Abstract
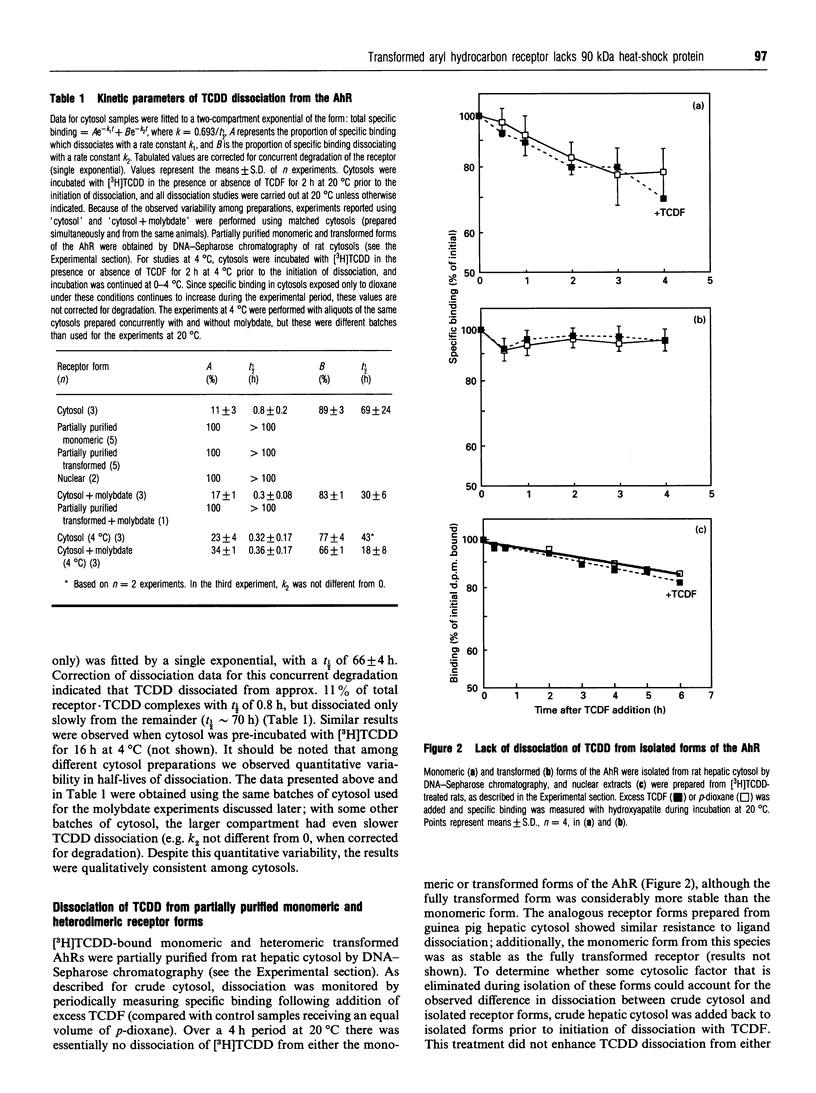
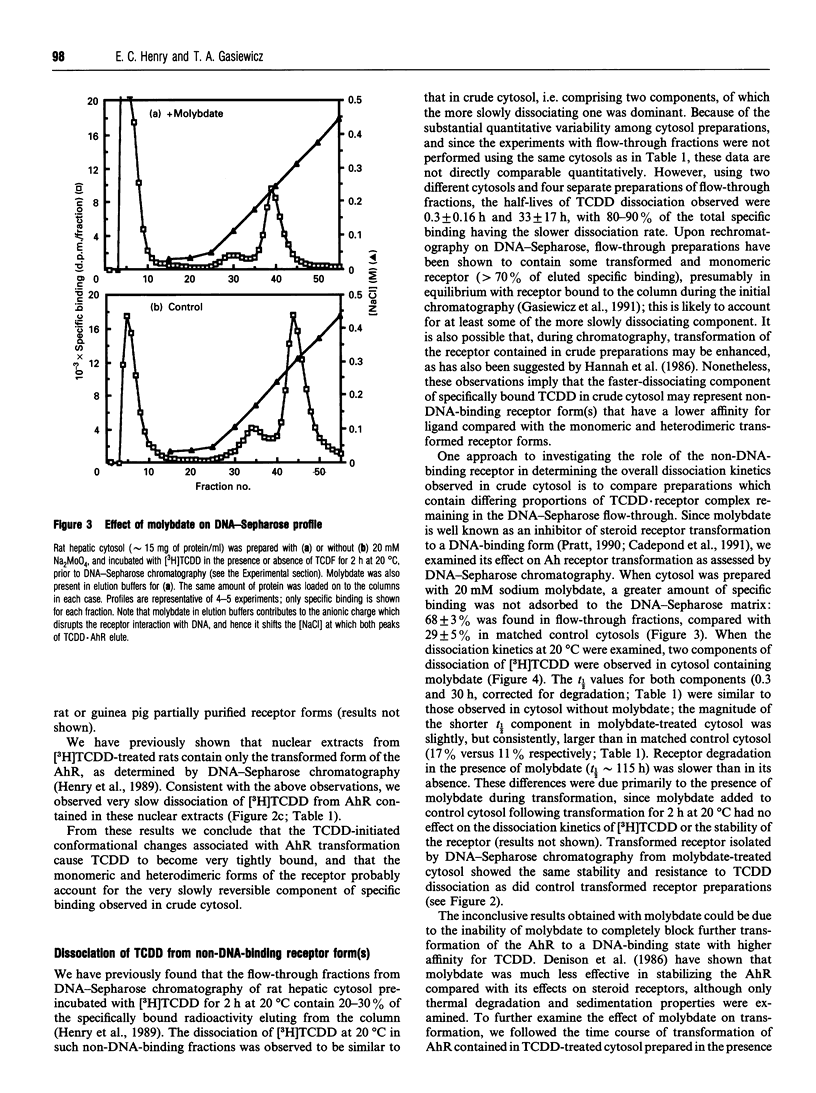
The binding of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) to the aryl hydrocarbon receptor (AhR) elicits a sequence of poorly defined molecular events that ultimately yield a heteromeric transformed AhR that is active as a transcription factor. We have previously developed a model of the ligand-initiated transformation of the AhR to the DNA-binding state based on characterization of several forms of the AhR with respect to their physicochemical properties and DNA-binding affinities. The present studies were designed to determine whether, and at what stage, this process of transformation alters the receptor's affinity for TCDD. In rat hepatic cytosol, approx. 10% of the TCDD specifically bound to the AhR rapidly dissociated (t1/2 approximately 1 h), while the remainder was only slowly dissociable (t1/2 approximately 70 h). The isolated DNA-binding forms of the receptor (monomeric and transformed) bound TCDD very tightly (t1/2 > 100 h), whereas TCDD was dissociable from the non-DNA-binding receptor form(s). A lower incubation temperature (0-4 degrees C) and the presence of molybdate partially stabilized the non-DNA-binding fraction of the TCDD.receptor complex and also enhanced TCDD dissociation in crude cytosol. Immunoprecipitation of the different AhR forms with an anti-AhR antibody and immunoblotting with antibody to the 90 kDa heat-shock protein (hsp90) demonstrated that hsp90 was associated with the unoccupied receptor complex as well as with a fraction of the non-DNA-binding TCDD.receptor complex; isolated DNA-binding forms did not contain detectable hsp90. We conclude that while hsp90 remains associated with the AhR, TCDD is readily dissociable; following release of hsp90, however, TCDD becomes very tightly bound, and remains so upon completion of transformation.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradfield C. A., Kende A. S., Poland A. Kinetic and equilibrium studies of Ah receptor-ligand binding: use of [125I]2-iodo-7,8-dibromodibenzo-p-dioxin. Mol Pharmacol. 1988 Aug;34(2):229–237. [PubMed] [Google Scholar]
- Bunce N. J., Landers J. P., Safe S. H. Kinetic models for association of 2,3,7,8-tetrachlorodibenzo-p-dioxin with the Ah receptor. Arch Biochem Biophys. 1988 Nov 15;267(1):384–397. doi: 10.1016/0003-9861(88)90044-6. [DOI] [PubMed] [Google Scholar]
- Cadepond F., Schweizer-Groyer G., Segard-Maurel I., Jibard N., Hollenberg S. M., Giguère V., Evans R. M., Baulieu E. E. Heat shock protein 90 as a critical factor in maintaining glucocorticosteroid receptor in a nonfunctional state. J Biol Chem. 1991 Mar 25;266(9):5834–5841. [PubMed] [Google Scholar]
- Dalman F. C., Sturzenbecker L. J., Levin A. A., Lucas D. A., Perdew G. H., Petkovitch M., Chambon P., Grippo J. F., Pratt W. B. Retinoic acid receptor belongs to a subclass of nuclear receptors that do not form "docking" complexes with hsp90. Biochemistry. 1991 Jun 4;30(22):5605–5608. doi: 10.1021/bi00236a038. [DOI] [PubMed] [Google Scholar]
- Denis M., Cuthill S., Wikström A. C., Poellinger L., Gustafsson J. A. Association of the dioxin receptor with the Mr 90,000 heat shock protein: a structural kinship with the glucocorticoid receptor. Biochem Biophys Res Commun. 1988 Sep 15;155(2):801–807. doi: 10.1016/s0006-291x(88)80566-7. [DOI] [PubMed] [Google Scholar]
- Denis M., Gustafsson J. A., Wikström A. C. Interaction of the Mr = 90,000 heat shock protein with the steroid-binding domain of the glucocorticoid receptor. J Biol Chem. 1988 Dec 5;263(34):18520–18523. [PubMed] [Google Scholar]
- Denison M. S., Fisher J. M., Whitlock J. P., Jr Inducible, receptor-dependent protein-DNA interactions at a dioxin-responsive transcriptional enhancer. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2528–2532. doi: 10.1073/pnas.85.8.2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denison M. S., Vella L. M., Okey A. B. Hepatic Ah receptor for 2,3,7,8-tetrachlorodibenzo-p-dioxin. Partial stabilization by molybdate. J Biol Chem. 1986 Aug 5;261(22):10189–10195. [PubMed] [Google Scholar]
- Elferink C. J., Gasiewicz T. A., Whitlock J. P., Jr Protein-DNA interactions at a dioxin-responsive enhancer. Evidence that the transformed Ah receptor is heteromeric. J Biol Chem. 1990 Nov 25;265(33):20708–20712. [PubMed] [Google Scholar]
- Farrell K., Safe S. Absence of positive co-operativity in the binding of 2,3,7,8-tetrachlorodibenzo-p-dioxin to its cytosolic receptor protein. Biochem J. 1987 Jun 15;244(3):539–546. doi: 10.1042/bj2440539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasiewicz T. A., Bauman P. A. Heterogeneity of the rat hepatic Ah receptor and evidence for transformation in vitro and in vivo. J Biol Chem. 1987 Feb 15;262(5):2116–2120. [PubMed] [Google Scholar]
- Gasiewicz T. A., Elferink C. J., Henry E. C. Characterization of multiple forms of the Ah receptor: recognition of a dioxin-responsive enhancer involves heteromer formation. Biochemistry. 1991 Mar 19;30(11):2909–2916. doi: 10.1021/bi00225a026. [DOI] [PubMed] [Google Scholar]
- Gasiewicz T. A., Neal R. A. The examination and quantitation of tissue cytosolic receptors for 2,3,7,8-tetrachlorodibenzo-p-dioxin using hydroxylapatite. Anal Biochem. 1982 Jul 15;124(1):1–11. doi: 10.1016/0003-2697(82)90212-3. [DOI] [PubMed] [Google Scholar]
- Gasiewicz T. A., Rucci G. Alpha-naphthoflavone acts as an antagonist of 2,3,7, 8-tetrachlorodibenzo-p-dioxin by forming an inactive complex with the Ah receptor. Mol Pharmacol. 1991 Nov;40(5):607–612. [PubMed] [Google Scholar]
- Hannah R. R., Lund J., Poellinger L., Gillner M., Gustafsson J. A. Characterization of the DNA-binding properties of the receptor for 2,3,7,8-tetrachlorodibenzo-p-dioxin. Eur J Biochem. 1986 Apr 15;156(2):237–242. doi: 10.1111/j.1432-1033.1986.tb09573.x. [DOI] [PubMed] [Google Scholar]
- Henry E. C., Gasiewicz T. A. Inhibition and reconstitution of Ah receptor transformation in vitro: role and partial characterization of a cytosolic factor(s). Arch Biochem Biophys. 1991 Jul;288(1):149–156. doi: 10.1016/0003-9861(91)90177-k. [DOI] [PubMed] [Google Scholar]
- Henry E. C., Kester J. E., Gasiewicz T. A. Effects of SH-modifying reagents on the rat hepatic Ah receptor: inhibition of ligand binding and transformation, and disruption of the ligand-receptor complex. Biochim Biophys Acta. 1988 Mar 17;964(3):361–376. doi: 10.1016/0304-4165(88)90037-2. [DOI] [PubMed] [Google Scholar]
- Henry E. C., Rucci G., Gasiewicz T. A. Characterization of multiple forms of the Ah receptor: comparison of species and tissues. Biochemistry. 1989 Jul 25;28(15):6430–6440. doi: 10.1021/bi00441a041. [DOI] [PubMed] [Google Scholar]
- Kester J. E., Gasiewicz T. A. Characterization of the in vitro stability of the rat hepatic receptor for 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD). Arch Biochem Biophys. 1987 Feb 1;252(2):606–625. doi: 10.1016/0003-9861(87)90067-1. [DOI] [PubMed] [Google Scholar]
- Landers J. P., Bunce N. J. The Ah receptor and the mechanism of dioxin toxicity. Biochem J. 1991 Jun 1;276(Pt 2):273–287. doi: 10.1042/bj2760273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landers J. P., Piskorska-Pliszczynska J., Zacharewski T., Bunce N. J., Safe S. Photoaffinity labeling of the nuclear Ah receptor from mouse Hepa 1c1c7 cells using 2,3,7,8-[3H]tetrachlorodibenzo-p-dioxin. J Biol Chem. 1989 Nov 5;264(31):18463–18471. [PubMed] [Google Scholar]
- Perdew G. H. Association of the Ah receptor with the 90-kDa heat shock protein. J Biol Chem. 1988 Sep 25;263(27):13802–13805. [PubMed] [Google Scholar]
- Perdew G. H. Chemical cross-linking of the cytosolic and nuclear forms of the Ah receptor in hepatoma cell line 1c1c7. Biochem Biophys Res Commun. 1992 Jan 15;182(1):55–62. doi: 10.1016/s0006-291x(05)80111-1. [DOI] [PubMed] [Google Scholar]
- Poland A., Glover E., Bradfield C. A. Characterization of polyclonal antibodies to the Ah receptor prepared by immunization with a synthetic peptide hapten. Mol Pharmacol. 1991 Jan;39(1):20–26. [PubMed] [Google Scholar]
- Poland A., Glover E., Ebetino F. H., Kende A. S. Photoaffinity labeling of the Ah receptor. J Biol Chem. 1986 May 15;261(14):6352–6365. [PubMed] [Google Scholar]
- Poland A., Glover E., Kende A. S. Stereospecific, high affinity binding of 2,3,7,8-tetrachlorodibenzo-p-dioxin by hepatic cytosol. Evidence that the binding species is receptor for induction of aryl hydrocarbon hydroxylase. J Biol Chem. 1976 Aug 25;251(16):4936–4946. [PubMed] [Google Scholar]
- Poland A., Knutson J. C. 2,3,7,8-tetrachlorodibenzo-p-dioxin and related halogenated aromatic hydrocarbons: examination of the mechanism of toxicity. Annu Rev Pharmacol Toxicol. 1982;22:517–554. doi: 10.1146/annurev.pa.22.040182.002505. [DOI] [PubMed] [Google Scholar]
- Pongratz I., Mason G. G., Poellinger L. Dual roles of the 90-kDa heat shock protein hsp90 in modulating functional activities of the dioxin receptor. Evidence that the dioxin receptor functionally belongs to a subclass of nuclear receptors which require hsp90 both for ligand binding activity and repression of intrinsic DNA binding activity. J Biol Chem. 1992 Jul 5;267(19):13728–13734. [PubMed] [Google Scholar]
- Pratt W. B. Interaction of hsp90 with steroid receptors: organizing some diverse observations and presenting the newest concepts. Mol Cell Endocrinol. 1990 Nov 12;74(1):C69–C76. doi: 10.1016/0303-7207(90)90198-h. [DOI] [PubMed] [Google Scholar]
- Reyes H., Reisz-Porszasz S., Hankinson O. Identification of the Ah receptor nuclear translocator protein (Arnt) as a component of the DNA binding form of the Ah receptor. Science. 1992 May 22;256(5060):1193–1195. doi: 10.1126/science.256.5060.1193. [DOI] [PubMed] [Google Scholar]
- Rucci G., Gasiewicz T. A. In vivo kinetics and DNA-binding properties of the Ah receptor in the golden Syrian hamster. Arch Biochem Biophys. 1988 Aug 15;265(1):197–207. doi: 10.1016/0003-9861(88)90385-2. [DOI] [PubMed] [Google Scholar]
- Wilhelmsson A., Cuthill S., Denis M., Wikström A. C., Gustafsson J. A., Poellinger L. The specific DNA binding activity of the dioxin receptor is modulated by the 90 kd heat shock protein. EMBO J. 1990 Jan;9(1):69–76. doi: 10.1002/j.1460-2075.1990.tb08081.x. [DOI] [PMC free article] [PubMed] [Google Scholar]



