Abstract
Leaky gut syndrome is a condition widely popularized in the lay literature, although it is not currently accepted as a formal medical diagnosis. Multiple gastrointestinal symptoms are ascribed to leaky gut syndrome, including diarrhea, bloating, distension, abdominal pain, and dyspeptic symptoms of early satiety, nausea, and postprandial fullness. The etiology and pathophysiology of leaky gut syndrome are multifactorial; a preceding gastrointestinal infection, inflammatory bowel disease, and certain medications may be relevant factors in some patients. The diagnosis of leaky gut syndrome is problematic. Although patients are frequently informed that the diagnosis can be readily made using results from blood work or stool studies, no validated test currently exists to make this diagnosis. Patients report a variety of myths about the etiology, diagnosis, and treatment of leaky gut syndrome, which can cause alarm and can frequently lead to expensive, unnecessary tests and unproven, sometimes dangerous treatments. This article reviews some of the most common myths about leaky gut syndrome and provides data from the scientific literature to correct these statements. Management strategies, based on data, are provided when available.
Keywords: Bloating, confocal laser endomicroscopy, glutamine, intestinal permeability, leaky gut syndrome, tight junctions
Case Study
A 42-year-old woman was referred for a second opinion in gastroenterology. She reported a 2-year history of lower abdominal pain and discomfort, bloating, and distension, as well as loose, watery, urgent bowel movements. Her symptoms began after an apparent episode of food poisoning. Her spouse had similar symptoms but recovered completely. Results of extensive laboratory tests, including a complete blood count, metabolic profile, liver chemistries, thyroid tests, celiac serologies, and C-reactive protein, were all normal on at least 2 separate occasions. Stool studies, including fecal lactoferrin and ova and parasites, were normal 3 separate times. Owing to persistent symptoms, she underwent an upper endoscopy and colonoscopy. Endoscopic findings and duodenal, gastric, terminal ileum, and colon biopsies, including special staining for mast cells, were all normal. Breath tests (lactulose, fructose, and then lactose) were normal. Her weight has remained stable since the onset of symptoms. Empiric trials of various probiotics, loperamide, cholestyramine, smooth muscle antispasmodics, eluxadoline, and a diet low in fermentable oligosaccharides, disaccharides, monosaccharides, and polyols have not been helpful. She recently saw a naturopathic doctor, who made a diagnosis of leaky gut syndrome based on her symptoms and a stool sample. The naturopathic doctor recommended a low-histamine diet, 2 different antihistamines, and a panel of supplements. The patient, a former dietitian, requested a second opinion.
Introduction
The discipline of gastroenterology is constantly evolving as new information, technology, ideas, and hypotheses come to light. This constant state of evolution leads not just to new treatments but often to new diagnoses. After rigorous testing using validated techniques, some new diagnoses remain (eg, postinfectious irritable bowel syndrome [IBS], central sensitization syndrome).1,2 Others, however, fade away, destined for the medical history books (eg, mucous colitis, female hysteria).3,4 Leaky gut syndrome is a recent diagnosis now popularized in the lay literature. It has been associated with a myriad of disorders (eg, Alzheimer disease, autism, dementia, diabetes, fibromyalgia),5-8 although scientific evidence using validated techniques to support these claims in humans is quite limited or nonexistent. The diagnosis of leaky gut syndrome is frequently made based on symptoms alone. In other cases, the diagnosis is made based on blood work or stool studies. Some health care providers frequently recommend courses of therapy without convincing or supportive scientific data. These practices have caused confusion for both patients and providers. Physicians who routinely evaluate patients referred for presumed leaky gut syndrome may hear a number of myths regarding this nebulous diagnosis. The goal of this article is to highlight some of the most common myths about leaky gut syndrome and provide scientific evidence from the recent literature, when available, to either support or refute these common views. Therapeutic options will also be discussed using data from the scientific literature.
Myth: Leaky Gut Syndrome Is Common and Causes Extraintestinal Symptoms
An extensive array of products marketed to consumers suggests that leaky gut syndrome is common; however, a lack of validated diagnostic methods for assessing leaky gut syndrome prevents accurate measurement of its true prevalence.9 Despite lay literature claims of gut permeability’s pathologic nature, it is important to understand that all individuals are subject to a baseline level of intestinal permeability, as normal intestinal physiology involves selectively permeable tight junctions that respond to a variety of biological factors (Figure 1).10 Empiric data support an association between impaired intestinal barrier function and only a limited number of conditions, including IBS, functional dyspepsia (FD), inflammatory bowel disease (IBD), graft-versus-host disease, type 1 diabetes, HIV/AIDS, and multiple organ dysfunction syndrome.11-18 Minimal to no reliable evidence exists to suggest that leaky gut syndrome maintains a causative role in the pathogenesis of conditions that are most commonly associated with it in lay discussion, including fibromyalgia, chronic fatigue syndrome, allergies, headache, and brain fog.11,19 All clinical studies to date examining these conditions in relation to leaky gut syndrome have solely provided correlative data without examination of causation. Furthermore, as intestinal permeability dynamically responds to diet, exercise, and infection, it remains impossible to reliably attribute these nonspecific symptomatologies to impaired intestinal barrier function.20
Figure 1.
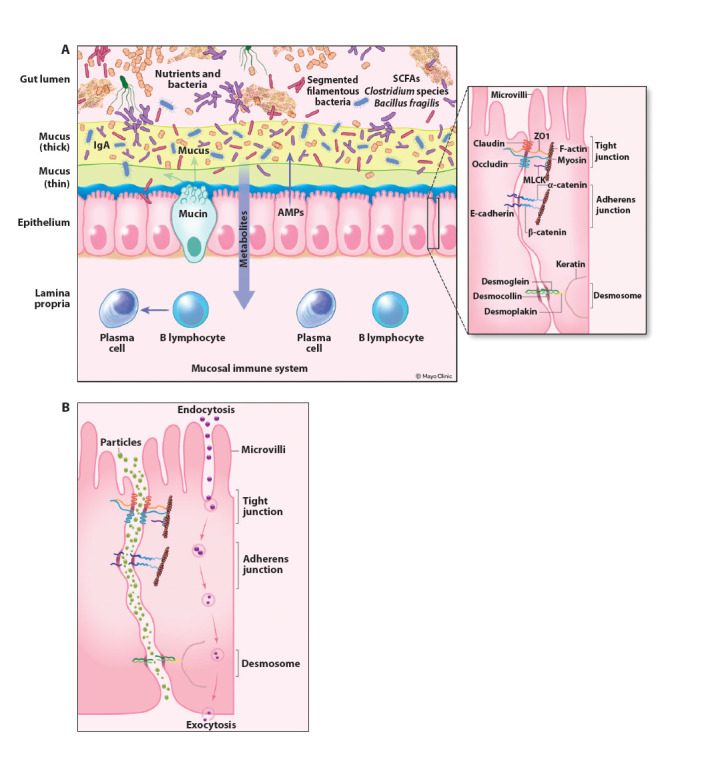
Normal gut barrier and disrupted barrier. The intestinal epithelial cells comprise the most important part of the intestinal barrier (A). Above this lies a layer of mucus; plasma cells may secrete IgA into the mucosal layer. Bacteria, viruses, and medications need to penetrate the mucus layer and the epithelial cell barrier to obtain access to the submucosal layer, where the intestinal immune system resides. Small particles normally cannot penetrate the tight junctions. However, after an insult to the epithelial cell barrier (eg, inflammation, ischemia, medications), tight junctions may open, allowing penetration of small particles with access to the submucosal region (B). The adherens junction and desmosomes help to stabilize cell-cell adhesion and interaction with the cytoskeleton.
AMPs, antimicrobial peptides; IgA, immunoglobulin A; MLCK, myosin light chain kinase; SCFAs, short-chain fatty acids; ZO1, zonula occludens-1.
Myth: Leaky Gut Syndrome Develops Owing to Stress and Eating Gluten
Evidence suggests that a variety of factors may influence intestinal permeability, including illness, antibiotic and drug use, alcohol consumption, and physical activity.1,21-24 Many such factors have been proposed to cause leaky gut syndrome, with stress and gluten consumption commonly highlighted.25-28 Despite the resulting plethora of lifestyle and diet recommendations that abound on the Internet, a closer examination of these claims alongside the existing literature raises doubts about their validity. Concerning stress, it is true that the gastrointestinal and nervous systems are tightly intertwined, interacting via what is often referred to as the gut-brain axis and, more recently, the microbiota-gut-brain axis.29,30 Relatedly, prolonged exposure to chronic stress has been linked to changes in gastrointestinal function and various digestive disorders, including IBS, gastroesophageal reflux disease, and FD.31
Although several studies have demonstrated stress-induced increases in intestinal permeability, the causal role of stress in the development of leaky gut syndrome remains unclear.32-34 Methodologically, the absence of reliable tools for diagnosing and quantifying leaky gut syndrome limits accurate research, and establishing causation would require isolating stress from the myriad factors influencing gastrointestinal health. In addition to stress, dietary choices are frequently implicated in the development of leaky gut syndrome, with gluten often cited as a culprit in lay literature.35,36 Confocal laser endomicroscopy has demonstrated changes in intestinal permeability in some patients with IBS, although recent data show that it may be less sensitive than initially thought.37,38 Although evidence does indicate that lifestyle and diet-related factors can increase intestinal permeability, especially in genetically susceptible individuals, the mere presence of increased intestinal permeability or altered barrier function has not been proven to directly cause symptoms in all patients.35,36 Likewise, research utilizing animal models has demonstrated that isolated impairments in intestinal barrier function do not definitively lead to the development of disease.11
Myth: Leaky Gut Syndrome Is Just a Break in the Gut Lining
The gastrointestinal mucosal lining represents the largest body surface area and therefore presents a unique opportunity for entry of pathogens into the body. Fortunately, intestinal barrier function depends on a complex system of integrated pathways, which, together, determine transport across the intestinal epithelium; leaky gut syndrome is not simply the result of a break in the epithelial monolayer. Although there are multiple different pathways by which components cross the intestinal epithelium, the paracellular and transcellular routes are the most relevant to the phenomenon of leaky gut syndrome.39 The paracellular transport pathway is the key means utilized by ions, water, and larger hydrophilic compounds to cross between epithelial cells (Figure 1). The paracellular route of transport depends largely on the function of tight junctions, which act to form a physical barrier that restricts diffusion across the epithelium and selectively allows transport of certain ion species. These tight junctions are constituted by transmembrane proteins, such as claudins and occludin, which interact with zonula occludens scaffold proteins (Figure 1).40 By contrast, the transcellular route is used by sugars, amino acids, and vitamins, as well as by larger proteins and bacterial components, via endocytosis and exocytosis of vesicles. Thus, increased intestinal transcellular permeability has been implicated as an important pathway by which bacteria, endotoxins, and lipopolysaccharide may cross the intestinal epithelium.
Myth: Leaky Gut Syndrome Can Be Confidently Diagnosed by Symptoms Alone
The validity of leaky gut syndrome can be challenged by the long list of associated symptoms, most of which overlap with a variety of other gastrointestinal and nongastrointestinal conditions.1,9,41 Reported symptoms typically include bloating, abdominal pain, fatigue, headache, and food sensitivities, among dozens of other symptoms.1,9 Such symptoms are nonspecific in nature, with 18% of the global population experiencing bloating at least once per week and more than 70% of individuals with IBS reporting bloating as a component of their symptom complex.42,43 Other prevalent symptoms such as brain fog, fatigue, and headache are characterized by vague and unclear etiologies. Additionally, although some data exist to suggest an association between food allergies and increased intestinal barrier permeability, adverse food reactions (whether allergy or intolerance) are likely multifactorial; thus, their mere presence cannot definitively implicate leaky gut syndrome.44 Amid the complex pathophysiology and nonspecific nature of most symptoms associated with leaky gut syndrome, it is impossible to discern this syndrome from others using symptoms alone. Furthermore, despite long lists of symptoms associated with leaky gut syndrome that are highlighted in the lay literature, currently no evidence exists to suggest that such symptoms are directly linked to increased intestinal permeability or leaky gut syndrome.36
Myth: Leaky Gut Syndrome Can Be Readily Diagnosed by Blood Work and Standard Endoscopy
Although symptoms cannot be used to reliably assess leaky gut syndrome, several methods exist for assessing intestinal permeability, including measurement via fractional urinary excretion of ingested saccharide probes, in vitro evaluation utilizing intestinal tissue biopsies, and in vivo techniques such as confocal laser endomicroscopy (Figure 2) and endoscopic mucosal impedance.1,45,46 Although available, the clinical utility of using orally ingested saccharide probes and in vitro techniques is limited partly owing to unknown normal values for comparison, lack of standardized protocols, and limited attempts at validation.47 Further highlighting the controversy in this area is the fact that, although the lactulose:mannitol ratio is used as a gold standard to identify abnormalities in intestinal permeability, it may not be as accurate as previously thought owing to low absorption in the colon. Future studies using the lactulose:mannitol test should include an additional form of analysis such as confocal laser endomicroscopy or analysis of transepithelial resistance in ex vivo samples. Regarding endoscopic methods, prior proof-of-concept studies demonstrating their utility in the evaluation of IBD and disorders of gut-brain interaction support the need for further investigation into their ability to diagnose leaky gut syndrome and other permeability-related conditions.48 As noted previously, confocal laser endomicroscopy may provide valuable information on intestinal permeability, although it may not be as sensitive as once thought for the evaluation of food sensitivities.45 For now, no validated tool exists to accurately diagnose leaky gut syndrome.39
Figure 2.
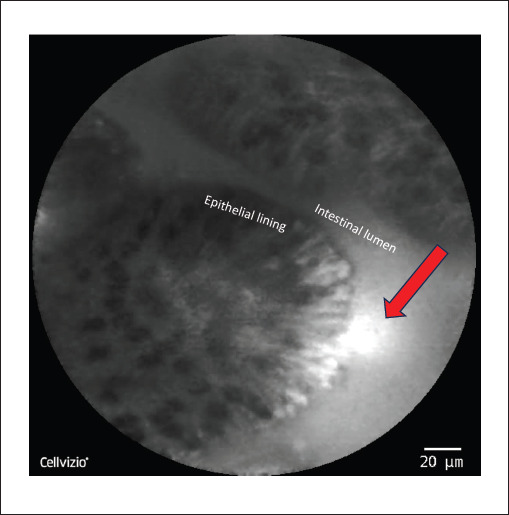
Representative confocal laser endomicroscopy image of the ileum. The intestinal lumen and epithelial lining are labeled. The red arrow indicates a break in the epithelial wall with hyperdense areas along the epithelial lining representing increased fluorescein uptake; leakage of fluorescein into the intestinal lumen is also noted by the red arrow.
Nevertheless, in recent years, direct-to-consumer blood panels for the diagnosis of leaky gut syndrome have been marketed, with an emphasis on measuring zonulin levels, as well as antibodies to zonulin, actin, and lipopolysaccharides. Although commercial zonulin assays have been used to assess intestinal permeability in a variety of gastrointestinal conditions, evidence suggests that the assays are methodologically flawed, and results do not correlate with disease states.49-51 The utility of measuring zonulin in over-the-counter kits is thus suspect. Other plasma biomarkers measuring bacterial translocation, such as lipopolysaccharide-binding protein, may correlate more reliably with intestinal permeability.52 Currently, no blood test to diagnose leaky gut syndrome exists, and further investigation is needed to identify valid, reliable, and clinically useful serum biomarkers of impaired intestinal barrier function.
Myth: Leaky Gut Syndrome Can Be Diagnosed by Stool Studies
In addition to blood testing, stool testing for leaky gut syndrome has been marketed to consumers by a multitude of alternative medicine and wellness companies. Readily accessible for purchase online, these tests allegedly measure a number of fecal biomarkers related to intestinal permeability, including zonulin levels, secretory immunoglobulin A, bacteria imbalances, Candida, and mold. Despite their availability, such tests are unregulated, and minimal evidence exists to support their use in the clinical setting. Furthermore, as the causal relationship between intestinal permeability and symptomatology is ill-defined, the clinical utility of stool testing in the context of leaky gut syndrome remains questionable.53 Even if testing were to reliably measure intestinal permeability, results would be of unclear significance, as no standard of care currently exists for treating and managing leaky gut syndrome. Although a number of additional potential biomarkers have been proposed in the literature, including both fecal calprotectin and fecal albumin, further investigation is needed to determine whether such forms of evaluation reliably measure intestinal permeability and can provide diagnostic value.54 Importantly, many of the same biomarkers are currently used to evaluate gastrointestinal disease activity, such as fecal calprotectin for the assessment of IBD.55 Although this overlap may challenge the utility of fecal biomarkers for discerning nonspecific leaky gut symptoms from other conditions, it may also support an association between impaired intestinal barrier function and gastrointestinal disease.
Myth: Leaky Gut Syndrome Can Be Cured by Diet and Probiotics
Diet would seem a logical target for the treatment of intestinal permeability given the inherent direct interaction between food, the gut epithelial lining, and potential changes in the integrity of the intestinal epithelial barrier. In addition to gluten (addressed previously), other dietary factors such as ethanol, bile acids, and emulsifiers have been shown to increase intestinal permeability via a number of different mechanisms in animal and/or human studies.56 Similarly, a number of dietary factors, including fiber, vitamins, and minerals (to be discussed in the following section), have been demonstrated to reduce intestinal permeability. However, it should be emphasized that the aforementioned dietary factors have not been studied specifically for the treatment of a pathologic disorder associated with leaky gut syndrome, and thus a direct link between diet and treatment of leaky gut syndrome has not been established.
That being said, fiber warrants attention as a potentially relevant dietary intervention to improve intestinal permeability. In addition to helping to maintain a healthy intestinal surface mucous membrane, dietary fiber also contains microbiota-accessible carbohydrates (MACs), which have been shown to enhance expression of tight junction proteins; furthermore, evidence suggests that short-chain fatty acids, a fermentation product of MACs, may improve intestinal barrier function by affecting T-cell–mediated neuroimmune functions within the gut.56,57 However, again, no study to date has assessed fiber as a treatment for leaky gut syndrome.
Similarly, although a vast array of probiotic strains exists, none have been studied thus far to specifically treat a pathologic condition associated with leaky gut syndrome. It is worth noting, however, that in a randomized, placebo-controlled trial in obese adults, 2 different strains of Bifidobacterium probiotics were found to improve markers of intestinal permeability (measured via sucralose:lactulose ratios following aspirin excretion).58 In addition, in a mouse model of colitis, treatment with VSL#3 was found to reduce intestinal epithelial permeability (measured in vivo), as well as prevent decreased expression of tight junction proteins.59
Myth: Nutritional Supplements Can Cure Leaky Gut Syndrome
A number of vitamins and minerals have also been studied in the context of intestinal permeability, including vitamins A and D, zinc, and glutamine (Table.).56 In experimental murine models of colitis, administration of vitamin D has been associated with improvement in intestinal barrier function via enhanced expression of zonula occludens, occludin, and claudin, whereas vitamin D receptor–deficient mice have been shown to be more susceptible to colitis and increased intestinal permeability.60 Additionally, in a randomized, double-blind, placebo-controlled study of patients with Crohn’s disease (n=27), treatment with 2000 international units of vitamin D per day for 3 months maintained intestinal permeability (as measured by the lactulose:mannitol urinary excretion test), compared with placebo, which resulted in increased intestinal permeability at 3 months.61 Similarly, in a randomized crossover study of 10 healthy volunteers, the coadministration of zinc carnosine with indomethacin appeared to prevent changes in intestinal permeability as measured by a lactulose:rhamnose urinary excretion test, compared with a significant increase in intestinal permeability that occurred when study participants received placebo with indomethacin.62
Table.
Therapeutic Interventions Evaluated in Humans for Leaky Gut Syndrome and/or Disordered Intestinal Permeability
| Intervention | Population studied | Dose and frequency | Results/comments |
|---|---|---|---|
| Probiotics | Obese adults8 |
Bifidobacterium adolescentis (1 × 109 CFU/daily); Bifidobacterium lactis (1 × 109 CFU/daily) |
Significant reduction in sucralose:lactulose ratio following aspirin excretion |
| Vitamin D | Crohn’s disease61 | Vitamin D3 2000 IU/daily | No difference in lactulose:mannitol ratio at 3 months in treatment group vs significant increase in lactulose:mannitol ratio in placebo group |
| Zinc | Healthy volunteers62 | Zinc carnosine 37.5 mg twice daily | No difference in lactulose:rhamnose ratio after 5 days of exposure to indomethacin 50 mg three times daily in treatment group vs significant increase in lactulose:rhamnose ratio in placebo group |
| Glutamine | Crohn’s disease63; postinfectious IBS-D64 | 0.5 mg/kg ideal body weight/day; 5 g three times daily | No difference in lactulose:mannitol ratio compared with control (whey protein).63 Significant improvement in lactulose:mannitol ratio compared with placebo64 |
| CL-C2 agonists | Healthy male volunteers67 | 24 µg/day for 28 days (lubiprostone) | Lactulose:mannitol ratio significantly lower in the lubiprostone group than in the untreated group after 28 days of treatment. Blood endotoxin activity exhibited almost no change over time in the lubiprostone and untreated groups |
| Larazotide | Celiac disease70 | 1, 4, or 8 mg three times daily | No difference in lactulose:mannitol ratio compared with placebo |
| Prednisone | Crohn’s disease82 | 40 mg/day for 3 weeks, tapered completely over the following 7 weeks | Lactulose:mannitol ratio was significantly decreased after treatment with prednisone |
| Infliximab | Crohn’s disease83 | 5 mg/kg body weight for 7 days | Significant decrease in lactulose:mannitol ratio following treatment with infliximab |
CFU, colony-forming unit; CL-C2, type-2 chloride channels; IBS-D, irritable bowel syndrome with diarrhea; IU, international units.
At present, the nutritional supplement with perhaps the most, albeit limited, data for treating intestinal permeability in the context of human disease is glutamine. In a randomized controlled trial of 28 patients with Crohn’s disease, glutamine significantly improved intestinal permeability, as measured by the lactulose:mannitol urinary excretion test, although improvement was similar in the control group that received whey protein.63 Moreover, in a randomized controlled trial of 106 patients with postinfectious IBS with diarrhea (IBS-D), treatment with glutamine significantly reduced intestinal permeability (as measured by the lactulose:mannitol urinary excretion test), as well as symptoms, bowel movement frequency, and consistency compared with placebo.64 Therefore, although several nutritional supplements have been suggested to improve intestinal permeability, only glutamine has been shown to improve leaky gut syndrome in the context of IBD and IBS. More data are needed before any nutritional supplements, including glutamine, can be recommended in clinical practice.
Myth: Medications Cannot Change Intestinal Permeability
Several medications can alter intestinal permeability, although none have been evaluated in prospective studies of patients diagnosed with leaky gut syndrome. It is worth highlighting that medications may potentially alter intestinal permeability as a direct effect (as discussed later), or indirectly via reducing generalized inflammation, which would then improve barrier function. Prednisone is a good example of the latter (Table 1). Lubiprostone, a prostaglandin E1 analog, binds to type-2 chloride channel receptors.65 It is approved by the US Food and Drug Administration for the treatment of IBS with constipation in women.66 In a prospective study of 28 healthy male volunteers treated with an anti-inflammatory agent (diclofenac), lubiprostone (24 µg daily) improved the lactulose:mannitol ratio compared with no therapy.67 The authors interpreted these results as indirect evidence of an improvement in intestinal permeability, although no placebo or comparator was provided. Larazotide, an 8–amino acid peptide, acts as a zonulin antagonist.68 It improves intestinal permeability in animal models and has been evaluated in clinical trials for the treatment of celiac disease.69,70 Three celiac disease clinical trials incorporated a lactulose-mannitol test, although a meta-analysis did not reveal any significant differences in the lactulose:mannitol ratio in patients treated with larazotide compared with those treated with placebo.71 Peripheral acting µ-opioid receptor antagonists (PAMORAs) are used to treat opioid-induced constipation.72 Morphine and other opioids may compromise intestinal barrier function, leading to localized immunosuppression and bacterial translocation.73-75 By acting on the µ-opioid receptor, PAMORAs improve survival in patients with advanced cancer on opioids, possibly by improving intestinal permeability.76
Myth: Once Leaky Gut Syndrome Develops, It Will Never Go Away
The natural history of any medical condition can be defined as the progression of that disorder in an individual, over time, without any type of therapeutic intervention. Understanding the natural history of a condition is important to help properly inform patients about whether diagnostic tests are necessary and discuss potential therapeutic options, if necessary. Properly characterizing the natural history of leaky gut syndrome would thus entail accurately diagnosing a large group of patients and then following that cohort prospectively over a long period to assess outcomes. However, no study has been performed to properly evaluate changes in symptoms and underlying pathophysiology over time; thus, the true natural history of leaky gut syndrome is unknown. Some insight into the natural history may be gleaned from studies of postinfectious IBS, which can involve alterations in the intestinal barrier of some, but not all, patients.77-79 Overall, the prognosis is favorable, with at least 50% of patients reporting resolution of symptoms 5 years after onset. In general, it appears that postinfectious IBS that develops after a viral illness improves more quickly than postinfectious IBS that develops after a bacterial infection.79 At present, for patients with concerns about leaky gut syndrome, the best approach is to educate them about the true possibility of this condition, inform them of more likely diagnoses, reassure them that the natural history is most likely benign, and focus treatment on the predominant symptom.
Case Resolution
The patient was diagnosed with IBS-D based on the chronicity of symptoms, the absence of warning signs on history and physical examination, normal laboratory and stool studies, and the Rome IV criteria.80 The patient was asked to reduce her fiber intake to less than 12 g per day, as fiber accelerates gastrointestinal transit and worsens bloating, and was started on low-dose amitriptyline each evening (10 mg).66 She reported a 40% improvement in global IBS symptoms on a phone call follow-up 2 weeks later, although she still had persistent symptoms of fecal urgency and loose stools. The provider suggested an increase in the dose of amitriptyline each night (25 mg followed 2 weeks later by an increase to 50 mg) and also initiated a trial of low-dose alosetron, a 5-HT3 antagonist, each morning (0.5 mg). On a follow-up phone call 2 weeks later, she noted further improvement in her IBS symptoms, including urgency and diarrhea. The dose of alosetron was increased to 1 mg each morning, and over the next month, while remaining on a low-fiber diet and amitriptyline each night (50 mg), the patient reported that her IBS symptoms had essentially resolved. Although no side effects were reported in this patient, alosetron has the potential to cause significant constipation in some patients and has been associated (rarely) with ischemic colitis. Introduction of the lower dose of alosetron to the market has helped improve the side-effect profile of the medication.81
Conclusion
Leaky gut syndrome is a condition fraught with myths and misunderstandings. It cannot be accurately diagnosed by symptoms, blood work, or stool studies. Although the term leaky gut syndrome implies changes in intestinal permeability, it is the rare patient who undergoes objective testing to identify changes in intestinal permeability. Thus, the term leaky gut syndrome should not be used by clinicians unless objective testing is performed to document changes in intestinal permeability. Importantly, changes in intestinal permeability are not always deleterious, and the relationship with symptoms is unclear. Changes in intestinal permeability have been identified in some patients with IBS and FD, although large, prospective studies documenting these changes have not been performed in patients appropriately diagnosed using standardized criteria.80 In summary, this is an intriguing area of research with more fallacies than facts. The issues posed in this article should drive researchers and clinicians to better elucidate this poorly described condition.
References
- Barbara G, Grover M, Bercik P et al. Rome Foundation working team report on post-infection irritable bowel syndrome. Gastroenterology. 2019;156(1):46–58.e7. doi: 10.1053/j.gastro.2018.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eller-Smith OC, Nicol AL, Christianson JA. Potential mechanisms underlying centralized pain and emerging therapeutic interventions. Front Cell Neurosci. 2018;12:35. doi: 10.3389/fncel.2018.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osler W. The Principles and Practice of Medicine. 1892. New York, NY: Appleton & Co.
- Tasca C, Rapetti M, Carta MG, Fadda B. Women and hysteria in the history of mental health. Clin Pract Epidemiol Ment Health. 2012;8:110–119. doi: 10.2174/1745017901208010110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Eufemia P, Celli M, Finocchiaro R et al. Abnormal intestinal permeability in children with autism. Acta Paediatr. 1996;85(9):1076–1079. doi: 10.1111/j.1651-2227.1996.tb14220.x. [DOI] [PubMed] [Google Scholar]
- de Magistris L, Familiari V, Pascotto A et al. Alterations of the intestinal barrier in patients with autism spectrum disorders and in their first-degree relatives. J Pediatr Gastroenterol Nutr. 2010;51(4):418–424. doi: 10.1097/MPG.0b013e3181dcc4a5. [DOI] [PubMed] [Google Scholar]
- Stadlbauer V, Engertsberger L, Komarova I et al. Dysbiosis, gut barrier dysfunction and inflammation in dementia: a pilot study. BMC Geriatr. 2020;20(1):248. doi: 10.1186/s12877-020-01644-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nah G, Park S-C, Kim K et al. Type-2 diabetics reduces spatial variation of microbiome based on extracellur vesicles from gut microbes across human body. Sci Rep. 2019;9(1):20136. doi: 10.1038/s41598-019-56662-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camilleri M. Leaky gut: mechanisms, measurement and clinical implications in humans. Gut. 2019;68(8):1516–1526. doi: 10.1136/gutjnl-2019-318427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groschwitz KR, Hogan SP. Intestinal barrier function: molecular regulation and disease pathogenesis. J Allergy Clin Immunol. 2009;124(1):3–20. doi: 10.1016/j.jaci.2009.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odenwald MA, Turner JR. Intestinal permeability defects: is it time to treat? Clin Gastroenterol Hepatol. 2013;11(9):1075–1083. doi: 10.1016/j.cgh.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanning N, Edwinson AL, Ceuleers H et al. Intestinal barrier dysfunction in irritable bowel syndrome: a systematic review. Therap Adv Gastroenterol. 2021;14:1756284821993586. doi: 10.1177/1756284821993586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nojkov B, Zhou SY, Dolan RD et al. Evidence of duodenal epithelial barrier impairment and increased pyroptosis in patients with functional dyspepsia on confocal laser endomicroscopy and “ex vivo” mucosa analysis. Am J Gastroenterol. 2020;115(11):1891–1901. doi: 10.14309/ajg.0000000000000827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson JE, Ekman T. Gut toxicity during hemopoietic stem cell transplantation may predict acute graft-versus-host disease severity in patients. Dig Dis Sci. 2007;52(9):2340–2345. doi: 10.1007/s10620-006-9404-x. [DOI] [PubMed] [Google Scholar]
- Bosi E, Molteni L, Radaelli MG et al. Increased intestinal permeability precedes clinical onset of type 1 diabetes. Diabetologia. 2006;49(12):2824–2827. doi: 10.1007/s00125-006-0465-3. [DOI] [PubMed] [Google Scholar]
- Sharpstone D, Neild P, Crane R et al. Small intestinal transit, absorption, and permeability in patients with AIDS with and without diarrhoea. Gut. 1999;45(1):70–76. doi: 10.1136/gut.45.1.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keating J, Bjarnason I, Somasundaram S et al. Intestinal absorptive capacity, intestinal permeability and jejunal histology in HIV and their relation to diarrhoea. Gut. 1995;37(5):623–629. doi: 10.1136/gut.37.5.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doig CJ, Sutherland LR, Sandham JD, Fick GH, Verhoef M, Meddings JB. Increased intestinal permeability is associated with the development of multiple organ dysfunction syndrome in critically ill ICU patients. Am J Respir Crit Care Med. 1998;158(2):444–451. doi: 10.1164/ajrccm.158.2.9710092. [DOI] [PubMed] [Google Scholar]
- Goebel A, Buhner S, Schedel R, Lochs H, Sprotte G. Altered intestinal permeability in patients with primary fibromyalgia and in patients with complex regional pain syndrome. Rheumatology (Oxford). 2008;47(8):1223–1227. doi: 10.1093/rheumatology/ken140. [DOI] [PubMed] [Google Scholar]
- Bischoff SC, Barbara G, Buurman W et al. Intestinal permeability—a new target for disease prevention and therapy. BMC Gastroenterol. 2014;14:189. doi: 10.1186/s12876-014-0189-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulstrup MV, Christensen EG, Carvalho V et al. Antibiotic treatment affects intestinal permeability and gut microbial composition in Wistar rats dependent on antibiotic class. PLoS One. 2015;10(12):e0144854. doi: 10.1371/journal.pone.0144854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smecuol E, Bai JC, Sugai E et al. Acute gastrointestinal permeability responses to different non-steroidal anti-inflammatory drugs. Gut. 2001;49(5):650–655. doi: 10.1136/gut.49.5.650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purohit V, Bode JC, Bode C et al. Alcohol, intestinal bacterial growth, intestinal permeability to endotoxin, and medical consequences: summary of a symposium. Alcohol. 2008;42(5):349–361. doi: 10.1016/j.alcohol.2008.03.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keirns BH, Koemel NA, Sciarrillo CM, Anderson KL, Emerson SR. Exercise and intestinal permeability: another form of exercise-induced hormesis? Am J Physiol Gastrointest Liver Physiol. 2020;319(4):G512–G518. doi: 10.1152/ajpgi.00232.2020. [DOI] [PubMed] [Google Scholar]
- Madison A, Kiecolt-Glaser JK. Stress, depression, diet, and the gut microbiota: human-bacteria interactions at the core of psychoneuroimmunology and nutrition. Curr Opin Behav Sci. 2019;28:105–110. doi: 10.1016/j.cobeha.2019.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aleman RS, Moncada M, Aryana KJ. Leaky gut and the ingredients that help treat it: a review. Molecules. 2023;28(2):619. doi: 10.3390/molecules28020619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasano A. All disease begins in the (leaky) gut: role of zonulin-mediated gut permeability in the pathogenesis of some chronic inflammatory diseases. F1000Res. 2020;9 doi: 10.12688/f1000research.20510.1. F1000 Faculty Rev-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cenni S, Sesenna V, Boiardi G et al. The role of gluten in gastrointestinal disorders: a review. Nutrients. 2023;15(7):1615. doi: 10.3390/nu15071615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer EA, Nance K, Chen S. The gut-brain axis. Annu Rev Med. 2022;73:439–453. doi: 10.1146/annurev-med-042320-014032. [DOI] [PubMed] [Google Scholar]
- Margolis KG, Cryan JF, Mayer EA. The microbiota-gut-brain axis: from motility to mood. Gastroenterology. 2021;160(5):1486–1501. doi: 10.1053/j.gastro.2020.10.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konturek PC, Brzozowski T, Konturek SJ. Stress and the gut: pathophysiology, clinical consequences, diagnostic approach and treatment options. J Physiol Pharmacol. 2011;62(6):591–599. [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Wilson SJ, Bailey ML et al. Marital distress, depression, and a leaky gut: translocation of bacterial endotoxin as a pathway to inflammation. Psychoneuroendocrinology. 2018;98:52–60. doi: 10.1016/j.psyneuen.2018.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanuytsel T, van Wanrooy S, Vanheel H et al. Psychological stress and corticotropin-releasing hormone increase intestinal permeability in humans by a mast cell-dependent mechanism. Gut. 2014;63(8):1293–1299. doi: 10.1136/gutjnl-2013-305690. [DOI] [PubMed] [Google Scholar]
- Zheng G, Wu SP, Hu Y, Smith DE, Wiley JW, Hong S. Corticosterone mediates stress-related increased intestinal permeability in a region-specific manner. Neurogastroenterol Motil. 2013;25(2):e127–e139. doi: 10.1111/nmo.12066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez-Roque MI, Camilleri M, Smyrk T et al. A controlled trial of gluten-free diet in patients with irritable bowel syndrome-diarrhea: effects on bowel frequency and intestinal function. Gastroenterology. 2013;144(5):903–911.e3. doi: 10.1053/j.gastro.2013.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camilleri M. Human intestinal barrier: effects of stressors, diet, prebiotics, and probiotics. Clin Transl Gastroenterol. 2021;12(1):e00308. doi: 10.14309/ctg.0000000000000308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritscher-Ravens A, Schuppan D, Ellrichmann M et al. Confocal endomicroscopy shows food-associated changes in the intestinal mucosa of patients with irritable bowel syndrome. Gastroenterology. 2014;147(5):1012–1020.e4. doi: 10.1053/j.gastro.2014.07.046. [DOI] [PubMed] [Google Scholar]
- Bojarski C, Tangermann P, Barmeyer C et al. Prospective, double-blind diagnostic multicentre study of confocal laser endomicroscopy for wheat sensitivity in patients with irritable bowel syndrome. Gut. 2022;71(8):1567–1576. doi: 10.1136/gutjnl-2021-325181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanuytsel T, Tack J, Farre R. The role of intestinal permeability in gastrointes tinal disorders and current methods of evaluation. Front Nutr. 2021;8:717925. doi: 10.3389/fnut.2021.717925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farré R, Vicario M. Abnormal barrier function in gastrointestinal disorders. Handb Exp Pharmacol. 2017;239:193–217. doi: 10.1007/164_2016_107. [DOI] [PubMed] [Google Scholar]
- Lacy BE, Cangemi D, Vazquez-Roque M. Management of chronic abdominal distension and bloating. Clin Gastroenterol Hepatol. 2021;19(2):219–231.e1. doi: 10.1016/j.cgh.2020.03.056. [DOI] [PubMed] [Google Scholar]
- Ballou S, Singh P, Nee J et al. Prevalence and associated factors of bloating: results from the Rome Foundation Global Epidemiology Study. Gastroenterology. 2023;165(3):647–655.e4. doi: 10.1053/j.gastro.2023.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringel Y, Williams RE, Kalilani L, Cook SF. Prevalence, characteristics, and impact of bloating symptoms in patients with irritable bowel syndrome. Clin Gastroenterol Hepatol. 2009;7(1):68–72. doi: 10.1016/j.cgh.2008.07.008. [DOI] [PubMed] [Google Scholar]
- Caminero A, Meisel M, Jabri B, Verdu EF. Mechanisms by which gut microorganisms influence food sensitivities. Nat Rev Gastroenterol Hepatol. 2019;16(1):7–18. doi: 10.1038/s41575-018-0064-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoultz I, Keita ÅV. The intestinal barrier and current techniques for the assessment of gut permeability. Cells. 2020;9(8):1909. doi: 10.3390/cells9081909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusticeanu M, Zimmer V, Lammert F. Visualising and quantifying intestinal permeability—where do we stand. Ann Hepatol. 2021;23:100266. doi: 10.1016/j.aohep.2020.09.010. [DOI] [PubMed] [Google Scholar]
- Khoshbin K, Khanna L, Maselli D et al. Development and validation of test for “leaky gut” small intestinal and colonic permeability using sugars in healthy adults. Gastroenterology. 2021;161(2):463–475.e13. doi: 10.1053/j.gastro.2021.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace MB, Vazquez-Roque M, Bojarski C, Schulzke JD. Imaging the leaky gut. Gastroenterology. 2014;147(5):952–954. doi: 10.1053/j.gastro.2014.09.027. [DOI] [PubMed] [Google Scholar]
- Ajamian M, Steer D, Rosella G, Gibson PR. Serum zonulin as a marker of intestinal mucosal barrier function: may not be what it seems. PLoS One. 2019;14(1):e0210728. doi: 10.1371/journal.pone.0210728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talley NJ, Holtmann GJ, Jones M et al. Zonulin in serum as a biomarker fails to identify the IBS, functional dyspepsia and non-coeliac wheat sensitivity. Gut. 2020;69(9):1–3. doi: 10.1136/gutjnl-2019-318664. [DOI] [PubMed] [Google Scholar]
- Massier L, Chakaroun R, Kovacs P, Heiker JT. Blurring the picture in leaky gut research: how shortcomings of zonulin as a biomarker mislead the field of intestinal permeability. Gut. 2021;70(9):1801–1802. doi: 10.1136/gutjnl-2020-323026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seethaler B, Basrai M, Neyrinck AM et al. Biomarkers for assessment of intestinal permeability in clinical practice. Am J Physiol Gastrointest Liver Physiol. 2021;321(1):G11–G17. doi: 10.1152/ajpgi.00113.2021. [DOI] [PubMed] [Google Scholar]
- Leech B, Schloss J, Steel A. Association between increased intestinal permeability and disease: a systematic review. Adv Integr Med. 2019;6(1):23–34. [Google Scholar]
- Perez-Diaz-Del-Campo N, Castelnuovo G, Ribaldone DG, Caviglia GP. Fecal and circulating biomarkers for the non-invasive assessment of intestinal permeability. Diagnostics (Basel). 2023;13(11):1976. doi: 10.3390/diagnostics13111976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsham NE, Sherwood RA. Fecal calprotectin in inflammatory bowel disease. Clin Exp Gastroenterol. 2016;9:21–29. doi: 10.2147/CEG.S51902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoshbin K, Camilleri M. Effects of dietary components on intestinal permeability in health and disease. Am J Physiol Gastrointest Liver Physiol. 2020;319(5):G589–G608. doi: 10.1152/ajpgi.00245.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camilleri M, Lyle BJ, Madsen KL, Sonnenburg J, Verbeke K, Wu GD. Role for diet in normal gut barrier function: developing guidance within the framework of food-labeling regulations. Am J Physiol Gastrointest Liver Physiol. 2019;317(1):G17–G39. doi: 10.1152/ajpgi.00063.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krumbeck JA, Rasmussen HE, Hutkins RW et al. Probiotic Bifidobacterium strains and galactooligosaccharides improve intestinal barrier function in obese adults but show no synergism when used together as synbiotics. Microbiome. 2018;6(1):121. doi: 10.1186/s40168-018-0494-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mennigen R, Nolte K, Rijcken E et al. Probiotic mixture VSL#3 protects the epithelial barrier by maintaining tight junction protein expression and preventing apoptosis in a murine model of colitis. Am J Physiol Gastrointest Liver Physiol. 2009;296(5):G1140–G1149. doi: 10.1152/ajpgi.90534.2008. [DOI] [PubMed] [Google Scholar]
- Vargas-Robles H, Castro-Ochoa KF, Citalán-Madrid AF, Schnoor M. Beneficial effects of nutritional supplements on intestinal epithelial barrier functions in experimental colitis models in vivo. World J Gastroenterol. 2019;25(30):4181–4198. doi: 10.3748/wjg.v25.i30.4181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raftery T, Martineau AR, Greiller CL et al. Effects of vitamin D supplementation on intestinal permeability, cathelicidin and disease markers in Crohn’s disease: results from a randomised double-blind placebo-controlled study. United European Gastroenterol J. 2015;3(3):294–302. doi: 10.1177/2050640615572176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmood A, FitzGerald AJ, Marchbank T et al. Zinc carnosine, a health food supplement that stabilises small bowel integrity and stimulates gut repair processes. Gut. 2007;56(2):168–175. doi: 10.1136/gut.2006.099929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin J, Makharia G, Ahuja V et al. Glutamine and whey protein improve intestinal permeability and morphology in patients with Crohn’s disease: a randomized controlled trial. Dig Dis Sci. 2012;57(4):1000–1012. doi: 10.1007/s10620-011-1947-9. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Verne ML, Fields JZ et al. Randomised placebo-controlled trial of dietary glutamine supplements for postinfectious irritable bowel syndrome. Gut. 2019;68(6):996–1002. doi: 10.1136/gutjnl-2017-315136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacy BE, Levy LC. Lubiprostone: a novel treatment for chronic constipation. Clin Interv Aging. 2008;3(2):357–364. doi: 10.2147/cia.s2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacy BE, Pimentel M, Brenner DM et al. ACG Clinical Guideline: management of irritable bowel syndrome. Am J Gastroenterol. 2021;116(1):17–44. doi: 10.14309/ajg.0000000000001036. [DOI] [PubMed] [Google Scholar]
- Kato T, Honda Y, Kurita Y et al. Lubiprostone improves intestinal permeability in humans, a novel therapy for the leaky gut: a prospective randomized pilot study in healthy volunteers. PLoS One. 2017;12(4):e0175626. doi: 10.1371/journal.pone.0175626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slifer ZM, Krishnan BR, Madan J, Blikslager AT. Larazotide acetate: a pharmacological peptide approach to tight junction regulation. Am J Physiol Gastrointest Liver Physiol. 2021;320(6):G983–G989. doi: 10.1152/ajpgi.00386.2020. [DOI] [PubMed] [Google Scholar]
- Leffler DA, Kelly CP, Abdallah HZ et al. A randomized, double-blind study of larazotide acetate to prevent the activation of celiac disease during gluten challenge. Am J Gastroenterol. 2012;107(10):1554–1562. doi: 10.1038/ajg.2012.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly CP, Green PH, Murray JA et al. Larazotide Acetate Celiac Disease Study Group. Larazotide acetate in patients with coeliac disease undergoing a gluten challenge: a randomised placebo-controlled study. Aliment Pharmacol Ther. 2013;37(2):252–262. doi: 10.1111/apt.12147. [DOI] [PubMed] [Google Scholar]
- Hoilat GJ, Altowairqi AK, Ayas MF et al. Larazotide acetate for treatment of celiac disease: a systematic review and meta-analysis of randomized controlled trials. Clin Res Hepatol Gastroenterol. 2022;46(1):101782. doi: 10.1016/j.clinre.2021.101782. [DOI] [PubMed] [Google Scholar]
- Pergolizzi JV, Jr, Christo PJ, LeQuang JA, Magnusson P. The use of peripheral μ-opioid receptor antagonists (PAMORA) in the management of opioid-induced constipation: an update on their efficacy and safety. Drug Des Devel Ther. 2020;14:1009–1025. doi: 10.2147/DDDT.S221278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng J, Yu H, Ma J et al. Morphine induces bacterial translocation in mice by compromising intestinal barrier function in a TLR-dependent manner. PLoS One. 2013;8(1):e54040. doi: 10.1371/journal.pone.0054040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacerdote P. Opioid-induced immunosuppression. Curr Opin Support Palliat Care. 2008;2(1):14–18. doi: 10.1097/SPC.0b013e3282f5272e. [DOI] [PubMed] [Google Scholar]
- Plein LM, Rittner HL. Opioids and the immune system—friend or foe. Br J Pharmacol. 2018;175(14):2717–2725. doi: 10.1111/bph.13750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janku F, Johnson LK, Karp DD, Atkins JT, Singleton PA, Moss J. Treatment with methylnaltrexone is associated with increased survival in patients with advanced cancer. Ann Oncol. 2016;27(11):2032–2038. doi: 10.1093/annonc/mdw317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiller RC, Jenkins D, Thornley JP et al. Increased rectal mucosal enteroendocrine cells, T lymphocytes, and increased gut permeability following acute Campylobacter enteritis and in post-dysenteric irritable bowel syndrome. Gut. 2000;47(6):804–811. doi: 10.1136/gut.47.6.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berumen A, Edwinson AL, Grover M. Post-infection irritable bowel syndrome. Gastroenterol Clin North Am. 2021;50(2):445–461. doi: 10.1016/j.gtc.2021.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghoshal UC. Postinfection irritable bowel syndrome. Gut Liver. 2022;16(3):331–340. doi: 10.5009/gnl210208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacy BE, Mearin F, Chang L et al. Bowel disorders. Gastroenterology. 2016;150(6):1393–1407. doi: 10.1053/j.gastro.2016.02.031. [DOI] [PubMed] [Google Scholar]
- Lacy BE, Nicandro JP, Chuang E, Earnest DL. Alosetron use in clinical practice: significant improvement in irritable bowel syndrome symptoms evaluated using the US Food and Drug Administration composite endpoint. Therap Adv Gastroenterol. 2018;11:1756284818771674. doi: 10.1177/1756284818771674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wild GE, Waschke KA, Bitton A, Thomson AB. The mechanisms of prednisone inhibition of inflammation in Crohn’s disease involve changes in intestinal permeability, mucosal TNFalpha production and nuclear factor kappa B expression. Aliment Pharmacol Ther. 2003;18(3):309–317. doi: 10.1046/j.1365-2036.2003.01611.x. [DOI] [PubMed] [Google Scholar]
- Noth R, Stüber E, Häsler R et al. Anti-TNF-α antibodies improve intestinal barrier function in Crohn’s disease. J Crohns Colitis. 2012;6(4):464–469. doi: 10.1016/j.crohns.2011.10.004. [DOI] [PubMed] [Google Scholar]


