Abstract
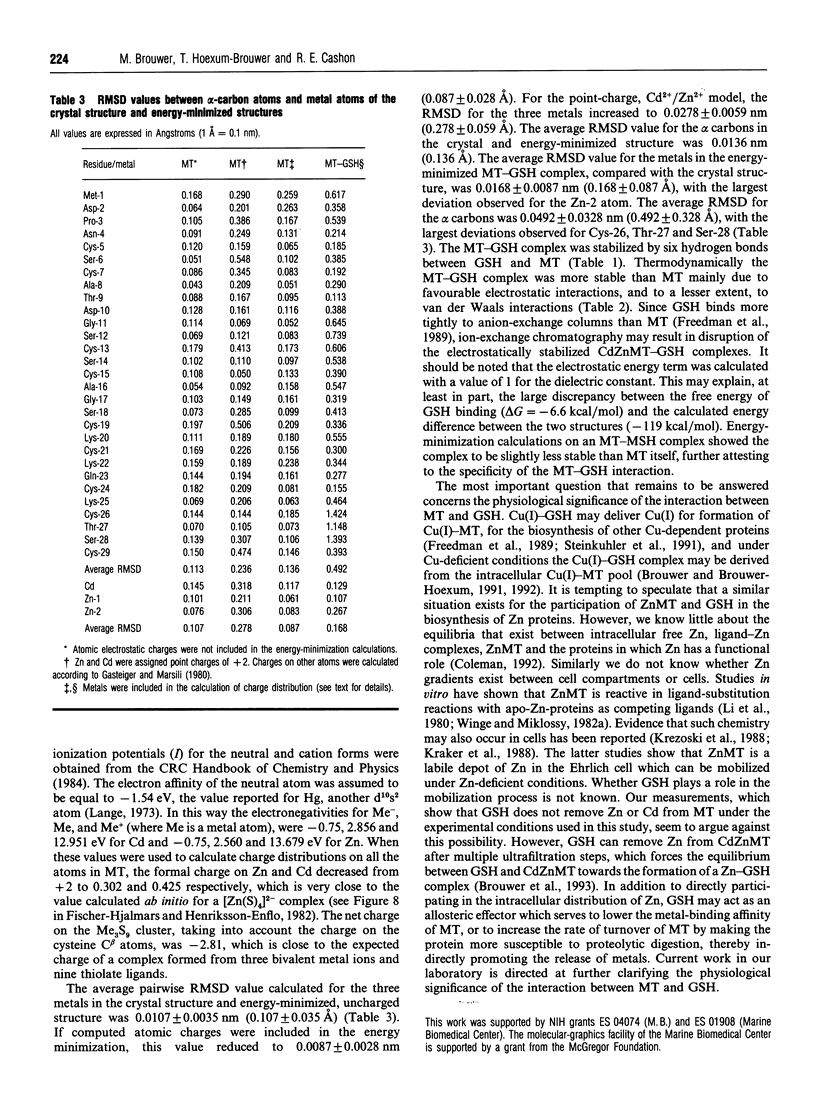
Glutathione (GSH) has been found to form a complex with both vertebrate and invertebrate copper-metallothionein (CuMT) [Freedman, Ciriolo and Peisach (1989) J. Biol. Chem. 264, 5598-5605; Brouwer and Brouwer-Hoexum (1991) Arch. Biochem. Biophys. 290, 207-213]. In this paper we report on the interaction of GSH with CdZnMT-I and CdZnMT-II from rabbit liver and with CdMT-I from Blue crab hepatopancreas. Ultrafiltration experiments showed that all three MTs combined with GSH. The measured binding data for the three MTs could be described by a single binding isotherm. The GSH/MT stoichiometry was 1.4 +/- 0.3 and Kdiss. = 14 +/- 6 microM. Partially Zn-depleted MT does not significantly bind GSH, indicating that the GSH-binding site is located on MT's Zn-containing N-terminal domain. The putative GSH-binding site on rabbit liver MT was investigated using molecular-graphics analysis. A cleft on the MT's N-terminal domain, which has the labile Zn-2 at its base, could easily accommodate GSH. Cysteine-ligand exchange between the terminal (non-bridging) Cys-26, bound to Zn-2, and the cysteine in GSH is stereochemically possible. Based on these considerations a model of MT-GSH was built in which GSH's cysteine replaces Cys-26 as a terminal Zn-2 ligand. This complex was energy-minimized by molecular-mechanics calculations, taking into account computed partial electrostatic charges on all atoms, including Cd and Zn. These calculations showed that the MT-GSH complex was thermodynamically more stable than MT, due to favourable non-bonded, electrostatic and van der Waals interactions. Six hydrogen bonds can form between GSH and MT. The average pairwise root-mean-square deviations (RMSD) of the metals in energy-minimized MT and MT-GSH, compared with the metals in the crystal structure, were 0.0087 +/- 0.0028 nm (0.087 +/- 0.028 A) and 0.0168 +/- 0.0087 nm (0.168 +/- 0.087 A) respectively. The RMSD values for the polypeptide-backbone alpha carbons were 0.0136 +/- 0.0060 nm (0.136 +/- 0.060 A) and 0.0491 +/- 0.0380 nm (0.491 +/- 0.380 A) respectively. No other docking sites for GSH were found. The energy-minimized structure of an MT-2-mercaptoethanol complex was somewhat less stable than the native MT domain, attesting to the specificity of the MT-GSH interaction. The possible physiological significance of the MT-GSH interaction is discussed.
Full text
PDF



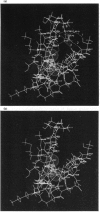
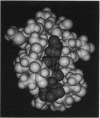
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrahams I. L., Bremner I., Diakun G. P., Garner C. D., Hasnain S. S., Ross I., Vasák M. Structural study of the copper and zinc sites in metallothioneins by using extended X-ray-absorption fine structure. Biochem J. 1986 Jun 1;236(2):585–589. doi: 10.1042/bj2360585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam J., Smith A. Heme-hemopexin-mediated induction of metallothionein gene expression. J Biol Chem. 1992 Aug 15;267(23):16379–16384. [PubMed] [Google Scholar]
- Andersen R. D., Taplitz S. J., Oberbauer A. M., Calame K. L., Herschman H. R. Metal-dependent binding of a nuclear factor to the rat metallothionein-I promoter. Nucleic Acids Res. 1990 Oct 25;18(20):6049–6055. doi: 10.1093/nar/18.20.6049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson M. E. Determination of glutathione and glutathione disulfide in biological samples. Methods Enzymol. 1985;113:548–555. doi: 10.1016/s0076-6879(85)13073-9. [DOI] [PubMed] [Google Scholar]
- Arseniev A., Schultze P., Wörgötter E., Braun W., Wagner G., Vasák M., Kägi J. H., Wüthrich K. Three-dimensional structure of rabbit liver [Cd7]metallothionein-2a in aqueous solution determined by nuclear magnetic resonance. J Mol Biol. 1988 Jun 5;201(3):637–657. doi: 10.1016/0022-2836(88)90644-4. [DOI] [PubMed] [Google Scholar]
- Banci L., Schröder S., Kollman P. A. Molecular dynamics characterization of the active cavity of carboxypeptidase A and some of its inhibitor adducts. Proteins. 1992 Aug;13(4):288–305. doi: 10.1002/prot.340130403. [DOI] [PubMed] [Google Scholar]
- Brouwer M., Brouwer-Hoexum T. Glutathione-mediated transfer of copper(I) into American lobster apohemocyanin. Biochemistry. 1992 Apr 28;31(16):4096–4102. doi: 10.1021/bi00131a028. [DOI] [PubMed] [Google Scholar]
- Brouwer M., Brouwer-Hoexum T. Interaction of copper-metallothionein from the American lobster, Homarus americanus, with glutathione. Arch Biochem Biophys. 1991 Oct;290(1):207–213. doi: 10.1016/0003-9861(91)90610-u. [DOI] [PubMed] [Google Scholar]
- Brouwer M., Schlenk D., Ringwood A. H., Brouwer-Hoexum T. Metal-specific induction of metallothionein isoforms in the blue crab Callinectes sapidus in response to single- and mixed-metal exposure. Arch Biochem Biophys. 1992 May 1;294(2):461–468. doi: 10.1016/0003-9861(92)90712-6. [DOI] [PubMed] [Google Scholar]
- Butler G., Thiele D. J. ACE2, an activator of yeast metallothionein expression which is homologous to SWI5. Mol Cell Biol. 1991 Jan;11(1):476–485. doi: 10.1128/mcb.11.1.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cismowski M. J., Huang P. C. Effect of cysteine replacements at positions 13 and 50 on metallothionein structure. Biochemistry. 1991 Jul 2;30(26):6626–6632. doi: 10.1021/bi00240a036. [DOI] [PubMed] [Google Scholar]
- Cismowski M. J., Narula S. S., Armitage I. M., Chernaik M. L., Huang P. C. Mutation of invariant cysteines of mammalian metallothionein alters metal binding capacity, cadmium resistance, and 113Cd NMR spectrum. J Biol Chem. 1991 Dec 25;266(36):24390–24397. [PubMed] [Google Scholar]
- Coleman J. E. Zinc proteins: enzymes, storage proteins, transcription factors, and replication proteins. Annu Rev Biochem. 1992;61:897–946. doi: 10.1146/annurev.bi.61.070192.004341. [DOI] [PubMed] [Google Scholar]
- Cousins R. J. Absorption, transport, and hepatic metabolism of copper and zinc: special reference to metallothionein and ceruloplasmin. Physiol Rev. 1985 Apr;65(2):238–309. doi: 10.1152/physrev.1985.65.2.238. [DOI] [PubMed] [Google Scholar]
- Creighton T. E. Disulfide bonds as probes of protein folding pathways. Methods Enzymol. 1986;131:83–106. doi: 10.1016/0076-6879(86)31036-x. [DOI] [PubMed] [Google Scholar]
- Freedman J. H., Ciriolo M. R., Peisach J. The role of glutathione in copper metabolism and toxicity. J Biol Chem. 1989 Apr 5;264(10):5598–5605. [PubMed] [Google Scholar]
- Godwin A. K., Meister A., O'Dwyer P. J., Huang C. S., Hamilton T. C., Anderson M. E. High resistance to cisplatin in human ovarian cancer cell lines is associated with marked increase of glutathione synthesis. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):3070–3074. doi: 10.1073/pnas.89.7.3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamer D. H. Metallothionein. Annu Rev Biochem. 1986;55:913–951. doi: 10.1146/annurev.bi.55.070186.004405. [DOI] [PubMed] [Google Scholar]
- Kille P., Lees W. E., Darke B. M., Winge D. R., Dameron C. T., Stephens P. E., Kay J. Sequestration of cadmium and copper by recombinant rainbow trout and human metallothioneins and by chimeric (mermaid and fishman) proteins with interchanged domains. J Biol Chem. 1992 Apr 25;267(12):8042–8049. [PubMed] [Google Scholar]
- Kini R. M., Evans H. J. Molecular modeling of proteins: a strategy for energy minimization by molecular mechanics in the AMBER force field. J Biomol Struct Dyn. 1991 Dec;9(3):475–488. doi: 10.1080/07391102.1991.10507930. [DOI] [PubMed] [Google Scholar]
- Kraker A. J., Krakower G., Shaw C. F., 3rd, Petering D. H., Garvey J. S. Zinc metabolism in Ehrlich cells: properties of a metallothionein-like zinc-binding protein. Cancer Res. 1988 Jun 15;48(12):3381–3388. [PubMed] [Google Scholar]
- Krezoski S. K., Villalobos J., Shaw C. F., 3rd, Petering D. H. Kinetic lability of zinc bound to metallothionein in Ehrlich cells. Biochem J. 1988 Oct 15;255(2):483–491. [PMC free article] [PubMed] [Google Scholar]
- Li T. Y., Kraker A. J., Shaw C. F., 3rd, Petering D. H. Ligand substitution reactions of metallothioneins with EDTA and apo-carbonic anhydrase. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6334–6338. doi: 10.1073/pnas.77.11.6334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messerle B. A., Schäffer A., Vasák M., Kägi J. H., Wüthrich K. Three-dimensional structure of human [113Cd7]metallothionein-2 in solution determined by nuclear magnetic resonance spectroscopy. J Mol Biol. 1990 Aug 5;214(3):765–779. doi: 10.1016/0022-2836(90)90291-S. [DOI] [PubMed] [Google Scholar]
- Nielson K. B., Atkin C. L., Winge D. R. Distinct metal-binding configurations in metallothionein. J Biol Chem. 1985 May 10;260(9):5342–5350. [PubMed] [Google Scholar]
- Otvos J. D., Armitage I. M. Structure of the metal clusters in rabbit liver metallothionein. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7094–7098. doi: 10.1073/pnas.77.12.7094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otvos J. D., Olafson R. W., Armitage I. M. Structure of an invertebrate metallothionein from Scylla serrata. J Biol Chem. 1982 Mar 10;257(5):2427–2431. [PubMed] [Google Scholar]
- Robbins A. H., McRee D. E., Williamson M., Collett S. A., Xuong N. H., Furey W. F., Wang B. C., Stout C. D. Refined crystal structure of Cd, Zn metallothionein at 2.0 A resolution. J Mol Biol. 1991 Oct 20;221(4):1269–1293. [PubMed] [Google Scholar]
- Singhal R. K., Anderson M. E., Meister A. Glutathione, a first line of defense against cadmium toxicity. FASEB J. 1987 Sep;1(3):220–223. doi: 10.1096/fasebj.1.3.2887478. [DOI] [PubMed] [Google Scholar]
- Steinkühler C., Sapora O., Carrì M. T., Nagel W., Marcocci L., Ciriolo M. R., Weser U., Rotilio G. Increase of Cu,Zn-superoxide dismutase activity during differentiation of human K562 cells involves activation by copper of a constantly expressed copper-deficient protein. J Biol Chem. 1991 Dec 25;266(36):24580–24587. [PubMed] [Google Scholar]
- Séguin C. A nuclear factor requires Zn2+ to bind a regulatory MRE element of the mouse gene encoding metallothionein-1. Gene. 1991 Jan 15;97(2):295–300. doi: 10.1016/0378-1119(91)90066-k. [DOI] [PubMed] [Google Scholar]
- Thiele D. J. Metal-regulated transcription in eukaryotes. Nucleic Acids Res. 1992 Mar 25;20(6):1183–1191. doi: 10.1093/nar/20.6.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasák M. Dynamic metal-thiolate cluster structure of metallothioneins. Environ Health Perspect. 1986 Mar;65:193–197. doi: 10.1289/ehp.65-1474703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasák M., Kägi J. H., Hill H. A. Zinc(II), cadmium(II), and mercury(II) thiolate transitions in metallothionein. Biochemistry. 1981 May 12;20(10):2852–2856. doi: 10.1021/bi00513a022. [DOI] [PubMed] [Google Scholar]
- Waalkes M. P., Goering P. L. Metallothionein and other cadmium-binding proteins: recent developments. Chem Res Toxicol. 1990 Jul-Aug;3(4):281–288. doi: 10.1021/tx00016a001. [DOI] [PubMed] [Google Scholar]
- Winge D. R., Miklossy K. A. Differences in the polymorphic forms of metallothionein. Arch Biochem Biophys. 1982 Mar;214(1):80–88. doi: 10.1016/0003-9861(82)90010-8. [DOI] [PubMed] [Google Scholar]