Abstract
Repetitive subconcussive head impacts (RSHI) are believed to induce sub‐clinical brain injuries, potentially resulting in cumulative, long‐term brain alterations. This study explores patterns of longitudinal brain white matter changes across sports with RSHI‐exposure. A systematic literature search identified 22 datasets with longitudinal diffusion magnetic resonance imaging data. Four datasets were centrally pooled to perform uniform quality control and data preprocessing. A total of 131 non‐concussed active athletes (American football, rugby, ice hockey; mean age: 20.06 ± 2.06 years) with baseline and post‐season data were included. Nonparametric permutation inference (one‐sample t tests, one‐sided) was applied to analyze the difference maps of multiple diffusion parameters. The analyses revealed widespread lateralized patterns of sports‐season‐related increases and decreases in mean diffusivity (MD), radial diffusivity (RD), and axial diffusivity (AD) across spatially distinct white matter regions. Increases were shown across one MD‐cluster (3195 voxels; mean change: 2.34%), one AD‐cluster (5740 voxels; mean change: 1.75%), and three RD‐clusters (817 total voxels; mean change: 3.11 to 4.70%). Decreases were shown across two MD‐clusters (1637 total voxels; mean change: −1.43 to −1.48%), two RD‐clusters (1240 total voxels; mean change: −1.92 to −1.93%), and one AD‐cluster (724 voxels; mean change: −1.28%). The resulting pattern implies the presence of strain‐induced injuries in central and brainstem regions, with comparatively milder physical exercise‐induced effects across frontal and superior regions of the left hemisphere, which need further investigation. This article highlights key considerations that need to be addressed in future work to enhance our understanding of the nature of observed white matter changes, improve the comparability of findings across studies, and promote data pooling initiatives to allow more detailed investigations (e.g., exploring sex‐ and sport‐specific effects).
Keywords: brain injuries, diffusion tensor imaging, multisite analysis, repetitive subconcussive head impacts, sport‐related mTBI, voxel‐based analysis
The combined analysis of existing longitudinal repetitive subconcussive head impacts studies showed widespread seasonal white matter changes across multiple diffusion parameters (i.e., mean diffusivity, radial diffusivity, and axial diffusivity) showing probable strain‐induced injuries and potential physical exercise effects.
Abbreviations
- AD
axial diffusivity
- BIDS
Brain Imaging Data Structure
- dMRI
diffusion magnetic resonance imaging
- DTI
diffusion tensor imaging
- FA
fractional anisotropy
- FSL
acronym for FMRIB software library
- FWE
family‐wise error
- IBM
International Business Machines Corporation
- JHU
Johns Hopkins University
- MD
mean diffusivity
- MNI
Montreal Neurological Institute
- MRI
magnetic resonance imaging
- mTBI
mild traumatic brain injury
- RD
radial diffusivity
- RSHI
repetitive subconcussive head impacts
- TBI
traumatic brain injury
- WM
white matter
1. INTRODUCTION
Sport‐related brain injuries are recognized as an important public health problem worldwide, affecting millions of athletes annually, particularly with mild traumatic brain injuries (mTBIs; Langlois et al., 2006). A growing interest in the field pertains to investigating repetitive subconcussive head impacts (RSHI) with particular concern regarding their possible cumulative adverse effects on the brain. RSHI are often considered “silent” impacts and may lack apparent concussion symptoms (Bailes et al., 2013); however, they have been associated with structural (Bazarian et al., 2012; Hirad et al., 2019; Koerte, Ertl‐Wagner, et al., 2012; Koerte, Kaufmann, et al., 2012), functional (Abbas et al., 2015; Breedlove et al., 2012; Talavage et al., 2014), and metabolic (Marchi et al., 2013; Poole et al., 2015) brain alterations. Potential accumulation of brain changes from RSHI may result from their frequent, silent nature, therefore hindering subtle injuries from completely healing before new ones arise. Across different degrees of traumatic brain injury, including sport‐related concussions, it is commonly seen that the stretching and tearing of axons lead to shear strain resulting in diffuse axonal injuries (Borich et al., 2013; Spain et al., 2010; Voelbel et al., 2012). Likewise, prolonged exposure to RSHI may lead to white matter (WM) changes in the brain, indicating axonal injuries.
Diffusion magnetic resonance imaging (dMRI) is an advanced, non‐invasive technique used to investigate the brain's WM (Goveas et al., 2015; Lebel & Deoni, 2018). Diffusion imaging metrics such as fractional anisotropy (FA) and mean diffusivity (MD) are sensitive to WM alterations arising for example from demyelination, axonal injury, or axonal degeneration (Alexander et al., 2011; Feldman et al., 2010; Jones et al., 2013) and have been widely used to study microstructural brain changes due to sport‐related concussion and RSHI‐exposure (Chamard & Lichtenstein, 2018; Gardner et al., 2012; Schneider et al., 2019). These long‐term WM alterations are commonly linked to reduced FA and increased MD, the two most reported diffusion metrics, indicating an overall rise in isotropic water diffusion. However, different scenarios causing WM changes can produce similar effects on diffusion parameters (Budde et al., 2011; Feldman et al., 2010; Figley et al., 2022). Therefore, when studying WM changes with dMRI, it is important to examine multiple diffusion metrics and their interrelations.
In recent research on various contact and collision sports (primarily based on pre‐ and post‐season data; e.g., Bahrami et al., 2016; Manning et al., 2020), longitudinal studies have been used to monitor and differentiate between subconcussive and concussive impacts. Schneider et al. (2019) found longitudinal changes in diffusion metrics in most of these studies. Still, the directionality of reported effects and affected regions are largely incongruent, most likely due to small sample sizes and large methodological differences across studies (e.g., analytical approach and selected region of interest). This heterogeneity and how study results are reported (e.g., lack of reporting peak coordinates and statistical quantities) make standard neuroimaging meta‐analytic approaches infeasible (Schneider et al., 2019). Consequently, a clear picture of global WM changes—potentially due to RSHI—in sports is lacking.
This study combines data from existing longitudinal dMRI studies on RSHI‐exposed contact and collision sports athletes. The goal is to explore characteristic patterns of WM changes linked to RSHI across sports while eliminating study‐specific effects due to methodological heterogeneity. By conducting a mega‐analysis (i.e., combining individual participant data in a single analysis) instead of the classical meta‐analytic approach (i.e., pooling site‐specific summary statistics in a second step; Zugman et al., 2022), this study was aimed at identifying potential sub‐thresholded WM changes that are not apparent in the individual studies. The brain's response to forces such as shear strain has been linked to diffuse axonal brain injuries (Borich et al., 2013; Li et al., 2010; Spain et al., 2010; Voelbel et al., 2012). Biomechanical studies using finite element modeling (i.e., simulating the mechanical response of the brain to head impacts) have shown that while strain waves propagate across the whole brain, the highest strains have been observed in the fornix, corpus callosum, midbrain, and other brainstem structures, pointing to a unique mechanical vulnerability of these WM structures to head impacts (Dashnaw et al., 2012; Ji et al., 2015; Ji et al., 2022; Zhao et al., 2019). Overall, the aim of this study was to explore whole brain WM changes using a data‐driven approach, under the hypothesis of widespread longitudinal WM changes. Crucially, the emphasis of this study was on athletes who have remained concussion‐free throughout the study period, allowing us to study the sole effects of RSHI.
2. MATERIALS AND METHODS
2.1. Literature search
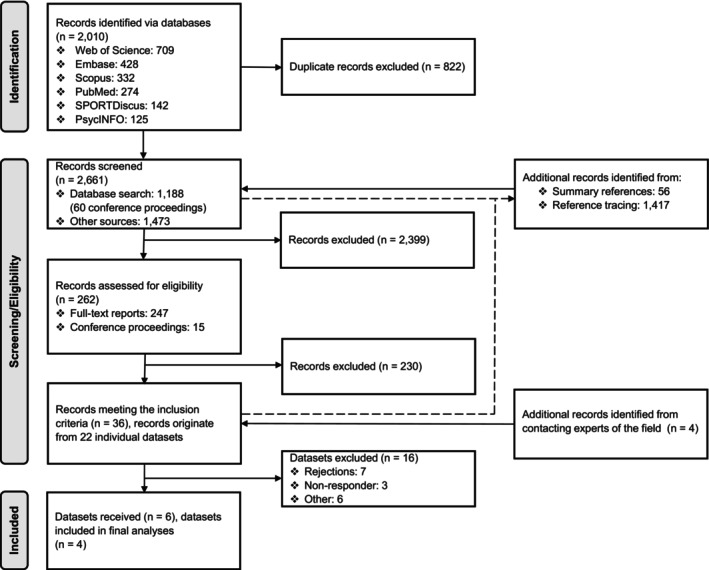
A literature search was performed and reported according to the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses guidelines (Page et al., 2021). It consisted of (1) an initial (database) search, and (2) a complemented search, including the screening of topic‐related summary references (i.e., reviews, meta‐analyses, book chapters, etc.) identified in the initial search, backward reference tracing by screening the reference lists of all studies meeting the inclusion criteria, and contacting experts in the field. The entire search process is illustrated in Figure 1. The following inclusion criteria were used: human subjects, original study, contact or collision sport athletes experiencing RSHI, including athletes who have not experienced a concussion during the study period, acquisition of dMRI data for at least two time points, and whole‐brain acquisition. Authors were contacted to request additional information if necessary.
FIGURE 1.
Flowchart illustrating the entire search process with number of records in‐/excluded.
The initial search was conducted on May 28, 2020, and March 15, 2021, using the online databases Web of Science, Embase, Scopus, PubMed, SPORTDiscus, and PsycINFO. A comprehensive search strategy was implemented to ensure that all studies with potentially suitable datasets were included (see Table 1 for an example and Supplementary Material S1 for the complete search strategy). Conference proceedings were included to identify unpublished datasets. All identified records were transferred to Endnote (Version X9; The EndNote Team, 2013), and duplicates were removed. For each record that passed the title and abstract screening, the full text was retrieved and checked for eligibility by two independent researchers (AK and CH). Disagreements between reviewers were resolved through joint discussion.
TABLE 1.
Example of the electronic search strategy performed via PubMed.
| Search number | Search terms |
|---|---|
| 1 | “diffusion tensor imaging” OR dti OR “diffusion tensor” OR “diffusion magnetic resonance imaging” OR “diffusion weighted imaging” OR DWI OR “fractional anisotropy” OR “mean diffusivity” OR “apparent diffusion coefficient” OR “radial diffusivity” OR “axial diffusivity” OR “white matter” |
| 2 | subconcuss* OR sub‐concuss* OR repetitive OR repeated OR recurrent OR multiple OR subclinical OR sub‐clinical OR cumulative OR chronic |
| 3 | impact* OR hit OR hits OR blow* OR event* |
| 4 | #2 AND #3 |
| 5 | “head injur*” OR “brain injur*” OR “head trauma*” OR “brain lesion*” OR concuss* OR “traumatic brain injury” OR “sport‐related concussion” OR heading |
| 6 | #4 OR #5 |
| 7 | sport* OR athlete* |
| 8 | #1 AND #6 AND #7 |
2.2. Data collection and data cleaning
Authors of eligible studies were contacted to request raw or pre‐processed dMRI data, T1‐anatomical images, and individual demographic details (i.e., age, sex, handedness, sports affiliation, lifetime concussions, and imaging dates to calculate individuals' scan intervals). Individual season dates were requested and/or complemented by publicly available information to provide study‐wise information on season and recreational intervals. Further details on interval definitions can be found in Supplementary Material B. For clarity and cross‐study comparison, this article refers to the first (pre‐ or in‐season) and second measurement time point as baseline and post‐season, respectively. Study‐specific details regarding level of play, country of data collection, concussion assessment within and prior to study participation, measurement time points, and related sport participation (with potential head impact exposure) before the baseline and within the recreational period were extracted from the original manuscripts. Subjects with incomplete imaging data and those who suffered a concussion during the respective study were removed from the dataset. For subjects who participated more than once (e.g., across multiple seasons), only their first data pair was included in the final dataset.
2.3. Quality assurance and data processing
Details on quality assurance and data processing steps are reported in Supplementary Material C. Quality assurance steps included: reorganizing the original data into the Brain Imaging Data Structure (BIDS) (Gorgolewski et al., 2016), visual inspection of raw imaging data, single‐subject and study‐wise automatic quality control, and repeated visual inspection of FA maps throughout preprocessing and registration steps. Standard preprocessing steps for raw dMRI data followed the ENIGMA‐DTI protocol (Hadded, 2022) and were performed using the MRtrix3 tool (Version 3.0.RC3, Tournier et al., 2019). Whole MRI acquisition details and preprocessing steps can be found in Supplementary Material D. Diffusion tensor maps were generated from pre‐processed images and used to extract the individual DTI metric maps (FA, MD, AD, RD). Successively, each T1‐weighted image was co‐registered to the unweighted diffusion volume (b = 0) and a brain extraction mask was derived. This mask was additionally eroded by one voxel at the resolution of the diffusion image and applied to each DTI metrics map to remove the bright “halo” of voxels due to eddy current distortions.
Brain extraction and registrations were performed using the Advanced Normalization Tools (Version 2.3.5; Tustison et al., 2021). Groupwise image registration of the individual FA maps was performed according to Schwarz et al. (2014) for improved specificity in avoiding false positives resulting from misregistration and increased sensitivity in detecting true changes compared to the conventional approach of tract‐based spatial statistics (Smith et al., 2006). Subsequently, all images were transformed to the Montreal Neurological Institute (MNI) standard space and resampled to a 2 × 2 × 2 mm resolution. Finally, difference maps were calculated for each of the DTI metrics using the fslmaths tool from FSL (Jenkinson et al., 2012) by subtracting post‐season to baseline images and vice versa. To limit statistical analyses to mostly WM regions, all individual FA maps (baseline and post‐season) were thresholded and combined to generate a WM mask. This mask included voxels with an FA value of 0.2 or higher in at least 50% of the images. The final mask included only the cerebrum, medulla, and pons to mitigate registration issues caused by missing cerebellum and peripheral brainstem regions in several dMRI images (due to bad positioning or simply lack of interest from the original investigators).
2.4. Statistical analyses
The samples' descriptives were calculated in IBM's Statistical Package for the Social Sciences (Version 29; IBM Corp, 2022). To examine sports‐season‐related changes in WM microstructure, a mega‐analytic approach was chosen, and voxel‐wise analyses were performed using FSL's randomise for nonparametric permutation inference (Winkler et al., 2014). For each metric, increases and decrease were tested separately using a one‐sample t test on the difference maps (raw difference between the post‐season and baseline measurement time point and vice versa) with 10,000 permutations. The curated dataset did not allow for examining sex‐ and sport‐specific effects due to their strong dependency on the specific research site (see Table 2), as it was not possible to sufficiently account for other site differences, such as variations in hardware or data acquisition. Nonetheless, we chose to address potential influences of sex. In the final analyses, the factors sex and site were de‐meaned and included as covariates. Age was not included, given that the overall sample exhibited a narrow age range that was comparable across all sites (mean = 20.06, SD = 2.06). The threshold‐free cluster enhancement method was applied, and results were FWE‐corrected to control for the family‐wise error rate. Statistical significance was defined as p < .025 (one‐sided testing). To ensure the reliability and robustness of the reported findings and to present the overall results concisely, a cluster size threshold of at least 50 voxels was chosen for reporting results. For clusters exceeding this threshold, the voxel‐wise and mean percentage change was calculated. Information on smaller clusters can be reviewed in the Supplementary Material E.
TABLE 2.
Descriptives of included studies.
| Dataset | Country | Sport | Level | Female/male | Mean age ± SD (years) | Mean lifetime concussion, range | Scan interval ± SD (days) | Season interval ± SD (days) | Recreational interval ± SD (days) |
|---|---|---|---|---|---|---|---|---|---|
| 1 a | CA | AF | University | 0/23 | 20.09 ± 1.31 | 0.91, 0–4 | 131.39 ± 26.67 | 60.91 ± 3.59 | 28.35 ± 7.53 |
| 2 b | US | AF | University | 0/36 | 19.67 ± 1.17 | ‐ | 96.86 ± 7.45 | 49.00 ± 0.00 c | 6.56 ± 5.57 |
| 3 d | CA | RU | University | 43/0 | 19.65 ± 1.33 | 0.60, 0–3 | 158.43 ± 26.44 | 44.16 ± 6.60 | 117.97 ± 39.78 |
| 4 e | CA | IH | University | 13/16 | 21.14 ± 3.51 | ‐ | 188.97 ± 20.16 | 132.14 ± 11.14 | 30.97 ± 7.24 |
| Total | 56/75 | 20.06 ± 2.06 | 0.71, 0–4 | 142.81 ± 40.56 | 67.91 ± 35.46 | 49.21 ± 50.87 |
Note: CA = Canada, US = United States, AF = American football, RU = rugby, IH = ice hockey, SD = standard deviation, scan interval = days between baseline and post‐season scan, season interval = days between season start and season end, recreational interval = days between season end and follow‐up scan.
Champagne et al. (2019).
Hirad et al. (2019).
Equal interval for all participants.
Manning et al. (2020).
Weber et al. (2018).
3. RESULTS
3.1. Literature search
The results from the literature search process are summarized in Figure 1. The database search yielded 2010 records, which were reduced to 1188 records after removing duplicate entries. The 56 additional records were identified from the summary references, and 1417 from the reference tracing. A total of 262 records passed the title and abstract screening, and 32 remained after the full‐text eligibility assessment. Four additional records were identified by experts in the field, eventually yielding 36 final records meeting the complete list of inclusion criteria. The 36 records originated from 22 individual datasets (see Supplementary Material F for a complete list of identified records).
3.2. Data collection, data cleaning, and quality assurance
Six datasets were available and were shared by the corresponding authors. Of these, only four datasets were comparable with respect to the data collection time frame (i.e., having acquired baseline and post‐season data) and were combined for the final analyses. Requests for the remaining datasets were declined mainly due to ethical or institutional regulations. Other exclusion reasons included ongoing data collection, missing data, and workload concerns. Three additional datasets were published on the Federal Interagency Traumatic Brain Injury Research data repository platform (https://fitbir.nih.gov/)—however, two remained inaccessible due to ongoing quality control procedures, and another lacked sufficient information to identify the data related to the subjects of interest.
Of the final sample, three groups shared raw imaging data, while the fourth shared pre‐processed imaging data (i.e., DTI metric maps). The final combined cohort included 135 non‐concussed athletes with complete baseline and post‐season data. Four athletes were excluded from the final analyses due to brain deformation (n = 1), acquisition error (n = 1), and failed preprocessing (n = 2), leaving a total of 131 athletes for final analyses. Of note, most athletes had their baseline measurement acquired during the pre‐season; however, for some athletes (17%), it was acquired after the start of the season.
3.3. Sample characteristics
The descriptives of individual studies and included athletes are shown in Table 2. The final cohort comprised 56 female and 75 male players with a mean age of 20.06 (SD = 2.06) years, including 59 American football players, 43 rugby players, and 29 ice hockey players. The assessment of handedness was limited to a small sample of athletes (n = 22), with the majority (91%) being right‐handed. Across all athletes, the scan interval was 142.81 (SD = 40.56, range 81–246) days. The average season interval was 67.91 (SD = 35.46, range 39–142) days, and the average recreational interval was 49.21 (SD = 50.87, range 0–243) days. All players practiced their sport at the university level. Further details on sample characteristics can be reviewed in Supplementary Material G and H.
3.4. Mega‐analyses results of DTI‐changes
Imaging results are visualized in Figures 2, 3, 4. Detailed results for significant clusters can be found in Table 3. Three clusters with significant MD changes throughout the sports season were found, one with increased MD values and two with decreased MD values. The largest cluster (MD1: 3195 voxels; mean change: 2.34%) exhibited widespread increases in central and brainstem regions. This cluster mainly extended across the anterior thalamic radiation (bilateral) and corticospinal tract (bilateral), with more pronounced changes in the right hemisphere. Clusters exhibiting MD decreases were located in the left hemisphere. The most notable one (MD2: 1498 voxels; mean change: −1.43%) showed sports‐season‐related changes mainly localized in the superior longitudinal fasciculus and corticospinal tract, extending further across the cingulum, inferior fronto‐occipital fasciculus, and inferior longitudinal fasciculus. Additionally, a smaller cluster (MD3; mean change: −1.48%) with MD decreases was found in the frontal parts, spanning over a region of overlapping tracts, which included the inferior fronto‐occipital fasciculus, uncinate fasciculus, forceps minor, and anterior thalamic radiation.
FIGURE 2.
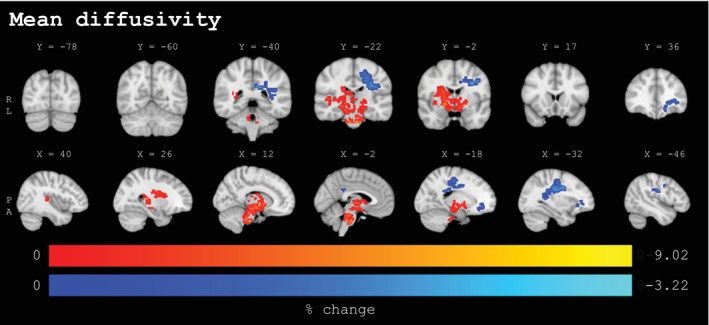
Percentage change values for clusters (≥50 voxels) with statistically significant baseline to post‐season changes from voxel‐wise analyses for mean diffusivity (n = 131, p < .025, FWE‐corrected, covariates: sex, site) in coronal (upper panel) and sagittal (lower panel) view. The lower panel extends from the left to the right hemisphere. Significant voxels underwent linear interpolation for enhanced visualization. Sport‐season‐related increases are depicted in red‐yellow, decreases are depicted in blue‐lightblue. Results are overlaid on the MNI152‐T1‐1mm standard image in radiological convention (right = subject's left). Details on individual clusters are given in Table 3. X/Y = standard space coordinate of slice (x/y‐direction), R = right, L = left, P = posterior, A = anterior.
FIGURE 3.
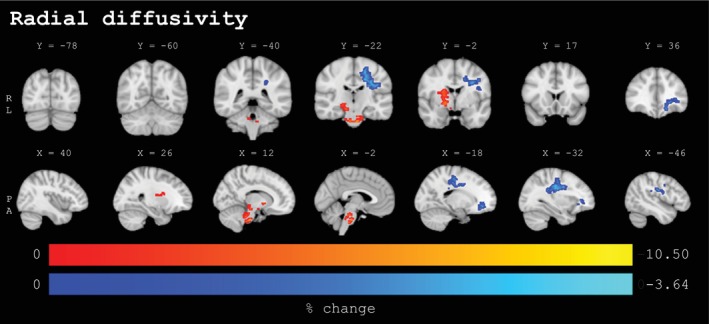
Percentage change values for clusters (≥50 voxels) with statistically significant baseline to post‐season changes from voxel‐wise analyses for radial diffusivity (n = 131, p < .025, FWE‐corrected, covariates: sex, site) in coronal (upper panel) and sagittal (lower panel) view. The lower panel extends from the left to the right hemisphere. Significant voxels underwent linear interpolation for enhanced visualization. Sport‐season‐related increases are depicted in red‐yellow, decreases are depicted in blue‐lightblue. Results are overlaid on the MNI152‐T1‐1mm standard image in radiological convention (right = subject's left). Details on individual clusters are given in Table 3. X/Y = standard space coordinate of slice (x/y‐direction), R = right, L = left, P = posterior, A = anterior.
FIGURE 4.
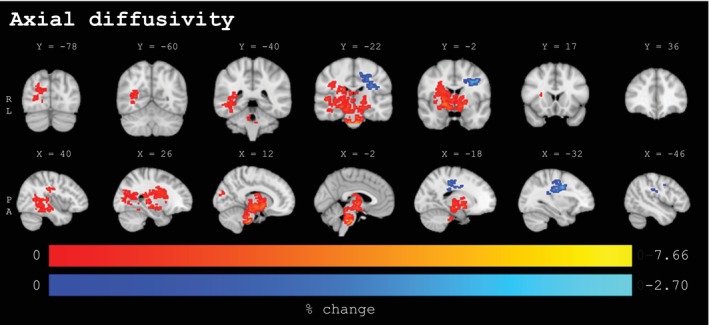
Percentage change values for clusters (≥50 voxels) with statistically significant baseline to post‐season changes from voxel‐wise analyses for axial diffusivity (n = 131, p < .025, FWE‐corrected, covariates: sex, site) in coronal (upper panel) and sagittal (lower panel) view. The lower panel extends from the left to the right hemisphere. Significant voxels underwent linear interpolation for enhanced visualization. Sport‐season‐related increases are depicted in red‐yellow, decreases are depicted in blue‐lightblue. Results are overlaid on the MNI152‐T1‐1mm standard image in radiological convention (right = subject's left). Details on individual clusters are given in Table 3. X/Y = standard space coordinate of slice (x/y‐direction), R = right, L = left, P = posterior, A = anterior.
TABLE 3.
Significant clusters of MD, RD, and AD with a size of ≥50 voxels.
| Cluster | Directionality | Number of voxels | p‐Value (max.) | MNI‐coordinates, region (max.) a | Subcomponents a |
|---|---|---|---|---|---|
| MD1 | Increase | 3195 | 0.001 | [18, 8, −2], Inferior fronto‐occipital fasciculus R | Anterior thalamic radiation L, Anterior thalamic radiation R, Corticospinal tract L, Corticospinal tract R, Forceps minor, Inferior fronto‐occipital fasciculus L, Inferior fronto‐occipital fasciculus R, Inferior longitudinal fasciculus R, Superior longitudinal fasciculus R, Uncinate fasciculus R, Superior longitudinal fasciculus (temporal part) R |
| MD2 | Decrease | 1498 | <0.001 | [−32, −12, 30], Superior longitudinal fasciculus L, Superior longitudinal fasciculus (temporal part) L | Anterior thalamic radiation L, Anterior thalamic radiation R, Corticospinal tract L, Cingulum (cingulate gyrus) L, Inferior fronto‐occipital fasciculus L, Inferior longitudinal fasciculus L, Superior longitudinal fasciculus L, Superior longitudinal fasciculus (temporal part) L |
| MD3 | Decrease | 139 | 0.011 | [−32, 36, 2], Inferior fronto‐occipital fasciculus L, Uncinate fasciculus L, Anterior thalamic radiation L | Anterior thalamic radiation L, Cingulum (cingulate gyrus) L, Forceps minor, Inferior fronto‐occipital fasciculus L, Uncinate fasciculus L, Superior longitudinal fasciculus (temporal part) L |
| RD1 | Increase | 383 | 0.004 | [8, −34, −30], Anterior thalamic radiation R, Corticospinal tract R | Anterior thalamic radiation L, Anterior thalamic radiation R, Corticospinal tract L, Corticospinal tract R |
| RD2 | Increase | 344 | 0.002 | [16, 8, −2], Anterior thalamic radiation R | Anterior thalamic radiation R, Corticospinal tract R, Inferior fronto‐occipital fasciculus R, Superior longitudinal fasciculus R, Superior longitudinal fasciculus (temporal part) R |
| RD3 | Increase | 90 | 0.014 | [22, −22, −8], Corticospinal tract R | Anterior thalamic radiation R, Corticospinal tract R |
| RD4 | Decrease | 1097 | <0.001 | [−32, −10, 30], Superior longitudinal fasciculus L, Superior longitudinal fasciculus (temporal part) L | Anterior thalamic radiation L, Corticospinal tract L, Cingulum (cingulate gyrus) L, Inferior fronto‐occipital fasciculus L, Inferior longitudinal fasciculus L, Superior longitudinal fasciculus L, Superior longitudinal fasciculus (temporal part) L |
| RD5 | Decrease | 143 | 0.014 | [−20, 42, −2], Forceps minor, Anterior thalamic radiation L, Inferior fronto‐occipital fasciculus L, Uncinate fasciculus L | Anterior thalamic radiation L, Cingulum (cingulate gyrus) L, Forceps minor, Inferior fronto‐occipital fasciculus L, Uncinate fasciculus L, Superior longitudinal fasciculus (temporal part) L |
| AD1 | Increase | 5740 | <0.001 | [20, 0, −6], Unclassified | Anterior thalamic radiation L, Anterior thalamic radiation R, Corticospinal tract L, Corticospinal tract R, Cingulum (hippocampus) R, Forceps major, Forceps minor, Inferior fronto‐occipital fasciculus L, Inferior fronto‐occipital fasciculus R, Inferior longitudinal fasciculus L, Inferior longitudinal fasciculus R, Superior longitudinal fasciculus R, Uncinate fasciculus R, Superior longitudinal fasciculus (temporal part) R |
| AD2 | Decrease | 724 | <0.001 | [−34, −6, 34], Superior longitudinal fasciculus L, Superior longitudinal fasciculus (temporal part) L | Anterior thalamic radiation L, Anterior thalamic radiation R, Corticospinal tract L, Cingulum (cingulate gyrus) L, Inferior fronto‐occipital fasciculus L, Superior longitudinal fasciculus L, Superior longitudinal fasciculus (temporal part) L |
Abbreviations: AD, axial diffusivity; L, left; Max., maximum; MD, mean diffusivity; MNI, Montreal Neurological Institute; R, right; RD, radial diffusivity.
The JHU WM tractography atlas (Hua et al., 2008) was used to identify brain regions at cluster peak coordinates and subcomponents, no significant clusters with ≥50 voxels were found for fractional anisotropy.
Radial diffusivity (RD) and axial diffusivity (AD) changes were spatially and directionally similar to the observed changes in MD. Notably, whereas in the left‐sided regions, both RD and AD changes were less pronounced (1240 and 724 total voxels, respectively) compared to changes in MD (1637 total voxels), in the central and brainstem regions, RD changes were considerably less (817 total voxels), and AD changes were considerably more pronounced (5740 total voxels) as compared to the observed changes in MD (3195 total voxels). Sports‐season‐related changes with increases in RD were spread across three spatially adjacent clusters (RD1‐3: ranging between 90 and 383 voxels and a mean change of 3.11 to 4.70%, respectively) and concentrated predominantly in the corticospinal tract (bilateral) and right anterior thalamic radiation. Decreases in RD were found in two clusters of the left hemisphere, a dominant one (RD4: 1097 voxels; mean change: −1.92%) in the superior longitudinal fasciculus and corticospinal tract, and a smaller cluster (RD5; mean change: −1.93%) across overlapping tracts in frontal regions, including the inferior fronto‐occipital fasciculus, uncinate fasciculus, forceps minor, and anterior thalamic radiation.
For AD, a single extensive cluster (AD1: 5740 voxels; mean change: 1.75%) with increased values and an additional cluster (AD2: 724 voxels; mean change: −1.28%) with AD decreases were observed. The former extended across a wide range of brainstem and central regions, including the following: anterior thalamic radiation (bilateral), corticospinal tract (bilateral), forceps major, inferior fronto‐occipital fasciculus (bilateral), inferior longitudinal fasciculus (bilateral), and right superior longitudinal fasciculus. Although AD increases were observed bilaterally, these were much more pronounced in the right hemisphere, where AD changes extended into the posterior parts of the tracts. Finally, the latter cluster was found in the left hemisphere spreading across the superior longitudinal fasciculus and corticospinal tract. Although no significant clusters with sports‐season‐related changes in FA reached a voxel size of 50, a similar lateralized pattern, as observed in the other DTI measures, emerged from the non‐thresholded results (see Supplementary Material I). However, this nonsignificant trend exhibited opposing directionality. Specifically, FA increases were predominantly spread bilaterally across the frontal lobe and superior regions of the left hemisphere, while decreases in FA were primarily concentrated in the midbrain, brainstem, and posterior brain regions, with more pronounced changes evident in the right hemisphere. Significant clusters for FA, MD, RD, and AD smaller than the 50‐voxel threshold appeared in close approximation to larger clusters and exhibited the same directional trend (for details, please review Supplementary Material E).
4. DISCUSSION
The primary goal of this study was to explore characteristic patterns of longitudinal brain WM changes resulting from RSHI‐exposure in contact and collision sports athletes. Previous attempts to conduct meta‐analyses combining available prospective studies failed due to considerable methodological differences and/or a lack of reporting peak coordinates and necessary statistical quantities (Koerte et al., 2023; Schneider et al., 2019). Through a systematic literature search, a total of 22 individual datasets from prospective dMRI studies were identified. Four datasets (131 athletes) were combined for joint analyses utilizing a mega‐analytic approach. Overall, the findings revealed a lateralized pattern of both sports‐season‐related increases and decreases (i.e., baseline to post‐season changes) in DTI parameters across spatially distinct brain WM regions.
Increases in MD, RD, and AD were found and primarily spread across different central and widespread brainstem regions, including the anterior thalamic radiation, corticospinal tract, and the inferior fronto‐occipital fasciculus. While there were noticeable increases in RD in the anterior thalamic radiation and corticospinal tract, increases in MD and AD appeared to be much more pronounced and widespread. Although increases in the three parameters were bilateral, results showed a distinct right‐dominance. The lack of significant changes in FA diverges from the general trend seen in previous studies on head impact exposure: a decrease in FA over time or in comparison with a control group that often coexists with an increase in the other DTI parameters (Koerte et al., 2023). However, some studies within the field have also reported no changes in FA while observing changes in other DTI parameters (Koerte, Ertl‐Wagner, et al., 2012; Myer et al., 2016; Yuan et al., 2018). Of note, the non‐thresholded FA results in the joint analyses showed a nonsignificant trend of widespread FA decreases that greatly overlapped with changes in the other three parameters. Figley et al. (2022) have highlighted that in the presence of crossing fibers, which is the case in most WM voxels, FA can remain stable even with significant alterations in the underlying tissue composition. This phenomenon occurs when the tensor eigenvalues change proportionally and could explain our observations.
The observed regional increases in MD, RD, and AD in the central and brainstem regions correspond closely with high‐strain regions identified in studies on biomechanics of head impacts and mTBI. Biomechanical studies simulating sport‐related concussive and/or subconcussive head impacts in professional American football players have indicated that maximum strain occurs at the core of the brain (i.e., fornix, diencephalon, and midbrain; Viano et al., 2005; Zhang et al., 2004; Zhao et al., 2017; Zhao et al., 2019). Most recently, the inferior fronto‐occipital fasciculus and cerebral peduncle (midbrain) have been identified among those regions to experience highest maximum principal strain (Zhao et al., 2019; Zhao et al., 2017). Further, the brainstem has been widely recognized as being particularly vulnerable to head impacts (Delano‐Wood et al., 2015; Gaetz, 2004), likely due to its geometric discontinuity with respect to the cerebrum (i.e., crossing the brain's rostro‐caudal axis at almost perpendicular angles; Arbogast & Margulies, 1998) and elevated pressure gradients terminating at the brainstem (Delano‐Wood et al., 2015; Ropper & Gorson, 2007). Supporting these findings, Kim et al. (2021) investigated volume changes using whole‐brain tensor‐based morphometry in mTBI patients, which exhibited significant loss in brainstem volume, while cerebral volume appeared to be preserved. Altogether, the regional correspondence of sports‐season‐related changes in the current study and previously identified high‐strain regions in response to head impacts suggests that the observed changes may reflect WM injuries deriving from their unique biomechanical vulnerability.
In the current findings, the greater expression of AD and MD changes in the central and brainstem regions compared to RD is an interesting observation that may help to better understand the nature of the underlying WM changes. AD changes were most pronounced and extended to the posterior projections of the inferior longitudinal fasciculus and forceps major within the right occipital lobe. Since MD reflects the average diffusivity across all tensors principal directions and is mathematically dependent on AD (Soares et al., 2013; Song et al., 2002), capturing diffusion along the primary direction, it appears that MD alterations are largely driven by changes in AD in these regions. Unlike FA, which is generally sensitive to WM organization changes but lacks specificity in identifying the type of change, AD and RD have been linked to direct microstructural changes (i.e., axonal and myelin injury, respectively; Alexander et al., 2011; Budde et al., 2011; Song et al., 2005). Another interesting observation is the right‐hemispheric dominance of the observed increases in DTI parameters. It is possible that an athlete's protective response, while anticipating a collision, could change brain accelerations and strain wave trajectories (i.e., how the brain moves and experiences strain within the scalp). For example, reflexive head movements toward one body side could change impact location inducing higher strain to the right hemisphere, and handedness may play an important role in this context. It is important to emphasize that this explanation is purely hypothetical and requires further investigation. Previous studies investigating sport‐related head impacts also observed lateralized patterns of DTI changes. However, while two studies observed changes exclusively in the left hemisphere (Cubon et al., 2011; Mayinger et al., 2018), other studies (Hirad et al., 2019; Koerte, Kaufmann, et al., 2012) reported changes in right brain regions only. Notably, data from three of these studies (Hirad et al., 2019; Koerte, Kaufmann, et al., 2012; Mayinger et al., 2018) were included in our joint analyses. The discrepancies may arise from methodological heterogeneities, such as variations in the sampled sports, and need additional research.
The final major finding was decreases in MD, RD, and AD across two left‐hemispheric WM regions: one located in superior and the other in frontal brain regions. Unlike the described changes in central and brainstem regions, these left‐sided changes were spatially distinct and showed opposing directionality, suggesting different underlying processes. Furthermore, changes were less pronounced in both spatial distribution and magnitude. Moreover, left‐sided MD changes appeared to be almost equally driven by RD and AD alterations, with AD changes slightly less pronounced. At first, there appears to be no clear explanation for why alterations exclusively manifest in the left hemisphere. However, while the affected tracts, particularly the corticospinal tract and superior longitudinal fasciculus, contribute to various cognitive and sensory functions, they also play a crucial role in motor functioning (e.g., planning, coordinating, and executing different motor tasks; Aralasmak et al., 2006; Forkel et al., 2022; Marmarou, 2011). One could speculate that these sports‐season‐related changes stem from extensive motor training and skill acquisition throughout the season, possibly related to handedness. For instance, improvements in the movement sequences of throwing techniques achieved through many repetitions with the dominant hand may have induced left‐sided structural changes through heightened stimulation of lateralized motor and sensory regions. Nonetheless, the nature of the changes warrants further investigation. Studies investigating the effects of physical exercise on brain WM in healthy subjects have yielded positive yet modest effects on global WM volume, local microstructural organization, and reductions in WM anomalies. Interestingly and similar to the current study findings, evidence links physical exercise to localized frontal lobe changes. However, due to several limitations, including the predominant focus on the aging brain, current results from this field are regarded as being preliminary (Maleki et al., 2022; Sexton et al., 2016). Essentially, the extent of physical exercise‐induced effects across the sports season on brain WM in contact and collision sport athletes, particularly in players with years of training, remains unclear.
In summary, the observed increases in MD, RD, and AD in the current study largely overlap with designated high‐strain regions and vulnerable structures as identified in the broader TBI literature. Therefore, these changes may reflect WM injuries that accumulate throughout the season. On the contrary, the spatially distinct decreases within the same parameters in the left‐hemisphere were less pronounced and are somewhat less elucidated, yet they might possibly reflect enhanced communication within the brain's motor network attributable to extensive motor training throughout the season. Furthermore, in accordance with several recommendations (e.g., Figley et al., 2022; Jones et al., 2013; Soares et al., 2013), we refrain from engaging in elaborate conjectures about the potential underlying biological correlates explaining the observed changes, and we strongly emphasize that the provided interpretations require confirmation from future studies. Despite being a promising tool to study in vivo WM microstructure, DTI tensor metrics are indirect measures, and interpreting them in isolation remains extremely complex (Figley et al., 2022). Specifically, both increases and decreases in DTI parameters have been shown to reflect positive and negative brain changes, respectively. The use of more advanced techniques (e.g., diffusion kurtosis imaging, neurite orientation dispersion and density imaging, tensor‐valued diffusion encoding, and novel fixed‐based analysis approaches; Figley et al., 2022) and multimodal approaches to support the interpretability of DTI findings (e.g., MR spectroscopy, functional MRI, amplified MRI, and brain‐derived blood biomarkers; Koerte et al., 2023) will be crucial to gain a better understanding of the pathophysiological and neuroplastic processes behind DTI changes emerging throughout the sports season.
While the curated dataset could not facilitate multimodal comparisons due to variations in MRI acquisition sequences, the studies of the constituent datasets have used imaging modalities complementary to dMRI. For example, similar to the overall results, one study (Manning et al., 2020) reported increases in MD, RD and AD across multiple WM tracts which were accompanied by hyperconnectivity in structurally and functionally related brain regions. As proposed in the study, this pattern could indicate the presence of ongoing neuroprotective or compensatory mechanisms triggered by the disruption of the underlying axonal organization. More precisely, the hyperconnectivity might signify an effort to maintain normal functioning by enlisting a broad range of brain regions. The growing utilization of multimodal imaging (e.g., Jarrett et al., 2016; Manning et al., 2020; Schranz et al., 2018; Weber et al., 2018; Wright et al., 2016) in conjunction with recent advancements in imaging and analytical techniques, holds promise to further enhance our understanding of the underlying processes contributing to alterations in the microstructure of WM resulting from sports‐related RSHI‐exposure.
4.1. Strengths and limitations
The creation of a curated RSHI dataset and joint analyses was an important achievement to characterize overall patterns of sports‐season‐related WM changes across different contact and collision sports. Given small sample sizes in most RSHI studies, the use of meta‐ and mega‐analytic approaches is of great importance for summarizing these changes and deriving robust conclusions about the potential harmfulness of sport‐related RSHI‐exposure. The availability of individual participant data has facilitated the adoption of a one‐stage (mega‐analytic) approach, offering several advantages over conventional neuroimaging meta‐analytic methods (e.g., increased statistical power, acceptable false‐positive rates, consistency in inclusion criteria, standardization of procedures, etc.; Zugman et al., 2022). A further strength of this study is the implementation of a special registration technique, enabling whole‐brain voxel‐wise analyses instead of TBSS (Schwarz et al., 2014). By leveraging these strengths, a comprehensive data‐driven investigation of the entire brain's WM was possible. This is particularly valuable when exploring overarching patterns of brain WM changes and unidentified regions that may have been overlooked in previous underpowered studies.
The results from the joint analyses should be regarded in light of some important limitations. First, the nature of our Mega‐Analysis necessitated the inclusion of all available studies, limiting our control over the acquisition setup across different sites. These variations could have introduced biases in signal intensity values and DTI indices. However, we implemented several strategies to substantially mitigate these potential confounding effects, thus allowing for meaningful interpretation of the data (i.e., conducting within‐subject comparisons between two scans under identical conditions, applying a standardized preprocessing protocol across sites, employing nonlinear registration to a group template, and controlling for site effects during the analyses). Similarly, the scope of the data analyzed also impacts the generalizability of our findings. While the data are representative of studies in the RSHI field, they predominantly originate from male American football players from the United States and Canada, and all athletes played at university level, the highest amateur level. Hence, the generalizability of our findings to a broader population of contact and collision sport athletes, including those at lower competitive levels (which likely constitute most active players) or from non‐represented sports such as soccer or lacrosse, as well as female athletes, or athletes from other countries and ethnic groups, may be limited.
Additionally, due to a lack of comparable cross‐study data, it was not possible to conduct group analyses using control‐group data, to include important covariates (e.g., number of lifetime concussions, handedness), and to correlate findings with head impact or clinical measures. As a result, for the current findings, it remains challenging to disentangle physical exercise‐induced changes and injury‐related effects, and to rule out alternative explanations (e.g., changes due to normal neurological development, although considered unlikely; Jang et al., 2019; Mayinger et al., 2018; Yuan et al., 2018). Nevertheless, part of the combined datasets utilized helmet accelerometer data to investigated WM changes across the sport season (Bazarian et al., 2014; Champagne et al., 2019; Hirad et al., 2019; Merchant‐Borna et al., 2016). Notably, Hirad et al. (2019) hypothesized and found declines in midbrain WM organization through region of interest analysis. This decline correlated inversely with the amount of experienced rotational accelerations from head impacts, supporting the notion that the observed WM changes in the overall findings within the same regions may indeed signify injury and repair processes due to repeated sports‐related RSHI‐exposure.
Second and previously mentioned, DTI is a useful but indirect measure of WM changes that does not directly elucidate the underlying biological mechanisms, and it changes dynamically in response to various processes. Following mTBI, studies have reported opposing directionality of DTI alterations, which are believed to indicate distinct processes during acute and chronic injury phases (Eierud et al., 2014; Pavlovic et al., 2019). In this regard, the repetitive nature of RSHI throughout the season adds another layer of complexity to the interpretation of longitudinal DTI changes, as these changes may reflect injury and repair processes at different stages. Also, it is worth noting that there was cross‐study heterogeneity regarding the exact measurement time points (e.g., pre‐ vs. in‐season baseline scan) and pre‐season head impact exposure. Given the dynamic nature of DTI, the proximity of head impact exposure to the time of measurement could potentially have influenced both the directionality and the magnitude of observed changes in diffusivity. This variability might have introduced biases into the interpretation of sports‐season‐related changes. Finally, it is important to consider the potential publication bias as studies without significant effects, particularly given the typically small sample sizes in RSHI studies, may have been less likely to be published.
4.2. Implications and future studies
Our findings have implications for characterizing and understanding brain microstructural changes related to RSHI‐exposure in sports that could guide future research exploring the biological correlates of these changes. The findings of this study indicate potential health risks in the form of acquired brain WM injuries within central and brainstem regions, which play a crucial role in vital brain functions. Injuries in these areas can cause a range of symptoms, including sleep disturbances, motor deficits, and balance problems (Benghanem et al., 2020; Boisgontier et al., 2017; Delano‐Wood et al., 2015). Eventually, these insights may be crucial to inform active athletes about potential health risks and to develop strategies to prevent long‐term adverse effects.
Furthermore, this study provides important insights into researchers' reasons for not sharing research data (e.g., missing data and workload concerns), which holds significant implications for future collective research initiatives aimed at promoting data pooling and enabling robust meta‐ and mega‐analyses. While researchers generally expressed their willingness to contribute, only 6 out of 22 datasets were received. Reasons for not sharing data, as well as incomprehensive data labeling of a published dataset, emphasize the importance of initially implementing thorough data management practices. These also include early consideration (ideally when planning and initiating new studies) of local ethical regulations pertaining to data sharing and reuse, for example, regarding the upload of research data on scientific repositories.
In addition to direct implications derived from our findings, further important considerations for future RSHI research have recently been discussed (Koerte et al., 2023) and aim to (1) facilitate the comparability of findings across studies and (2) improve interpretability of DTI changes regarding their diagnostic value and relation to underlying biological changes. These considerations include establishing standard dMRI acquisition and analysis protocols, multimodal imaging, as well as the investigation of interactive effects between physical exercise and strain‐induced injuries. Regarding the latter, it will be crucial to investigate physical exercise‐related brain changes specific to contact and collision sports athletes. Current research on physical exercise and brain WM changes has primarily focused on older adults, moderate intensity levels, and individualized sports such as walking and cycling (Maleki et al., 2022; Sexton et al., 2016). However, contact‐ and collision sports are predominantly favored by younger individuals (i.e., children, young adults), at various intensity levels, playing for several years, and demanding diverse cognitive capabilities required for complex motor functioning and team‐oriented play (Draheim et al., 2022; Eime et al., 2016; McNeese et al., 2016). Additionally, while sports‐season‐related WM alterations seem to reverse during recreational periods, it has been assumed that the initiated repair processes may not have the capacity to fully reverse the damage (Mayinger et al., 2018; Myer et al., 2019). Only one of the reviewed studies followed athletes across multiple seasons (Manning et al., 2020). However, given that athletes usually practice their sport for years, it will be important to investigate the effects of sport‐related RSHI‐exposure over longer timeframes. Finally, with the availability of more data, it would be interesting to investigate potential characteristic patterns that are specific to male and female athletes, as well as different sports.
CONCLUSION
This study reveals widespread sports‐season‐related brain WM changes in contact and collision sports athletes. The observed changes in central and brainstem regions (particularly in the anterior thalamic radiation, corticospinal tract, and inferior fronto‐occipital fasciculus) overlap with designated high‐strain regions as determined by biomechanical and brain morphometry studies. These changes likely reflect microstructural brain WM changes as a consequence of season RSHI‐exposure. While less prominent, the opposing pattern of WM‐changes in the left hemisphere may reflect physical exercise‐induced effects from extensive motor training and skill acquisition throughout the season. Overall, the results highlight that both positive and negative sports‐season‐related effects likely co‐occur, potentially affecting similar brain areas, while region‐specific effects emerge where one dominates. DTI is sensitive but limited to the investment of overall patterns of brain WM changes. The observed patterns can guide future research exploring the biological correlates of these changes, requiring advanced models and multimodal approaches. To draw reliable conclusions regarding potential health risks and their severity for active athletes, future studies should resolve several limitations, address important confounding factors (e.g., physical exercise‐induced effects and last impact exposure), and follow athletes across multiple seasons. The latter is crucial for understanding how strain‐induced injuries reverse during off‐season recreational periods and to what extent residual changes accumulate across several seasons.
AUTHOR CONTRIBUTIONS
Anna Kwiatkowski: Conceptualization, methodology, software, formal analysis, investigation, data curation, writing—original draft, visualization, project administration, funding acquisition. Carmen Weidler: Formal analysis, writing—review and editing. Ute Habel: Writing—review and editing, supervision. Nicole S. Coverdale: Investigation, resources, writing—review and editing. Adnan A. Hirad: Investigation, resources, writing—review and editing. Kathryn Y. Manning: Investigation, resources, writing—review and editing. Alexander Rauscher: Investigation, resources, writing—review and editing. Jeffrey J. Bazarian: Investigation, resources, writing—review and editing. Douglas J. Cook: Investigation, resources, writing—review and editing. David K. B. Li: Investigation, resources, writing—review and editing. Bradford Z. Mahon: Investigation, resources, writing—review and editing. Ravi S. Menon: Investigation, resources, writing—review and editing. Jack Taunton: Investigation, resources, writing—review and editing. Kathrin Reetz: Writing—review and editing; supervision. Sandro Romanzetti: Methodology, software, investigation, writing—review and editing. Charlotte Huppertz: Conceptualization, investigation, writing—review and editing, supervision, funding acquisition.
CONFLICT OF INTEREST STATEMENT
AAH and BZM hold IP related to predicting injury from mTBI using MRI (US‐20220039732‐A1). The remaining authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this article.
Supporting information
Data S1: Supporting Information.
Figure S1: Supporting Information.
ACKNOWLEDGMENTS
This work was supported by the CNS—Hannelore Kohl Foundation. The authors are grateful to Prof. Dr. Simon Eickhoff, Dr. rer. medic. Veronika Müller, and their research groups from the Research Centre Jülich (Jülich, Germany) for their expertise and constructive feedback, which significantly enhanced the methodology and strengthened the overall quality of this work. This work was further supported by the Brain Imaging Facility of the Interdisciplinary Center for Clinical Research (IZKF) Aachen within the Faculty of Medicine at RWTH Aachen University. Open Access funding enabled and organized by Projekt DEAL.
Kwiatkowski, A. , Weidler, C. , Habel, U. , Coverdale, N. S. , Hirad, A. A. , Manning, K. Y. , Rauscher, A. , Bazarian, J. J. , Cook, D. J. , Li, D. K. B. , Mahon, B. Z. , Menon, R. S. , Taunton, J. , Reetz, K. , Romanzetti, S. , & Huppertz, C. (2024). Uncovering the hidden effects of repetitive subconcussive head impact exposure: A mega‐analytic approach characterizing seasonal brain microstructural changes in contact and collision sports athletes. Human Brain Mapping, 45(12), e26811. 10.1002/hbm.26811
Sandro Romanzetti and Charlotte Huppertz contributed equally to this study.
DATA AVAILABILITY STATEMENT
The data are available upon reasonable request.
REFERENCES
- Abbas, K. , Shenk, T. E. , Poole, V. N. , Breedlove, E. L. , Leverenz, L. J. , Nauman, E. A. , Talavage, T. M. , & Robinson, M. E. (2015). Alteration of default mode network in high school football athletes due to repetitive subconcussive mild traumatic brain injury: A resting‐state functional magnetic resonance imaging study. Brain Connectivity, 5, 91–101. 10.1089/brain.2014.0279 [DOI] [PubMed] [Google Scholar]
- Alexander, A. L. , Hurley, S. A. , Samsonov, A. A. , Adluru, N. , Hosseinbor, A. P. , Mossahebi, P. , Tromp, D. P. M. , Zakszewski, E. , & Field, A. S. (2011). Characterization of cerebral white matter properties using quantitative magnetic resonance imaging stains. Brain Connectivity, 1, 423–446. 10.1089/brain.2011.0071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aralasmak, A. , Ulmer, J. L. , Kocak, M. , Salvan, C. V. , Hillis, A. E. , & Yousem, D. M. (2006). Association, commissural, and projection pathways and their functional deficit reported in literature. Journal of Computer Assisted Tomography, 30, 695–715. 10.1097/01.rct.0000226397.43235.8b [DOI] [PubMed] [Google Scholar]
- Arbogast, K. B. , & Margulies, S. S. (1998). Material characterization of the brainstem from oscillatory shear tests. Journal of Biomechanics, 31, 801–807. 10.1016/S0021-9290(98)00068-2 [DOI] [PubMed] [Google Scholar]
- Bahrami, N. , Sharma, D. , Rosenthal, S. , Davenport, E. M. , Urban, J. E. , Wagner, B. , Jung, Y. , Vaughan, C. G. , Gioia, G. A. , Stitzel, J. D. , Whitlow, C. T. , & Maldjian, J. A. (2016). Subconcussive head impact exposure and white matter tract changes over a single season of youth football. Radiology, 281, 919–926. 10.1148/radiol.2016160564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailes, J. E. , Petraglia, A. L. , Omalu, B. I. , Nauman, E. , & Talavage, T. (2013). Role of subconcussion in repetitive mild traumatic brain injury. Journal of Neurosurgery, 119, 1235–1245. 10.3171/2013.7.JNS121822 [DOI] [PubMed] [Google Scholar]
- Bazarian, J. J. , Zhu, T. , Blyth, B. , Borrino, A. , & Zhong, J. (2012). Subject‐specific changes in brain white matter on diffusion tensor imaging after sports‐related concussion. Magnetic Resonance Imaging, 30, 171–180. 10.1016/j.mri.2011.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazarian, J. J. , Zhu, T. , Zhong, J. , Janigro, D. , Rozen, E. , Roberts, A. , Javien, H. , Merchant‐Borna, K. , Abar, B. , & Blackman, E. G. (2014). Persistent, long‐term cerebral white matter changes after sports‐related repetitive head impacts. PLoS One, 9, e94734. 10.1371/journal.pone.0094734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benghanem, S. , Mazeraud, A. , Azabou, E. , Chhor, V. , Shinotsuka, C. R. , Claassen, J. , Rohaut, B. , & Sharshar, T. (2020). Brainstem dysfunction in critically ill patients. Critical Care, 24, e5. 10.1186/s13054-019-2718-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boisgontier, M. P. , Cheval, B. , Chalavi, S. , van Ruitenbeek, P. , Leunissen, I. , Levin, O. , Nieuwboer, A. , & Swinnen, S. P. (2017). Individual differences in brainstem and basal ganglia structure predict postural control and balance loss in young and older adults. Neurobiology of Aging, 50, 47–59. 10.1016/j.neurobiolaging.2016.10.024 [DOI] [PubMed] [Google Scholar]
- Borich, M. , Makan, N. , Boyd, L. , & Virji‐Babul, N. (2013). Combining whole‐brain voxel‐wise analysis with in vivo tractography of diffusion behavior after sports‐related concussion in adolescents: A preliminary report. Journal of Neurotrauma, 30, 1243–1249. 10.1089/neu.2012.2818 [DOI] [PubMed] [Google Scholar]
- Breedlove, E. L. , Robinson, M. , Talavage, T. M. , Morigaki, K. E. , Yoruk, U. , O'Keefe, K. , King, J. , Leverenz, L. J. , Gilger, J. W. , & Nauman, E. A. (2012). Biomechanical correlates of symptomatic and asymptomatic neurophysiological impairment in high school football. Journal of Biomechanics, 45, 1265–1272. 10.1016/j.jbiomech.2012.01.034 [DOI] [PubMed] [Google Scholar]
- Budde, M. D. , Janes, L. , Gold, E. , Turtzo, L. C. , & Frank, J. A. (2011). The contribution of gliosis to diffusion tensor anisotropy and tractography following traumatic brain injury: Validation in the rat using Fourier analysis of stained tissue sections. Brain, 134, 2248–2260. 10.1093/brain/awr161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamard, E. , & Lichtenstein, J. D. (2018). A systematic review of neuroimaging findings in children and adolescents with sports‐related concussion. Brain Injury, 32, 816–831. 10.1080/02699052.2018.1463106 [DOI] [PubMed] [Google Scholar]
- Champagne, A. A. , Peponoulas, E. , Terem, I. , Ross, A. , Tayebi, M. , Chen, Y. , Coverdale, N. S. , Nielsen, P. M. F. , Wang, A. , Shim, V. , Holdsworth, S. J. , & Cook, D. J. (2019). Novel strain analysis informs about injury susceptibility of the corpus callosum to repeated impacts. Brain Communications, 1, e21. 10.1093/braincomms/fcz021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cubon, V. A. , Putukian, M. , Boyer, C. , & Dettwiler, A. (2011). A diffusion tensor imaging study on the white matter skeleton in individuals with sports‐related concussion. Journal of Neurotrauma, 28, 189–201. 10.1089/neu.2010.1430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dashnaw, M. L. , Petraglia, A. L. , & Bailes, J. E. (2012). An overview of the basic science of concussion and subconcussion: Where we are and where we are going. Neurosurgical Focus, 33, 1–9. 10.3171/2012.10.FOCUS12284 [DOI] [PubMed] [Google Scholar]
- Delano‐Wood, L. , Bangen, K. J. , Sorg, S. F. , Clark, A. L. , Schiehser, D. M. , Luc, N. , Bondi, M. W. , Werhane, M. , Kim, R. T. , & Bigler, E. D. (2015). Brainstem white matter integrity is related to loss of consciousness and postconcussive symptomatology in veterans with chronic mild to moderate traumatic brain injury. Brain Imaging and Behavior, 9, 500–512. 10.1007/s11682-015-9432-2 [DOI] [PubMed] [Google Scholar]
- Draheim, C. , Pak, R. , Draheim, A. A. , & Engle, R. W. (2022). The role of attention control in complex real‐world tasks. Psychonomic Bulletin & Review, 29, 1143–1197. 10.3758/s13423-021-02052-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eierud, C. , Craddock, R. C. , Fletcher, S. , Aulakh, M. , King‐Casas, B. , Kuehl, D. , & LaConte, S. M. (2014). Neuroimaging after mild traumatic brain injury: Review and meta‐analysis. NeuroImage: Clinical, 4, 283–294. 10.1016/j.nicl.2013.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eime, R. M. , Harvey, J. T. , Charity, M. J. , Casey, M. M. , Westerbeek, H. , & Payne, W. R. (2016). Age profiles of sport participants. BMC Sports Science, Medicine and Rehabilitation, 8, e6. 10.1186/s13102-016-0031-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman, H. M. , Yeatman, J. D. , Lee, E. S. , Barde, L. H. F. , & Gaman‐Bean, S. (2010). Diffusion tensor imaging: A review for pediatric researchers and clinicians. Journal of Developmental and Behavioral Pediatrics, 31, 346–356. 10.1097/DBP.0b013e3181dcaa8b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figley, C. R. , Uddin, M. N. , Wong, K. , Kornelsen, J. , Puig, J. , & Figley, T. D. (2022). Potential pitfalls of using fractional anisotropy, axial diffusivity, and radial diffusivity as biomarkers of cerebral white matter microstructure. Frontiers in Neuroscience, 15, e799576. 10.3389/fnins.2021.799576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forkel, S. J. , Friedrich, P. , Thiebaut de Schotten, M. , & Howells, H. (2022). White matter variability, cognition, and disorders: A systematic review. Brain Structure & Function, 227, 529–544. 10.1007/s00429-021-02382-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaetz, M. (2004). The neurophysiology of brain injury. Clinical Neurophysiology, 115, 4–18. 10.1016/S1388-2457(03)00258-X [DOI] [PubMed] [Google Scholar]
- Gardner, A. , Kay‐Lambkin, F. , Stanwell, P. , Donnelly, J. , Williams, W. H. , Hiles, A. , Schofield, P. , Levi, C. , & Jones, D. K. (2012). A systematic review of diffusion tensor imaging findings in sports‐related concussion. Journal of Neurotrauma, 29, 2521–2538. 10.1089/neu.2012.2628 [DOI] [PubMed] [Google Scholar]
- Gorgolewski, K. J. , Auer, T. , Calhoun, V. D. , Craddock, R. C. , Das, S. , Duff, E. P. , Flandin, G. , Ghosh, S. S. , Glatard, T. , Halchenko, Y. O. , Handwerker, D. A. , Hanke, M. , Keator, D. , Li, X. , Michael, Z. , Maumet, C. , Nichols, B. N. , Nichols, T. E. , Pellman, J. , … Poldrack, R. A. (2016). The brain imaging data structure, a format for organizing and describing outputs of neuroimaging experiments. Scientific Data, 3, e160044. 10.1038/sdata.2016.44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goveas, J. , O'Dwyer, L. , Mascalchi, M. , Cosottini, M. , Diciotti, S. , De Santis, S. , Passamonti, L. , Tessa, C. , Toschi, N. , & Giannelli, M. (2015). Diffusion‐MRI in neurodegenerative disorders. Magnetic Resonance Imaging, 33, 853–876. 10.1016/j.mri.2015.04.006 [DOI] [PubMed] [Google Scholar]
- Hadded, L. (2022). ENIGMA_DTI_01_Preprocessing. Retrieved from https://github.com/ENIGMA-git/ENIGMA_DTI_01_Preprocessing
- Hirad, A. A. , Bazarian, J. J. , Merchant‐Borna, K. , Garcea, F. E. , Heilbronner, S. , Paul, D. , Hintz, E. B. , van Wijngaarden, E. , Schifitto, G. , Wright, D. W. , Espinoza, T. R. , & Mahon, B. Z. (2019). A common neural signature of brain injury in concussion and subconcussion. Science Advances, 5, e3460. 10.1126/sciadv.aau3460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua, K. , Zhang, J. , Wakana, S. , Jiang, H. , Li, X. , Reich, D. S. , Calabresi, P. A. , Pekar, J. J. , van Zijl, P. C. M. , & Mori, S. (2008). Tract probability maps in stereotaxic spaces: Analyses of white matter anatomy and tract‐specific quantification. NeuroImage, 39, 336–347. 10.1016/j.neuroimage.2007.07.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- IBM Corp . (2022). IBM SPSS statistics for windows (version 29). Armonk, NY. Retrieved from https://www.ibm.com/
- Jang, I. , Chun, I. Y. , Brosch, J. R. , Bari, S. , Zou, Y. , Cummiskey, B. R. , Lee, T. A. , Lycke, R. J. , Poole, V. N. , Shenk, T. E. , Svaldi, D. O. , Tamer, G. G. , Dydak, U. , Leverenz, L. J. , Nauman, E. A. , & Talavage, T. M. (2019). Every hit matters: White matter diffusivity changes in high school football athletes are correlated with repetitive head acceleration event exposure. NeuroImage: Clinical, 24, e101930. 10.1016/j.nicl.2019.101930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrett, M. , Tam, R. , Hernández‐Torres, E. , Martin, N. , Perera, W. , Zhao, Y. , Shahinfard, E. , Dadachanji, S. , Taunton, J. , Li, D. K. B. , & Rauscher, A. (2016). Prospective pilot investigation of brain volume, white matter hyperintensities, and hemorrhagic lesions after mild traumatic brain injury. Frontiers in Neurology, 7, e11. 10.3389/fneur.2016.00011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson, M. , Beckmann, C. F. , Behrens, T. E. J. , Woolrich, M. W. , & Smith, S. M. (2012). Fsl. NeuroImage, 62, 782–790. 10.1016/j.neuroimage.2011.09.015 [DOI] [PubMed] [Google Scholar]
- Ji, S. , Ghajari, M. , Mao, H. , Kraft, R. H. , Hajiaghamemar, M. , Panzer, M. B. , Willinger, R. , Gilchrist, M. D. , Kleiven, S. , & Stitzel, J. D. (2022). Use of brain biomechanical models for monitoring impact exposure in contact sports. Annals of Biomedical Engineering, 50, 1389–1408. 10.1007/s10439-022-02999-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji, S. , Zhao, W. , Ford, J. C. , Beckwith, J. G. , Bolander, R. P. , Greenwald, R. M. , Flashman, L. A. , Paulsen, K. D. , & McAllister, T. W. (2015). Group‐wise evaluation and comparison of white matter fiber strain and maximum principal strain in sports‐related concussion. Journal of Neurotrauma, 32, 441–454. 10.1089/neu.2013.3268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, D. K. , Knösche, T. R. , & Turner, R. (2013). White matter integrity, fiber count, and other fallacies: The do's and don'ts of diffusion MRI. NeuroImage, 73, 239–254. 10.1016/j.neuroimage.2012.06.081 [DOI] [PubMed] [Google Scholar]
- Kim, E. , Seo, H. G. , Lee, H. H. , Lee, S. H. , Choi, S. H. , Yoo, R.‐E. , Cho, W.‐S. , Yun, S. J. , Kang, M.‐G. , & Oh, B.‐M. (2021). Reduced brainstem volume after mild traumatic brain injury. American Journal of Physical Medicine & Rehabilitation, 100, 473–482. 10.1097/PHM.0000000000001580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koerte, I. K. , Ertl‐Wagner, B. , Reiser, M. , Zafonte, R. , & Shenton, M. E. (2012). White matter integrity in the brains of professional soccer players without a symptomatic concussion. JAMA, 308, 1859–1861. 10.1001/jama.2012.13735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koerte, I. K. , Kaufmann, D. , Hartl, E. , Bouix, S. , Pasternak, O. , Kubicki, M. , Rauscher, A. , Li, D. K. B. , Dadachanji, S. B. , Taunton, J. A. , Forwell, L. A. , Johnson, A. M. , Echlin, P. S. , & Shenton, M. E. (2012). A prospective study of physician‐observed concussion during a varsity university hockey season: White matter integrity in ice hockey players. Part 3 of 4. Neurosurgical Focus, 33, 1–7. 10.3171/2012.10.FOCUS12303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koerte, I. K. , Wiegand, T. L. T. , Bonke, E. M. , Kochsiek, J. , & Shenton, M. E. (2023). Diffusion imaging of sport‐related repetitive head impacts—A systematic review. Neuropsychology Review, 33, 122–143. 10.1007/s11065-022-09566-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langlois, J. A. , Rutland‐Brown, W. , & Wald, M. M. (2006). The epidemiology and impact of traumatic brain injury. Journal of Head Trauma Rehabilitation, 21, 375–378. 10.1097/00001199-200609000-00001 [DOI] [PubMed] [Google Scholar]
- Lebel, C. , & Deoni, S. (2018). The development of brain white matter microstructure. NeuroImage, 182, 207–218. 10.1016/j.neuroimage.2017.12.097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, X. Y. , Li, J. , Feng, D. F. , & Gu, L. (2010). Diffuse axonal injury induced by simultaneous moderate linear and angular head accelerations in rats. Neuroscience, 169, 357–369. 10.1016/j.neuroscience.2010.04.075 [DOI] [PubMed] [Google Scholar]
- Maleki, S. , Hendrikse, J. , Chye, Y. , Caeyenberghs, K. , Coxon, J. P. , Oldham, S. , Suo, C. , & Yücel, M. (2022). Associations of cardiorespiratory fitness and exercise with brain white matter in healthy adults: A systematic review and meta‐analysis. Brain Imaging and Behavior, 16, 2402–2425. 10.1007/s11682-022-00693-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning, K. Y. , Brooks, J. S. , Dickey, J. P. , Harriss, A. , Fischer, L. , Jevremovic, T. , Blackney, K. , Barreira, C. , Brown, A. , Bartha, R. , Doherty, T. , Fraser, D. , Holmes, J. , Dekaban, G. A. , & Menon, R. S. (2020). Longitudinal changes of brain microstructure and function in nonconcussed female rugby players. Neurology, 95, 402–412. 10.1212/WNL.0000000000009821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchi, N. , Bazarian, J. J. , Puvenna, V. , Janigro, M. , Ghosh, C. , Zhong, J. , Zhu, T. , Blackman, E. , Stewart, D. , Ellis, J. , Butler, R. , & Janigro, D. (2013). Consequences of repeated blood‐brain barrier disruption in football players. PLoS One, 8, e56805. 10.1371/journal.pone.0056805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmarou, C. R. (2011). Cortical motor pathways. In Kreutzer J. S., DeLuca J., & Caplan B. (Eds.), Encyclopedia of clinical neuropsychology (pp. 721–722). Springer. [Google Scholar]
- Mayinger, M. C. , Merchant‐Borna, K. , Hufschmidt, J. , Muehlmann, M. , Weir, I. R. , Rauchmann, B.‐S. , Shenton, M. E. , Koerte, I. K. , & Bazarian, J. J. (2018). White matter alterations in college football players: A longitudinal diffusion tensor imaging study. Brain Imaging and Behavior, 12, 44–53. 10.1007/s11682-017-9672-4 [DOI] [PubMed] [Google Scholar]
- McNeese, N. , Cooke, N. J. , Fedele, M. , & Gray, R. (2016). Perspectives on team cognition and team sports. In Sport and exercise psychology research (pp. 123–141). Elsevier. 10.1016/B978-0-12-803634-1.00006-6 [DOI] [Google Scholar]
- Merchant‐Borna, K. , Asselin, P. , Narayan, D. , Abar, B. , Jones, C. M. C. , & Bazarian, J. J. (2016). Novel method of weighting cumulative helmet impacts improves correlation with brain white matter changes after one football season of sub‐concussive head blows. Annals of Biomedical Engineering, 44, 3679–3692. 10.1007/s10439-016-1680-9 [DOI] [PubMed] [Google Scholar]
- Myer, G. D. , Barber Foss, K. , Thomas, S. , Galloway, R. , DiCesare, C. A. , Dudley, J. , Gadd, B. , Leach, J. , Smith, D. , Gubanich, P. , Meehan, W. P., III , Altaye, M. , Lavin, P. , & Yuan, W. (2019). Altered brain microstructure in association with repetitive subconcussive head impacts and the potential protective effect of jugular vein compression: A longitudinal study of female soccer athletes. British Journal of Sports Medicine, 53, 1539–1551. 10.1136/bjsports-2018-099571 [DOI] [PubMed] [Google Scholar]
- Myer, G. D. , Yuan, W. , Barber Foss, K. D. , Smith, D. , Altaye, M. , Reches, A. , Leach, J. , Kiefer, A. W. , Khoury, J. C. , Weiss, M. , Thomas, S. , Dicesare, C. , Adams, J. , Gubanich, P. J. , Geva, A. , Clark, J. F. , Meehan, W. P. , Mihalik, J. P. , & Krueger, D. (2016). The effects of external jugular compression applied during head impact exposure on longitudinal changes in brain neuroanatomical and neurophysiological biomarkers: A preliminary investigation. Frontiers in Neurology, 7, e74. 10.3389/fneur.2016.00074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page, M. J. , McKenzie, J. E. , Bossuyt, P. M. , Boutron, I. , Hoffmann, T. C. , Mulrow, C. D. , Shamseer, L. , Tetzlaff, J. M. , Akl, E. A. , Brennan, S. E. , Chou, R. , Glanville, J. , Grimshaw, J. M. , Hróbjartsson, A. , Lalu, M. M. , Li, T. , Loder, E. W. , Mayo‐Wilson, E. , McDonald, S. , … Moher, D. (2021). The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. British Medical Journal, 372, e71. 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlovic, D. , Pekic, S. , Stojanovic, M. , & Popovic, V. (2019). Traumatic brain injury: Neuropathological, neurocognitive and neurobehavioral sequelae. Pituitary, 22, 270–282. 10.1007/s11102-019-00957-9 [DOI] [PubMed] [Google Scholar]
- Poole, V. N. , Breedlove, E. L. , Shenk, T. E. , Abbas, K. , Robinson, M. E. , Leverenz, L. J. , Nauman, E. A. , Dydak, U. , & Talavage, T. M. (2015). Sub‐concussive hit characteristics predict deviant brain metabolism in football athletes. Developmental Neuropsychology, 40, 12–17. 10.1080/87565641.2014.984810 [DOI] [PubMed] [Google Scholar]
- Ropper, A. H. , & Gorson, K. C. (2007). Concussion. New England Journal of Medicine, 356, 166–172. 10.1056/NEJMcp064645 [DOI] [PubMed] [Google Scholar]
- Schneider, D. K. , Galloway, R. , Bazarian, J. J. , Diekfuss, J. A. , Dudley, J. , Leach, J. L. , Mannix, R. , Talavage, T. M. , Yuan, W. , & Myer, G. D. (2019). Diffusion tensor imaging in athletes sustaining repetitive head impacts: A systematic review of prospective studies. Journal of Neurotrauma, 36, 2831–2849. 10.1089/neu.2019.6398 [DOI] [PubMed] [Google Scholar]
- Schranz, A. L. , Manning, K. Y. , Dekaban, G. A. , Fischer, L. , Jevremovic, T. , Blackney, K. , Barreira, C. , Doherty, T. J. , Fraser, D. D. , Brown, A. , Holmes, J. , Menon, R. S. , & Bartha, R. (2018). Reduced brain glutamine in female varsity rugby athletes after concussion and in non‐concussed athletes after a season of play. Human Brain Mapping, 39, 1489–1499. 10.1002/hbm.23919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz, C. G. , Reid, R. I. , Gunter, J. L. , Senjem, M. L. , Przybelski, S. A. , Zuk, S. M. , Whitwell, J. L. , Vemuri, P. , Josephs, K. A. , Kantarci, K. , Thompson, P. M. , Petersen, R. C. , & Jack, C. R. (2014). Improved DTI registration allows voxel‐based analysis that outperforms tract‐based spatial statistics. NeuroImage, 94, 65–78. 10.1016/j.neuroimage.2014.03.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sexton, C. E. , Betts, J. F. , Demnitz, N. , Dawes, H. , Ebmeier, K. P. , & Johansen‐Berg, H. (2016). A systematic review of MRI studies examining the relationship between physical fitness and activity and the white matter of the ageing brain. NeuroImage, 131, 81–90. 10.1016/j.neuroimage.2015.09.071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, S. M. , Jenkinson, M. , Johansen‐Berg, H. , Rueckert, D. , Nichols, T. E. , Mackay, C. E. , Watkins, K. E. , Ciccarelli, O. , Cader, M. Z. , Matthews, P. M. , & Behrens, T. E. J. (2006). Tract‐based spatial statistics: Voxelwise analysis of multi‐subject diffusion data. NeuroImage, 31, 1487–1505. 10.1016/j.neuroimage.2006.02.024 [DOI] [PubMed] [Google Scholar]
- Soares, J. M. , Marques, P. , Alves, V. , & Sousa, N. (2013). A hitchhiker's guide to diffusion tensor imaging. Frontiers in Neuroscience, 7, e31. 10.3389/fnins.2013.00031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, S.‐K. , Sun, S.‐W. , Ramsbottom, M. J. , Chang, C. , Russell, J. , & Cross, A. H. (2002). Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. NeuroImage, 17, 1429–1436. 10.1006/nimg.2002.1267 [DOI] [PubMed] [Google Scholar]
- Song, S.‐K. , Yoshino, J. , Le, T. Q. , Lin, S.‐J. , Sun, S.‐W. , Cross, A. H. , & Armstrong, R. C. (2005). Demyelination increases radial diffusivity in corpus callosum of mouse brain. NeuroImage, 26, 132–140. 10.1016/j.neuroimage.2005.01.028 [DOI] [PubMed] [Google Scholar]
- Spain, A. , Daumas, S. , Lifshitz, J. , Rhodes, J. , Andrews, P. J. D. , Horsburgh, K. , & Fowler, J. H. (2010). Mild fluid percussion injury in mice produces evolving selective axonal pathology and cognitive deficits relevant to human brain injury. Journal of Neurotrauma, 27, 1429–1438. 10.1089/neu.2010.1288 [DOI] [PubMed] [Google Scholar]
- Talavage, T. M. , Nauman, E. A. , Breedlove, E. L. , Yoruk, U. , Dye, A. E. , Morigaki, K. E. , Feuer, H. , & Leverenz, L. J. (2014). Functionally‐detected cognitive impairment in high school football players without clinically‐diagnosed concussion. Journal of Neurotrauma, 31, 327–338. 10.1089/neu.2010.1512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- The EndNote Team . (2013). EndNote (Version X9). Philadelphia. EndNote.
- Tournier, J.‐D. , Smith, R. , Raffelt, D. , Tabbara, R. , Dhollander, T. , Pietsch, M. , Christiaens, D. , Jeurissen, B. , Yeh, C.‐H. , & Connelly, A. (2019). MRtrix3: A fast, flexible and open software framework for medical image processing and visualisation. NeuroImage, 202, e116137. 10.1016/j.neuroimage.2019.116137 [DOI] [PubMed] [Google Scholar]
- Tustison, N. J. , Cook, P. A. , Holbrook, A. J. , Johnson, H. J. , Muschelli, J. , Devenyi, G. A. , Duda, J. T. , Das, S. R. , Cullen, N. C. , Gillen, D. L. , Yassa, M. A. , Stone, J. R. , Gee, J. C. , & Avants, B. B. (2021). The ANTsX ecosystem for quantitative biological and medical imaging. Scientific Reports, 11, e9068. 10.1038/s41598-021-87564-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viano, D. C. , Casson, I. R. , Pellman, E. J. , Zhang, L. , King, A. I. , & Yang, K. H. (2005). Concussion in professional football: Brain responses by finite element analysis: Part 9. Neurosurgery, 57, 891–916. 10.1227/01.NEU.0000186950.54075.3B [DOI] [PubMed] [Google Scholar]
- Voelbel, G. T. , Genova, H. M. , Chiaravalotti, N. D. , & Hoptman, M. J. (2012). Diffusion tensor imaging of traumatic brain injury review: Implications for neurorehabilitation. NeuroRehabilitation, 31, 281–293. 10.3233/NRE-2012-0796 [DOI] [PubMed] [Google Scholar]
- Winkler, A. M. , Ridgway, G. R. , Webster, M. A. , Smith, S. M. , & Nichols, T. E. (2014). Permutation inference for the general linear model. NeuroImage, 92, 381–397. 10.1016/j.neuroimage.2014.01.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright, A. D. , Jarrett, M. , Vavasour, I. , Shahinfard, E. , Kolind, S. , van Donkelaar, P. , Taunton, J. , Li, D. , & Rauscher, A. (2016). Myelin water fraction is transiently reduced after a single mild traumatic brain injury—A prospective cohort study in collegiate hockey players. PLoS One, 11, e0150215. 10.1371/journal.pone.0150215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan, W. , Barber Foss, K. D. , Thomas, S. , DiCesare, C. A. , Dudley, J. A. , Kitchen, K. , Gadd, B. , Leach, J. L. , Smith, D. , Altaye, M. , Gubanich, P. , Galloway, R. T. , McCrory, P. , Bailes, J. E. , Mannix, R. , Meehan, W. P. , & Myer, G. D. (2018). White matter alterations over the course of two consecutive high‐school football seasons and the effect of a jugular compression collar: A preliminary longitudinal diffusion tensor imaging study. Human Brain Mapping, 39, 491–508. 10.1002/hbm.23859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, L. , Yang, K. H. , & King, A. I. (2004). A proposed injury threshold for mild traumatic brain injury. Journal of Biomechanical Engineering, 126, 226–236. 10.1115/1.1691446 [DOI] [PubMed] [Google Scholar]
- Zhao, W. , Bartsch, A. , Benzel, E. , Miele, V. , Stemper, B. D. , & Ji, S. (2019). Regional brain injury vulnerability in football from two finite element models of the human head. In: 2019 IRCOBI Conference Proceedings. Florence, pp. 619–621.
- Zhao, W. , Cai, Y. , Li, Z. , & Ji, S. (2017). Injury prediction and vulnerability assessment using strain and susceptibility measures of the deep white matter. Biomechanics and Modeling in Mechanobiology, 16, 1709–1727. 10.1007/s10237-017-0915-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zugman, A. , Harrewijn, A. , Cardinale, E. M. , Zwiebel, H. , Freitag, G. F. , Werwath, K. E. , Bas‐Hoogendam, J. M. , Groenewold, N. A. , Aghajani, M. , Hilbert, K. , Cardoner, N. , Porta‐Casteràs, D. , Gosnell, S. , Salas, R. , Blair, K. S. , Blair, J. R. , Hammoud, M. Z. , Milad, M. , Burkhouse, K. , … Winkler, A. M. (2022). Mega‐analysis methods in ENIGMA: The experience of the generalized anxiety disorder working group. Human Brain Mapping, 43, 255–277. 10.1002/hbm.25096 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1: Supporting Information.
Figure S1: Supporting Information.
Data Availability Statement
The data are available upon reasonable request.


