasemnogene abeparvovec gene therapy for symptomatic infantile-onset spinal muscular atrophy in patients with two copies of SMN2 (STR1VE): an open-label, single-arm, multicentre, phase 3 trial. Lancet Neurol. 2021;20(4):284–293. doi: 10.1016/S1474-4422(21)00001-6. [DOI] [PubMed] [Google Scholar]
- 127.Hoy S.M. Delandistrogene moxeparvovec: first approval. Drugs. 2023;83(14):1323–1329. doi: 10.1007/s40265-023-01929-x. [DOI] [PubMed] [Google Scholar]
- 128.Ling Q., Herstine J.A., Bradbury A., Gray S.J. AAV-based in vivo gene therapy for neurological disorders. Nat Rev Drug Discov. 2023;22(10):789–806. doi: 10.1038/s41573-023-00766-7. [DOI] [PubMed] [Google Scholar]
- 129.Byun S., Lee M., Kim M. Gene therapy for huntington's disease: the final strategy for a cure? J Mov Disord. 2022;15(1):15–20. doi: 10.14802/jmd.21006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zhang L., Wang Y. Gene therapy in epilepsy. Biomed Pharmacother. 2021;143 doi: 10.1016/j.biopha.2021.112075. [DOI] [PubMed] [Google Scholar]
- 131.O'Connor D.M., Boulis N.M. Gene therapy for neurodegenerative diseases. Trends Mol Med. 2015;21(8):504–512. doi: 10.1016/j.molmed.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 132.Rafii M.S., Tuszynski M.H., Thomas R.G., Barba D., Brewer J.B., Rissman R.A., et al. Adeno-associated viral vector (serotype 2)-nerve growth factor for patients with alzheimer disease: a randomized clinical trial. JAMA Neurol. 2018;75(7):834–841. doi: 10.1001/jamaneurol.2018.0233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Castle M.J., Baltanas F.C., Kovacs I., Nagahara A.H., Barba D., Tuszynski M.H. Postmortem analysis in a clinical trial of AAV2-NGF gene therapy for alzheimer's disease identifies a need for improved vector delivery. Hum Gene Ther. 2020;31(7-8):415–422. doi: 10.1089/hum.2019.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kaplitt M.G., Feigin A., Tang C., Fitzsimons H.L., Mattis P., Lawlor P.A., et al. Safety and tolerability of gene therapy with an adeno-associated virus (AAV) borne GAD gene for Parkinson's disease: an open label, phase I trial. Lancet. 2007;369(9579):2097–2105. doi: 10.1016/S0140-6736(07)60982-9. [DOI] [PubMed] [Google Scholar]
- 135.LeWitt P.A., Rezai A.R., Leehey M.A., Ojemann S.G., Flaherty A.W., Eskandar E.N., et al. AAV2-GAD gene therapy for advanced Parkinson's disease: a double-blind, sham-surgery controlled, randomised trial. Lancet Neurol. 2011;10(4):309–319. doi: 10.1016/S1474-4422(11)70039-4. [DOI] [PubMed] [Google Scholar]
- 136.Eberling J.L., Jagust W.J., Christine C.W., Starr P., Larson P., Bankiewicz K.S., et al. Results from a phase I safety trial of hAADC gene therapy for Parkinson disease. Neurology. 2008;70(21):1980–1983. doi: 10.1212/01.wnl.0000312381.29287.ff. [DOI] [PubMed] [Google Scholar]
- 137.Mittermeyer G., Christine C.W., Rosenbluth K.H., Baker S.L., Starr P., Larson P., et al. Long-term evaluation of a phase 1 study of AADC gene therapy for Parkinson's disease. Hum Gene Ther. 2012;23(4):377–381. doi: 10.1089/hum.2011.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Christine C.W., Starr P.A., Larson P.S., Eberling J.L., Jagust W.J., Hawkins R.A., et al. Safety and tolerability of putaminal AADC gene therapy for Parkinson disease. Neurology. 2009;73(20):1662–1669. doi: 10.1212/WNL.0b013e3181c29356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Christine C.W., Richardson R.M., Van Laar A.D., Thompson M.E., Fine E.M., Khwaja O.S., et al. Safety of AADC gene therapy for moderately advanced Parkinson disease: three-year outcomes from the PD-1101 trial. Neurology. 2022;98(1):e40–e50. doi: 10.1212/WNL.0000000000012952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Palfi S., Gurruchaga J.M., Ralph G.S., Lepetit H., Lavisse S., Buttery P.C., et al. Long-term safety and tolerability of ProSavin, a lentiviral vector-based gene therapy for Parkinson's disease: a dose escalation, open-label, phase 1/2 trial. Lancet. 2014;383(9923):1138–1146. doi: 10.1016/S0140-6736(13)61939-X. [DOI] [PubMed] [Google Scholar]
- 141.Marks W.J., Jr., Ostrem J.L., Verhagen L., Starr P.A., Larson P.S., Bakay R.A., et al. Safety and tolerability of intraputaminal delivery of CERE-120 (adeno-associated virus serotype 2-neurturin) to patients with idiopathic Parkinson's disease: an open-label, phase I trial. Lancet Neurol. 2008;7(5):400–408. doi: 10.1016/S1474-4422(08)70065-6. [DOI] [PubMed] [Google Scholar]
- 142.Warren Olanow C., Bartus R.T., Baumann T.L., Factor S., Boulis N., Stacy M., et al. Gene delivery of neurturin to putamen and substantia nigra in Parkinson disease: a double-blind, randomized, controlled trial. Ann Neurol. 2015;78(2):248–257. doi: 10.1002/ana.24436. [DOI] [PubMed] [Google Scholar]
- 143.Marks W.J., Jr., Bartus R.T., Siffert J., Davis C.S., Lozano A., Boulis N., et al. Gene delivery of AAV2-neurturin for Parkinson's disease: a double-blind, randomised, controlled trial. Lancet Neurol. 2010;9(12):1164–1172. doi: 10.1016/S1474-4422(10)70254-4. [DOI] [PubMed] [Google Scholar]
- 144.Bennett C.F., Kordasiewicz H.B., Cleveland D.W. Antisense drugs make sense for neurological diseases. Annu Rev Pharmacol Toxicol. 2021;61:831–852. doi: 10.1146/annurev-pharmtox-010919-023738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Singh N.N., Howell M.D., Androphy E.J., Singh R.N. How the discovery of ISS-N1 led to the first medical therapy for spinal muscular atrophy. Gene Ther. 2017;24(9):520–526. doi: 10.1038/gt.2017.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Neil E.E., Bisaccia E.K. Nusinersen: a novel antisense oligonucleotide for the treatment of spinal muscular atrophy. J Pediatr Pharmacol Therapeut. 2019;24(3):194–203. doi: 10.5863/1551-6776-24.3.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Mathew V., Wang A.K. Inotersen: new promise for the treatment of hereditary transthyretin amyloidosis. Drug Des Dev Ther. 2019;13:1515–1525. doi: 10.2147/DDDT.S162913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Shirley M. Casimersen: first approval. Drugs. 2021;81(7):875–879. doi: 10.1007/s40265-021-01512-2. [DOI] [PubMed] [Google Scholar]
- 149.Roshmi R.R., Yokota T. Viltolarsen: from preclinical studies to FDA approval. Methods Mol Biol. 2023;2587:31–41. doi: 10.1007/978-1-0716-2772-3_2. [DOI] [PubMed] [Google Scholar]
- 150.Heo Y.A. Golodirsen: first approval. Drugs. 2020;80(3):329–333. doi: 10.1007/s40265-020-01267-2. [DOI] [PubMed] [Google Scholar]
- 151.Edwards A.L., Collins J.A., Junge C., Kordasiewicz H., Mignon L., Wu S., et al. Exploratory tau biomarker results from a multiple ascending-dose study of BIIB080 in alzheimer disease: a randomized clinical trial. JAMA Neurol. 2023;80(12):1344–1352. doi: 10.1001/jamaneurol.2023.3861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Mummery C.J., Borjesson-Hanson A., Blackburn D.J., Vijverberg E.G.B., De Deyn P.P., Ducharme S., et al. Tau-targeting antisense oligonucleotide MAPT(Rx) in mild Alzheimer's disease: a phase 1b, randomized, placebo-controlled trial. Nat Med. 2023;29(6):1437–1447. doi: 10.1038/s41591-023-02326-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Garber K. Alnylam terminates revusiran program, stock plunges. Nat Biotechnol. 2016;34(12):1213–1214. doi: 10.1038/nbt1216-1213. [DOI] [PubMed] [Google Scholar]
- 154.McColgan P., Thobhani A., Boak L., Schobel S.A., Nicotra A., Palermo G., et al. Tominersen in adults with manifest huntington's disease. N Engl J Med. 2023;389(23):2203–2205. doi: 10.1056/NEJMc2300400. [DOI] [PubMed] [Google Scholar]
- 155.Miller T.M., Cudkowicz M.E., Genge A., Shaw P.J., Sobue G., Bucelli R.C., et al. Trial of antisense oligonucleotide tofersen for SOD1 ALS. N Engl J Med. 2022;387(12):1099–1110. doi: 10.1056/NEJMoa2204705. [DOI] [PubMed] [Google Scholar]
- 156.Germain N.D., Chung W.K., Sarmiere P.D. RNA interference (RNAi)-based therapeutics for treatment of rare neurologic diseases. Mol Aspect Med. 2023;91 doi: 10.1016/j.mam.2022.101148. [DOI] [PubMed] [Google Scholar]
- 157.Bravo-Hernandez M., Tadokoro T., Navarro M.R., Platoshyn O., Kobayashi Y., Marsala S., et al. Spinal subpial delivery of AAV9 enables widespread gene silencing and blocks motoneuron degeneration in ALS. Nat Med. 2020;26(1):118–130. doi: 10.1038/s41591-019-0674-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Li J., Peng H., Zhang W., Li M., Wang N., Peng C., et al. Enhanced nose-to-brain delivery of combined small interfering RNAs using lesion-recognizing nanoparticles for the synergistic therapy of alzheimer's disease. ACS Appl Mater Interfaces. 2023;15(46):53177–53188. doi: 10.1021/acsami.3c08756. [DOI] [PubMed] [Google Scholar]
- 159.Singer O., Marr R.A., Rockenstein E., Crews L., Coufal N.G., Gage F.H., et al. Targeting BACE1 with siRNAs ameliorates Alzheimer disease neuropathology in a transgenic model. Nat Neurosci. 2005;8(10):1343–1349. doi: 10.1038/nn1531. [DOI] [PubMed] [Google Scholar]
- 160.Harper S.Q., Staber P.D., He X., Eliason S.L., Martins I.H., Mao Q., et al. RNA interference improves motor and neuropathological abnormalities in a Huntington's disease mouse model. Proc Natl Acad Sci USA. 2005;102(16):5820–5825. doi: 10.1073/pnas.0501507102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Feldman E.L., Goutman S.A., Petri S., Mazzini L., Savelieff M.G., Shaw P.J., et al. Amyotrophic lateral sclerosis. Lancet. 2022;400(10360):1363–1380. doi: 10.1016/S0140-6736(22)01272-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Mueller C., Berry J.D., McKenna-Yasek D.M., Gernoux G., Owegi M.A., Pothier L.M., et al. SOD1 suppression with adeno-associated virus and MicroRNA in familial ALS. N Engl J Med. 2020;383(2):151–158. doi: 10.1056/NEJMoa2005056. [DOI] [PubMed] [Google Scholar]
- 163.Kalola U.K., Pellegrini M.V. StatPearls [Internet]; 2024. Patisiran.https://www.ncbi.nlm.nih.gov/pubmed/36972364 June 27, 2024. [PubMed] [Google Scholar]
- 164.Nie T., Heo Y.A., Shirley M. Vutrisiran: a review in polyneuropathy of hereditary transthyretin-mediated amyloidosis. Drugs. 2023;83(15):1425–1432. doi: 10.1007/s40265-023-01943-z. [DOI] [PubMed] [Google Scholar]
- 165.Benabdellah K., Sanchez-Hernandez S., Aguilar-Gonzalez A., Maldonado-Perez N., Gutierrez-Guerrero A., Cortijo-Gutierrez M., et al. Genome-edited adult stem cells: next-generation advanced therapy medicinal products. Stem Cells Transl Med. 2020;9(6):674–685. doi: 10.1002/sctm.19-0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Maeder M.L., Gersbach C.A. Genome-editing technologies for gene and cell therapy. Mol Ther. 2016;24(3):430–446. doi: 10.1038/mt.2016.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Zhang H.X., Zhang Y., Yin H. Genome editing with mRNA encoding ZFN, TALEN, and Cas9. Mol Ther. 2019;27(4):735–746. doi: 10.1016/j.ymthe.2019.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Bae K.H., Kwon Y.D., Shin H.C., Hwang M.S., Ryu E.H., Park K.S., et al. Human zinc fingers as building blocks in the construction of artificial transcription factors. Nat Biotechnol. 2003;21(3):275–280. doi: 10.1038/nbt796. [DOI] [PubMed] [Google Scholar]
- 169.Beerli R.R., Barbas C.F., 3rd Engineering polydactyl zinc-finger transcription factors. Nat Biotechnol. 2002;20(2):135–141. doi: 10.1038/nbt0202-135. [DOI] [PubMed] [Google Scholar]
- 170.Segal D.J., Dreier B., Beerli R.R., Barbas C.F., 3rd Toward controlling gene expression at will: selection and design of zinc finger domains recognizing each of the 5'-GNN-3' DNA target sequences. Proc Natl Acad Sci USA. 1999;96(6):2758–2763. doi: 10.1073/pnas.96.6.2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Boch J., Scholze H., Schornack S., Landgraf A., Hahn S., Kay S., et al. Breaking the code of DNA binding specificity of TAL-type III effectors. Science. 2009;326(5959):1509–1512. doi: 10.1126/science.1178811. [DOI] [PubMed] [Google Scholar]
- 172.Moscou M.J., Bogdanove A.J. A simple cipher governs DNA recognition by TAL effectors. Science. 2009;326(5959):1501. doi: 10.1126/science.1178817. [DOI] [PubMed] [Google Scholar]
- 173.Gaj T., Gersbach C.A., Barbas C.F., 3rd ZFN, TALEN, and CRISPR/Cas-based methods for genome engineering. Trends Biotechnol. 2013;31(7):397–405. doi: 10.1016/j.tibtech.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Guan L., Han Y., Yang C., Lu S., Du J., Li H., et al. CRISPR-Cas9-Mediated gene therapy in neurological disorders. Mol Neurobiol. 2022;59(2):968–982. doi: 10.1007/s12035-021-02638-w. [DOI] [PubMed] [Google Scholar]
- 175.Li H., Yang Y., Hong W., Huang M., Wu M., Zhao X. Applications of genome editing technology in the targeted therapy of human diseases: mechanisms, advances and prospects. Signal Transduct Targeted Ther. 2020;5(1):1. doi: 10.1038/s41392-019-0089-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Wegmann S., DeVos S.L., Zeitler B., Marlen K., Bennett R.E., Perez-Rando M., et al. Persistent repression of tau in the brain using engineered zinc finger protein transcription factors. Sci Adv. 2021;7(12) doi: 10.1126/sciadv.abe1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Garriga-Canut M., Agustin-Pavon C., Herrmann F., Sanchez A., Dierssen M., Fillat C., et al. Synthetic zinc finger repressors reduce mutant huntingtin expression in the brain of R6/2 mice. Proc Natl Acad Sci USA. 2012;109(45):E3136–E3145. doi: 10.1073/pnas.1206506109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.An M.C., Zhang N., Scott G., Montoro D., Wittkop T., Mooney S., et al. Genetic correction of Huntington's disease phenotypes in induced pluripotent stem cells. Cell Stem Cell. 2012;11(2):253–263. doi: 10.1016/j.stem.2012.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Ababneh N.A., Scaber J., Flynn R., Douglas A., Barbagallo P., Candalija A., et al. Correction of amyotrophic lateral sclerosis related phenotypes in induced pluripotent stem cell-derived motor neurons carrying a hexanucleotide expansion mutation in C9orf72 by CRISPR/Cas9 genome editing using homology-directed repair. Hum Mol Genet. 2020;29(13):2200–2217. doi: 10.1093/hmg/ddaa106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Philippidis A. CRISPR therapeutics, vertex complete rolling biologics license applications for exa-cel in sickle cell disease, beta thalassemia. Hum Gene Ther. 2023;34(9-10):341–344. doi: 10.1089/hum.2023.29241.bfs. [DOI] [PubMed] [Google Scholar]
- 181.Lee J., Lim K., Kim A., Mok Y.G., Chung E., Cho S.I., et al. Prime editing with genuine Cas9 nickases minimizes unwanted indels. Nat Commun. 2023;14(1):1786. doi: 10.1038/s41467-023-37507-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Villiger L., Joung J., Koblan L., Weissman J., Abudayyeh O.O., Gootenberg J.S. CRISPR technologies for genome, epigenome and transcriptome editing. Nat Rev Mol Cell Biol. 2024;25(6):464–487. doi: 10.1038/s41580-023-00697-6. [DOI] [PubMed] [Google Scholar]
- 183.Ingusci S., Verlengia G., Soukupova M., Zucchini S., Simonato M. Gene therapy tools for brain diseases. Front Pharmacol. 2019;10:724. doi: 10.3389/fphar.2019.00724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Choudhury S.R., Hudry E., Maguire C.A., Sena-Esteves M., Breakefield X.O., Grandi P. Viral vectors for therapy of neurologic diseases. Neuropharmacology. 2017;120:63–80. doi: 10.1016/j.neuropharm.2016.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Tasset A., Bellamkonda A., Wang W., Pyatnitskiy I., Ward D., Peppas N., et al. Overcoming barriers in non-viral gene delivery for neurological applications. Nanoscale. 2022;14(10):3698–3719. doi: 10.1039/d1nr06939j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Durymanov M., Reineke J. Non-viral delivery of nucleic acids: insight into mechanisms of overcoming intracellular barriers. Front Pharmacol. 2018;9:971. doi: 10.3389/fphar.2018.00971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Wang S., Olumolade O.O., Sun T., Samiotaki G., Konofagou E.E. Noninvasive, neuron-specific gene therapy can be facilitated by focused ultrasound and recombinant adeno-associated virus. Gene Ther. 2015;22(1):104–110. doi: 10.1038/gt.2014.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Stavarache M.A., Petersen N., Jurgens E.M., Milstein E.R., Rosenfeld Z.B., Ballon D.J., et al. Safe and stable noninvasive focal gene delivery to the mammalian brain following focused ultrasound. J Neurosurg. 2018;130(3):989–998. doi: 10.3171/2017.8.JNS17790. [DOI] [PubMed] [Google Scholar]
- 189.Fan C.H., Ting C.Y., Lin C.Y., Chan H.L., Chang Y.C., Chen Y.Y., et al. Noninvasive, targeted, and non-viral ultrasound-mediated GDNF-plasmid delivery for treatment of Parkinson's disease. Sci Rep. 2016;6 doi: 10.1038/srep19579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Hsu P.H., Wei K.C., Huang C.Y., Wen C.J., Yen T.C., Liu C.L., et al. Noninvasive and targeted gene delivery into the brain using microbubble-facilitated focused ultrasound. PLoS One. 2013;8(2) doi: 10.1371/journal.pone.0057682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Huang Q., Deng J., Wang F., Chen S., Liu Y., Wang Z., et al. Targeted gene delivery to the mouse brain by MRI-guided focused ultrasound-induced blood-brain barrier disruption. Exp Neurol. 2012;233(1):350–356. doi: 10.1016/j.expneurol.2011.10.027. [DOI] [PubMed] [Google Scholar]
- 192.Burgess A., Huang Y., Querbes W., Sah D.W., Hynynen K. Focused ultrasound for targeted delivery of siRNA and efficient knockdown of Htt expression. J Contr Release. 2012;163(2):125–129. doi: 10.1016/j.jconrel.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Thevenot E., Jordao J.F., O'Reilly M.A., Markham K., Weng Y.Q., Foust K.D., et al. Targeted delivery of self-complementary adeno-associated virus serotype 9 to the brain, using magnetic resonance imaging-guided focused ultrasound. Hum Gene Ther. 2012;23(11):1144–1155. doi: 10.1089/hum.2012.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Weber-Adrian D., Thevenot E., O'Reilly M.A., Oakden W., Akens M.K., Ellens N., et al. Gene delivery to the spinal cord using MRI-guided focused ultrasound. Gene Ther. 2015;22(7):568–577. doi: 10.1038/gt.2015.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Chirmule N., Propert K., Magosin S., Qian Y., Qian R., Wilson J. Immune responses to adenovirus and adeno-associated virus in humans. Gene Ther. 1999;6(9):1574–1583. doi: 10.1038/sj.gt.3300994. [DOI] [PubMed] [Google Scholar]
- 196.Lowenstein P.R., Castro M.G. Inflammation and adaptive immune responses to adenoviral vectors injected into the brain: peculiarities, mechanisms, and consequences. Gene Ther. 2003;10(11):946–954. doi: 10.1038/sj.gt.3302048. [DOI] [PubMed] [Google Scholar]
- 197.Hudry E., Vandenberghe L.H. Therapeutic AAV gene transfer to the nervous system: a clinical reality. Neuron. 2019;101(5):839–862. doi: 10.1016/j.neuron.2019.02.017. [DOI] [PubMed] [Google Scholar]
- 198.Linden R.M., Ward P., Giraud C., Winocour E., Berns K.I. Site-specific integration by adeno-associated virus. Proc Natl Acad Sci USA. 1996;93(21):11288–11294. doi: 10.1073/pnas.93.21.11288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Kotin R.M., Siniscalco M., Samulski R.J., Zhu X.D., Hunter L., Laughlin C.A., et al. Site-specific integration by adeno-associated virus. Proc Natl Acad Sci U S A. 1990;87(6):2211–2215. doi: 10.1073/pnas.87.6.2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Chandler R.J., Sands M.S., Venditti C.P. Recombinant adeno-associated viral integration and genotoxicity: insights from animal models. Hum Gene Ther. 2017;28(4):314–322. doi: 10.1089/hum.2017.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Nault J.C., Datta S., Imbeaud S., Franconi A., Mallet M., Couchy G., et al. Recurrent AAV2-related insertional mutagenesis in human hepatocellular carcinomas. Nat Genet. 2015;47(10):1187–1193. doi: 10.1038/ng.3389. [DOI] [PubMed] [Google Scholar]
- 202.Srivastava A., Carter B.J. AAV infection: protection from cancer. Hum Gene Ther. 2017;28(4):323–327. doi: 10.1089/hum.2016.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Leone P., Shera D., McPhee S.W., Francis J.S., Kolodny E.H., Bilaniuk L.T., et al. Long-term follow-up after gene therapy for canavan disease. Sci Transl Med. 2012;4(165) doi: 10.1126/scitranslmed.3003454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Janson C., McPhee S., Bilaniuk L., Haselgrove J., Testaiuti M., Freese A., et al. Clinical protocol. Gene therapy of Canavan disease: AAV-2 vector for neurosurgical delivery of aspartoacylase gene (ASPA) to the human brain. Hum Gene Ther. 2002;13(11):1391–1412. doi: 10.1089/104303402760128612. [DOI] [PubMed] [Google Scholar]
- 205.Duque S., Joussemet B., Riviere C., Marais T., Dubreil L., Douar A.M., et al. Intravenous administration of self-complementary AAV9 enables transgene delivery to adult motor neurons. Mol Ther. 2009;17(7):1187–1196. doi: 10.1038/mt.2009.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Mendell J.R., Al-Zaidy S., Shell R., Arnold W.D., Rodino-Klapac L.R., Prior T.W., et al. Single-dose gene-replacement therapy for spinal muscular atrophy. N Engl J Med. 2017;377(18):1713–1722. doi: 10.1056/NEJMoa1706198. [DOI] [PubMed] [Google Scholar]
- 207.Chen X., Lim D.A., Lawlor M.W., Dimmock D., Vite C.H., Lester T., et al. Biodistribution of adeno-associated virus gene therapy following cerebrospinal fluid-directed administration. Hum Gene Ther. 2023;34(3-4):94–111. doi: 10.1089/hum.2022.163. [DOI] [PubMed] [Google Scholar]
- 208.Hordeaux J., Jeffrey B.A., Jian J., Choudhury G.R., Michalson K., Mitchell T.W., et al. Efficacy and safety of a Krabbe disease gene therapy. Hum Gene Ther. 2022;33(9-10):499–517. doi: 10.1089/hum.2021.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Milone M.C., O'Doherty U. Clinical use of lentiviral vectors. Leukemia. 2018;32(7):1529–1541. doi: 10.1038/s41375-018-0106-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Palfi S., Gurruchaga J.M., Lepetit H., Howard K., Ralph G.S., Mason S., et al. Long-term follow-up of a phase I/II study of ProSavin, a lentiviral vector gene therapy for Parkinson's disease. Hum Gene Ther Clin Dev. 2018;29(3):148–155. doi: 10.1089/humc.2018.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Wolfe D., Mata M., Fink D.J. A human trial of HSV-mediated gene transfer for the treatment of chronic pain. Gene Ther. 2009;16(4):455–460. doi: 10.1038/gt.2009.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Sung Y.K., Kim S.W. Recent advances in the development of gene delivery systems. Biomater Res. 2019;23:8. doi: 10.1186/s40824-019-0156-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Duckert B., Vinkx S., Braeken D., Fauvart M. Single-cell transfection technologies for cell therapies and gene editing. J Contr Release. 2021;330:963–975. doi: 10.1016/j.jconrel.2020.10.068. [DOI] [PubMed] [Google Scholar]
- 214.Zhou J., Shao Z., Liu J., Duan Q., Wang X., Li J., et al. From endocytosis to nonendocytosis: the emerging era of gene delivery. ACS Appl Bio Mater. 2020;3(5):2686–2701. doi: 10.1021/acsabm.9b01131. [DOI] [PubMed] [Google Scholar]
- 215.Thomas T.J., Tajmir-Riahi H.A., Pillai C.K.S. Biodegradable polymers for gene delivery. Molecules. 2019;24(20) doi: 10.3390/molecules24203744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Buck J., Grossen P., Cullis P.R., Huwyler J., Witzigmann D. Lipid-based DNA therapeutics: hallmarks of non-viral gene delivery. ACS Nano. 2019;13(4):3754–3782. doi: 10.1021/acsnano.8b07858. [DOI] [PubMed] [Google Scholar]
- 217.Hadianamrei R., Zhao X. Current state of the art in peptide-based gene delivery. J Contr Release. 2022;343:600–619. doi: 10.1016/j.jconrel.2022.02.010. [DOI] [PubMed] [Google Scholar]
- 218.Cardoso A.L., Simoes S., de Almeida L.P., Plesnila N., Pedroso de Lima M.C., Wagner E., et al. Tf-lipoplexes for neuronal siRNA delivery: a promising system to mediate gene silencing in the CNS. J Contr Release. 2008;132(2):113–123. doi: 10.1016/j.jconrel.2008.08.014. [DOI] [PubMed] [Google Scholar]
- 219.Yuan Z., Zhao L., Zhang Y., Li S., Pan B., Hua L., et al. Inhibition of glioma growth by a GOLPH3 siRNA-loaded cationic liposomes. J Neuro Oncol. 2018;140(2):249–260. doi: 10.1007/s11060-018-2966-6. [DOI] [PubMed] [Google Scholar]
- 220.Bender H., Noyes N., Annis J.L., Hitpas A., Mollnow L., Croak K., et al. PrPC knockdown by liposome-siRNA-peptide complexes (LSPCs) prolongs survival and normal behavior of prion-infected mice immunotolerant to treatment. PLoS One. 2019;14(7) doi: 10.1371/journal.pone.0219995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Dos Santos Rodrigues B., Lakkadwala S., Kanekiyo T., Singh J. Development and screening of brain-targeted lipid-based nanoparticles with enhanced cell penetration and gene delivery properties. Int J Nanomed. 2019;14:6497–6517. doi: 10.2147/IJN.S215941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Yu C., Li L., Hu P., Yang Y., Wei W., Deng X., et al. Recent advances in stimulus-responsive nanocarriers for gene therapy. Adv Sci. 2021;8(14) doi: 10.1002/advs.202100540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Chang E.L., Ting C.Y., Hsu P.H., Lin Y.C., Liao E.C., Huang C.Y., et al. Angiogenesis-targeting microbubbles combined with ultrasound-mediated gene therapy in brain tumors. J Contr Release. 2017;255:164–175. doi: 10.1016/j.jconrel.2017.04.010. [DOI] [PubMed] [Google Scholar]
- 224.Mead B.P., Kim N., Miller G.W., Hodges D., Mastorakos P., Klibanov A.L., et al. Novel focused ultrasound gene therapy approach noninvasively restores dopaminergic neuron function in a rat Parkinson's disease model. Nano Lett. 2017;17(6):3533–3542. doi: 10.1021/acs.nanolett.7b00616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Cui Y., Li X., Zeljic K., Shan S., Qiu Z., Wang Z. Effect of PEGylated magnetic PLGA-PEI nanoparticles on primary hippocampal neurons: reduced nanoneurotoxicity and enhanced transfection efficiency with magnetofection. ACS Appl Mater Interfaces. 2019;11(41):38190–38204. doi: 10.1021/acsami.9b15014. [DOI] [PubMed] [Google Scholar]
- 226.Keam S.J. Elivaldogene autotemcel: first approval. Mol Diagn Ther. 2021;25(6):803–809. doi: 10.1007/s40291-021-00555-1. [DOI] [PubMed] [Google Scholar]
- 227.Eichler F., Duncan C., Musolino P.L., Orchard P.J., De Oliveira S., Thrasher A.J., et al. Hematopoietic stem-cell gene therapy for cerebral adrenoleukodystrophy. N Engl J Med. 2017;377(17):1630–1638. doi: 10.1056/NEJMoa1700554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Fumagalli F., Calbi V., Natali Sora M.G., Sessa M., Baldoli C., Rancoita P.M.V., et al. Lentiviral haematopoietic stem-cell gene therapy for early-onset metachromatic leukodystrophy: long-term results from a non-randomised, open-label, phase 1/2 trial and expanded access. Lancet. 2022;399(10322):372–383. doi: 10.1016/S0140-6736(21)02017-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Gentner B., Tucci F., Galimberti S., Fumagalli F., De Pellegrin M., Silvani P., et al. Hematopoietic stem- and progenitor-cell gene therapy for hurler syndrome. N Engl J Med. 2021;385(21):1929–1940. doi: 10.1056/NEJMoa2106596. [DOI] [PubMed] [Google Scholar]
- 230.Kinsella J.L., Wynn R.F., Bigger B., Thrasher A.J., Booth C., Buckland K., et al. Ex-vivo autologous stem cell gene therapy clinical trial for mucopolysaccharidosis type IIIA: trial in progress- NCT04201405. Blood. 2020;136:15–16. [Google Scholar]
- 231.McGinley L.M., Sims E., Lunn J.S., Kashlan O.N., Chen K.S., Bruno E.S., et al. Human cortical neural stem cells expressing insulin-like growth factor-I: a novel cellular therapy for alzheimer's disease. Stem Cells Transl Med. 2016;5(3):379–391. doi: 10.5966/sctm.2015-0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Chen K.S., Noureldein M.H., McGinley L.M., Hayes J.M., Rigan D.M., Kwentus J.F., et al. Human neural stem cells restore spatial memory in a transgenic Alzheimer's disease mouse model by an immunomodulating mechanism. Front Aging Neurosci. 2023;15 doi: 10.3389/fnagi.2023.1306004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Bayarsaikhan E., Bayarsaikhan D., Lee J., Son M., Oh S., Moon J., et al. Microglial AGE-albumin is critical for neuronal death in Parkinson's disease: a possible implication for theranostics. Int J Nanomed. 2015:281–292. doi: 10.2147/IJN.S95077. 10 Spec Iss(Spec Iss) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Lee J., Bayarsaikhan D., Arivazhagan R., Park H., Lim B., Gwak P., et al. CRISPR/Cas9 edited sRAGE-MSCs protect neuronal death in Parkinson's disease model. Int J Stem Cells. 2019;12(1):114–124. doi: 10.15283/ijsc18110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Tuszynski M.H., Thal L., Pay M., Salmon D.P., U H.S., Bakay R., et al. A phase 1 clinical trial of nerve growth factor gene therapy for Alzheimer disease. Nat Med. 2005;11(5):551–555. doi: 10.1038/nm1239. [DOI] [PubMed] [Google Scholar]
- 236.Baloh R.H., Johnson J.P., Avalos P., Allred P., Svendsen S., Gowing G., et al. Transplantation of human neural progenitor cells secreting GDNF into the spinal cord of patients with ALS: a phase 1/2a trial. Nat Med. 2022;28(9):1813–1822. doi: 10.1038/s41591-022-01956-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Hagell P., Piccini P., Bjorklund A., Brundin P., Rehncrona S., Widner H., et al. Dyskinesias following neural transplantation in Parkinson's disease. Nat Neurosci. 2002;5(7):627–628. doi: 10.1038/nn863. [DOI] [PubMed] [Google Scholar]
- 238.Chen Y., Xiong M., Dong Y., Haberman A., Cao J., Liu H., et al. Chemical control of grafted human PSC-derived neurons in a mouse model of Parkinson's disease. Cell Stem Cell. 2016;18(6):817–826. doi: 10.1016/j.stem.2016.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Haworth R., Sharpe M. Accept or reject: the role of immune tolerance in the development of stem cell therapies and possible future approaches. Toxicol Pathol. 2021;49(7):1308–1316. doi: 10.1177/0192623320918241. [DOI] [PubMed] [Google Scholar]
- 240.Xu H., Wang B., Ono M., Kagita A., Fujii K., Sasakawa N., et al. Targeted disruption of HLA genes via CRISPR-cas9 generates iPSCs with enhanced immune compatibility. Cell Stem Cell. 2019;24(4):566–578 e7. doi: 10.1016/j.stem.2019.02.005. [DOI] [PubMed] [Google Scholar]
- 241.Cubo E., Gonzalez M., del Puerto I., de Yebenes J.G., Arconada O.F., Gabriel y Galan J.M., et al. Placebo effect characteristics observed in a single, international, longitudinal study in Huntington's disease. Mov Disord. 2012;27(3):439–442. doi: 10.1002/mds.24062. [DOI] [PubMed] [Google Scholar]
- 242.Simmons Z. Can we eliminate placebo in ALS clinical Trials? Muscle Nerve. 2009;39(6):861–865. doi: 10.1002/mus.21358. [DOI] [PubMed] [Google Scholar]
- 243.Lidstone S.C. Great expectations: the placebo effect in Parkinson's disease. Handb Exp Pharmacol. 2014;225:139–147. doi: 10.1007/978-3-662-44519-8_8. [DOI] [PubMed] [Google Scholar]
- 244.Goetz C.G., Wuu J., McDermott M.P., Adler C.H., Fahn S., Freed C.R., et al. Placebo response in Parkinson's disease: comparisons among 11 trials covering medical and surgical interventions. Mov Disord. 2008;23(5):690–699. doi: 10.1002/mds.21894. [DOI] [PubMed] [Google Scholar]
- 245.McRae C., Cherin E., Yamazaki T.G., Diem G., Vo A.H., Russell D., et al. Effects of perceived treatment on quality of life and medical outcomes in a double-blind placebo surgery trial. Arch Gen Psychiatr. 2004;61(4):412–420. doi: 10.1001/archpsyc.61.4.412. [DOI] [PubMed] [Google Scholar]
- 246.Benedetti F., Mayberg H.S., Wager T.D., Stohler C.S., Zubieta J.K. Neurobiological mechanisms of the placebo effect. J Neurosci. 2005;25(45):10390–10402. doi: 10.1523/JNEUROSCI.3458-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]