Abstract
The interactions between the TM (transmembrane) domains of many membrane proteins are important for their proper functioning. Mutations of residues into positively charged ones within TM domains were reported to be involved in many genetic diseases, possibly because these mutations affect the self- and/or hetero-assembly of the corresponding proteins. To our knowledge, despite significant progress in understanding the role of various amino acids in TM–TM interactions in vivo, the direct effect of positively charged residues on these interactions has not been studied. To address this issue, we employed the N-terminal TM domain of the aspartate receptor (Tar-1) as a dimerization model system. We expressed within the ToxR TM assembly system several Tar-1 constructs that dimerize via polar- or non-polar amino acid motifs, and mutated these by replacement with a single arginine residue. Our results have revealed that a mutation in each of the motifs significantly reduced the ability of the TMs to dimerize. Furthermore, a Tar-1 construct that contained two arginine residues was unable to correctly integrate itself into the membrane. Nevertheless, an exogenous synthetic Tar-1 peptide containing these two arginine residues was able to inhibit in vivo the marked dimerization of a mutant Tar-1 construct that contained two glutamate residues at similar positions. This indicates that hetero-assembly of TM domains can be mediated by the interaction of two oppositely charged residues, probably by formation of ion pairs. This study broadens our knowledge regarding the effect of positively charged residues on TM–TM interactions in vivo, and provides a potential therapeutic approach to inhibit uncontrolled dimerization of TM domains caused by mutations of polar amino acids.
Keywords: assembly, peptide, positively charged residues, Tar receptor, transmembrane domain
Abbreviations: DMF, dimethylformamide; Fmoc, fluoren-9-ylmethoxycarbonyl; GPA, glycophorin A; PE/PG, phosphatidylethanolamine/phosphatidylglycerol; TM, transmembrane; WT, wild-type
INTRODUCTION
Positively charged amino acids, which are localized within the TM (transmembrane) domains of membrane proteins, are known to have both functional and structural roles regarding the activity of these proteins. Examples include the involvement of positively charged residues in substrate recognition [1], their ability to determine the electrogenicity of the H,K-ATPases [2], and their involvement in mitochondrial citrate transport [3]. Moreover, mutations that introduce positively charged residues into TM domains have been shown previously to be involved in human genetic diseases [4,5], including cystic fibrosis, Charcot-Marie-Tooth disease, Wilson's disease, dominant renal hypomagnesaemia [4–6], and many others. TM domains are known to be involved in self- and hetero-assembly of membrane proteins, and therefore such mutations might interfere with these interactions. To our knowledge, despite significant progress in understanding the role of various amino acids in TM–TM interactions, the direct effect of positively charged residues on these interactions has not been studied in vivo. Interestingly, most of these disease-causing mutations introduce an arginine residue, and not lysine, into the TM domains. The first study that showed a correlation between a positively charged mutation and the disruption of TM–TM self-assembly was performed in vitro using synthetic peptides. It was shown that a peptide containing a Gly→Arg mutation within the TM region of the sodium pump γ-subunit abrogates the oligomerization of the γ-TM peptide [7]. This suggests that a gain of positively charged residues can result in abnormal self-assembly, which can eventually lead to protein malfunction.
To investigate whether the insertion of an arginine residue within a TM domain can directly affect dimerization in vivo, we utilized the ToxR–MalE system, which can assess TM–TM interactions within the Escherichia coli natural membrane [8]. In the present study, we have employed the N-terminal TM domain of the E. coli aspartate receptor (Tar) as a dimerization model system. This receptor is one of the main chemotaxis receptors found in bacteria, and it forms a homodimer complex in which each subunit is composed of two TM helices (Tar-1 and Tar-2) separated by a substantial periplasmic domain. Although the dimerization of the receptor is mainly mediated by its periplasmic or cytoplasmic domains [9–12], biochemical studies have demonstrated that the N-terminal TM domain of the receptor (Tar-1) is also able to dimerize [13–15]. Note that a single amino acid substitution (from alanine to lysine) within the TM domain of the full-length Tar receptor was shown previously to result in the loss of the chemotaxis function. However, neither membrane integration nor the aspartate-binding ability of the receptor was affected [16].
The dimerization of Tar-1 was previously shown to be driven by a polar amino acid motif (G22xxS25, where ‘x’ represents any amino acid residue) [14]. Interestingly, one of the mutated constructs, which contained two glutamic acid residues (E/E) at positions 22 and 25 that are localized at the interaction surfaces [13], was found to have significantly increased dimerization.
In the present study we have examined the effect of positively charged residues on the self-assembly of Tar-1 TM domains in vivo by substituting the amino acids at positions 22 or 25 with arginine. This was done in three different constructs, each of which performs its self-assembly through different interaction motifs: (i) a construct containing the Tar-1 native sequence that dimerizes through interactions between its two polar residues Gln22 and Ser25; (ii) a construct containing the Tar-1 mutant in which Gln22 and Ser25 were mutated to Trp22 and Trp25, which drives dimerization probably via π–π stacking interactions; and (iii) a construct in which Gln22 and Ser25 were mutated to Gly22 and Gly25, which, together with native Gly26, create a GxxxG motif, which has been shown to drive the association of several membrane proteins [17–19]. Furthermore, the role of arginine in heterodimerization was also investigated by examining the ability of synthetic peptides that mimic the Tar-1 domain and its analogues to disrupt the dimerization of a Glu22–Glu25 construct (E/E), which demonstrated enhanced dimerization compared with the WT (wild-type) construct [14].
The results are discussed with regard to a possible role of mutation into arginine in preventing homodimer, but not heterodimer, formation, and provide a potential novel therapeutic approach to modulate the activity of these proteins.
EXPERIMENTAL
Construction of the ToxR chimaeras
A NheI/BamHI TM-DNA cassette encoding 16 residues of the Tar-1 WT TM domain (M13VLGVFALLQLISGSL28) was inserted between the ToxR transcription activator and the E. coli maltose-binding protein (MalE) within the ToxR–MalE plasmid. The mutants contained the same sequence as the Tar-1 WT TM domain, except for the replacement of the glutamine and/or the serine residue(s) at positions 22 and 25 respectively (shown underlined in the sequence above; Table 1). All the constructs were confirmed by DNA sequencing.
Table 1. Sequences of the TM domain that were inserted between the ToxR transcription activator and the maltose-binding protein in the ToxR–MalE plasmid.
Amino acids are numbered according to their position in the WT protein (SwissProt p07017). Mutations in the Tar-1 TM domain are shown in bold and underlined. The nomenclature of the TM domains represents the two amino acids replacing the original polar residues, glutamine and serine, of the WT sequence at positions 22 and 25 respectively.
| TM domain | Sequence |
|---|---|
| Tar-1 WT | M13VLGVFALLQLISGSL28 |
| Tar-1 R/R | M13VLGVFALLRLIRGSL28 |
| Tar-1 Q/R | M13VLGVFALLQLIRGSL28 |
| Tar-1 R/S | M13VLGVFALLRLISGSL28 |
| Tar-1 W/W | M13VLGVFALLWLIWGSL28 |
| Tar-1 W/R | M13VLGVFALLWLIRGSL28 |
| Tar-1 R/W | M13VLGVFALLRLIWGSL28 |
| Tar-1 G/G | M13VLGVFALLGLIGGSL28 |
| Tar-1 G/R | M13VLGVFALLGLIRGSL28 |
| Tar-1 R/G | M13VLGVFALLRLIGGSL28 |
| Tar-1 E/E | M13VLGVFALLELIEGSL28 |
Maltose complementation assay
Membrane insertion and correct orientation were examined as described previously [20]. Briefly, PD28 cells transformed with the different plasmids were cultured overnight. The cells were then washed twice with PBS, and used to inoculate M9 minimal medium including 0.4% maltose at a 200-fold dilution. The growth of the cells was measured at different times by attenuance at 650 nm.
ToxR–Tar-1 protein expression levels
Western blot analysis was performed in order to determine whether the change in one or two polar amino acids in the sequence of the TM domain affects the expression level of the chimaeric protein. Specifically, aliquots of 10 μl of FHK12 cells, each with a different plasmid, were mixed with a sample buffer, boiled for 5 min, subjected to SDS/12%-PAGE, and then transferred to nitrocellulose. The primary antibody used was anti-(maltose binding protein) antibody (New England Biolabs). Detection was achieved using the Phototope-HRP Western blot detection system from Cell Signaling Technology.
Peptide synthesis and purification
The Tar-1 WT, E/E and R/R peptides (see Table 1 for the details of peptide nomenclature) were synthesized using the Fmoc (fluoren-9-ylmethoxycarbonyl) solid-phase method on Rink Amide MBHA resin. The peptides were cleaved from the resin with trifluoroacetic acid, and were purified by RP (reversed-phase)-HPLC on a C4 reverse-phase Bio-Rad semi-preparative column (250 mm×10 mm, 30 nm pore size, 5 μm particle size). The purified peptides were shown to be homogeneous (>95%) by analytical HPLC. The peptides' compositions were confirmed by electrospray mass spectrometry. Lysine residues were added to the N- and C-termini of the peptides to confer water solubility to the hydrophobic TM domains. This addition was shown previously to have no effect on the oligomerization of the peptide [15,19,21].
Fluorescent labelling of peptides
The Fmoc-protecting group was removed from the N-terminus of the resin-bound peptides by incubation with piperidine (20%) in DMF (dimethylformamide) for 12 min, whereas all the other reactive amine groups of the attached peptides were kept protected. The resin-bound peptides were washed twice with DMF, and then treated with 2 mol of rhodamine in anhydrous DMF containing 2% (v/v) DIEA (N,N-di-isopropylethylamine), leading to the formation of a resin-bound N-rhodamine peptide. After 24 h, the resin was washed thoroughly with DMF, and then with methylene chloride. The labelled peptides were cleaved from the resin and purified as described previously [19].
Detection in vivo of homo- and hetero-dimerization of TM domains within the membrane
The ToxR transcription activator can be used successfully to assess weak protein–protein interactions within the E. coli inner membrane. A Tar-1 TM domain encoding the DNA cassette was grafted between the ToxR transcription activator and the maltose-binding protein in the ToxR–MalE plasmid. The plasmid was then transformed into E. coli FHK12 cells, which contain β-galactosidase, under the control of a ctx promoter. Dimerization of the TM domains in this system results in association and activation of the ToxR transcription activator, which then becomes active and is able to bind the ctx promoter [8]. Quantification of the amount of homodimerization was done by measuring the activity of the β-galactosidase reporter gene, and by normalizing it to the cell content (D590; measured in Miller units). The baseline activity of a negative control (ToxR'A16), which remains a monomer, was subtracted from all the results [8]. The transformed cells were grown in the presence of chloramphenicol for 18 h at 37 °C. β-Galactosidase activities were quantified in crude cell lysates after adding o-nitrophenyl galactosidase and monitoring the reaction at 405 nm for 20 min, at intervals of 30 s at 28 °C with a Molecular Devices kinetic reader [8,22]. Specific β-galactosidase activities were computed from the Vmax of the reaction.
Hetero-association was detected using ToxR–Tar-1-expressing bacteria grown in the presence of an exogenous peptide, as has been described previously [22]. Inhibition was calculated as follows:
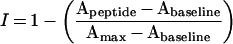 |
(1) |
where I represents the inhibitory ability of the peptide, Apeptide is the activity of ToxR–Tar-1 in the presence of peptide, Amax is the maximal activity of ToxR–Tar-1 without the peptide, and Abaseline is the baseline activity of the monomer A16 plasmid [8]. We fitted normalized data to the Langmuir equation (eqn 3) using the Origin 6.0 commercial program [23].
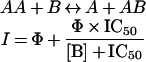 |
(2) |
where A represents the ToxR–Tar-1 transcription activator, B represents the exogenous peptide, and I represents the inhibition of β-galactosidase activity. This model has a scaling constant (Φ), which takes into account the fact that exogenous peptides can homo-oligomerize into several oligomeric states. Differences between the exogenous peptides will most likely result in a different equilibrium between the different homo-oligomerization states. If one of these oligomers can interact with the dimer ToxR receptor, it may inactivate the receptor. Thus the effective concentration of the active receptor available for interaction with the monomeric form of the peptide can vary. Consequently, this will lead to different saturation levels for the inhibition by different peptides.
CD spectroscopy
The CD spectra of the peptides were measured in an Aviv 202 spectropolarimeter. The spectra were scanned with a thermostat-controlled quartz optical cell with a path length of 1 mm. Each spectrum was recorded at 0.2 nm/s intervals with an average time of 10 s, at a wavelength range of 190–260 nm. The peptides were scanned at a 50 μM concentration in 1% (w/v) SDS micelles. Fractional helicities [24,25] were calculated as follows:
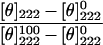 |
(4) |
where [θ]222 is the experimentally observed mean residue ellipticity at 222 nm, and values for [θ]0222 and [θ]100222, corresponding to 0% and 100% helix content respectively at 222 nm, are estimated to be −2000 and −32000 deg·cm2·dmol−1 respectively [24].
Confocal imaging of peptide binding and insertion into the E. coli membrane
Partitioning of rhodamine/TAMRA [5(6)-carboxytetramethylrhodamine]-labelled peptides into the inner membrane of E. coli was determined by confocal laser scanning microscopy. Confocal images were obtained using an Olympus IX70 FV500 confocal laser-scanning microscope. Care was taken so that any photobleaching did not compromise the interpretation, and laser irradiation and other types of illumination were prevented between images. The confocal images were obtained at 12-bit resolution.
Binding analysis using a BIAcore biosensor
Biosensor experiments were carried out with a BIAcore X analytical system (Biacore, Uppsala, Sweden) using an L1 sensor chip (Biacore). The L1 sensor chip is composed of long-chain alkanethiol molecules covalently linked to a gold surface to form a hydrophobic bilayer. The running buffer used for all the experiments was PBS without Ca2+ and Mg2+ (pH 6.8). The washing solution was 40 mM N-octyl β-D-glucopyranoside. All solutions were freshly prepared, degassed and filtered through 0.22 μm pores. All the experiments were performed at a temperature of 25 °C. After cleaning the system according to the manufacturer's instructions, the BIAcore X instrument was left running overnight using Milli-Q water as the eluent to thoroughly wash all liquid handling parts of the instrument. The L1 chip was then installed, and the alkane-thiol surface was cleaned by an injection of the non-ionic detergent N-octyl β-D-glucopyranoside (40 mM; 25 μl) at a flow rate of 5 μl/min. An aliquot (80 μl) of 0.5 mM PE/PG (phosphatidylethanolamine/phosphatidylglycerol; 7:3, w/w) vesicles was then applied to the chip surface at a low flow rate (2 μl/min). To remove any multilamellar structures from the lipid surface, 50 μl of 10 mM NaOH was injected at a flow rate of 50 μl/min, which resulted in a stable baseline corresponding to the lipid monolayer linked to the chip surface. This bilayer, linked to the chip surface, was then used as a model membrane surface to study the peptide–membrane binding. In a typical experiment, peptide solutions (15 μl of a PBS solution containing 0.1–10 μM peptide) were injected on to the lipid surface at a flow rate of 5 μl/min. PBS alone then replaced the peptide solution for 1200 s to allow peptide dissociation.
RESULTS
To investigate the role of positively charged amino acids in TM–TM interactions, we utilized synthetic peptides corresponding to the Tar-1 TM domain, together with the ToxR–MalE system [8]. This system can detect TM–TM interaction within the inner membrane of E. coli. The dimeric state of the chimaeric protein is indicated by the activity (Vmax) of the β-galactosidase reporter gene. The Tar-1 TM domain, which was used as our model system for TM–TM dimerization, was grafted within the ToxR–MalE chimaeric protein recently [14], and showed marked association activity compared with a known homodimer, such as glycophorin A.
Arginine mutations within the polar dimerization motif of the WT Tar-1 TM domain
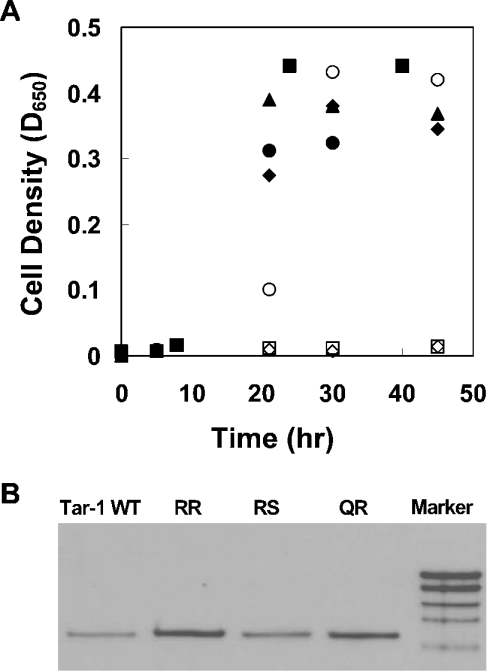
Each residue at position 22 and/or position 25 of the WT Tar-1 dimerization motif (G22xxS25) was replaced by an arginine [i.e. the possible pairings of residues 22/25 were Q/R, R/S and R/R]. Since the presence of charged amino acids within TM domains could affect membrane insertion, the mutated chimaeric proteins were first tested for their ability to correctly integrate into the bacterial inner membrane by analysing their ability to functionally complement a MalE-deficient E. coli strain (PD28) [20]. PD28 cells are unable to grow on a minimal medium with maltose as the only carbon source. Therefore only cells that express the chimaeric protein in the right orientation (MalE pointed towards the periplasm) will be able to utilize maltose, and thus will allow cell growth. Q/R and R/S constructs showed cell growth rates similar to Tar-1 WT, GPA (glycophorin A) and A16, indicating proper membrane integration (Figure 1A). However, the R/R construct showed no growth up to 48 h. A construct with a deleted TM domain (ΔTM) served as a negative control, since it was expected to reside in the cytoplasm. The control was unable to complement the MalE deficiency (Figure 1A), as expected. In order to exclude the possibility that the growth conditions affected the correct integration of the chimaeric proteins into the membrane, we examined their correct insertion into the membrane using a protease-accessibility assay [26]. We found that only ToxR-TM–MalE chimaeric proteins that are correctly integrated into the membrane are susceptible to protease digestion in spheroplasts, but not in whole cells. The results of this assay are in agreement with the maltose complementation assay (results not shown).
Figure 1. Membrane insertion and chimaeric protein expression.
(A) Correct integration of the ToxR-TM–MalE chimaeric proteins was examined by their ability to functionally complement the MalE deficiency of PD28 cells. PD28 cells were transformed with the different plasmids and were grown in minimal medium containing maltose. Only cells that expressed periplasmic MalE were able to grow with maltose as the only carbon source. Tar-1 Q/R and R/S constructs showed similar growth curves as Tar-1 WT, indicating proper membrane integration. The double arginine mutation and the negative control with the deleted TM domain (ΔTM) showed no growth. ◆, Tar-1 WT; ○, GPA; ■, A16; □, Tar R/R; ▲, Q/R; ●, R/S; and ◇, ΔTM. (B) Comparison of the ToxR-TM–MalE chimaeric proteins' expression levels (shown by the bands at ≈65 kDa). Samples of FHK12 cells containing different sequences of Tar-1 within the ToxR–MalE chimaeric protein were lysed in SDS sample buffer, separated on SDS/12%-PAGE and immunoblotted using anti-(maltose binding protein) antibody (New England Biolabs). The chimaeric protein mutants showed expression levels similar to that of the WT TM domain. Sizes of the markers from the bottom to the top are 60, 80, 100, 140 and 200 kDa.
To determine whether the inability of the R/R construct to grow on minimal medium resulted from low expression levels of the chimaeric protein, or alternatively from its failure to properly insert into the membrane, we performed Western blotting. The data revealed that the R/R mutant expression level is similar to that of the Tar-1 WT construct (Figure 1B), indicating that the R/R mutant chimaeric protein is unable to insert into the membrane. Q/R and R/S showed protein expression levels similar to those of Tar-1 WT. The correctly inserted Q/R and R/S constructs (Figure 1) were then examined for their ability to form dimers. Interestingly, the data revealed an approximate 80% decrease in dimer formation of the mutants compared with Tar-1 WT (Figure 2). These results indicate that the presence of an arginine residue within the TM domain can disrupt and destabilize self-assembly.
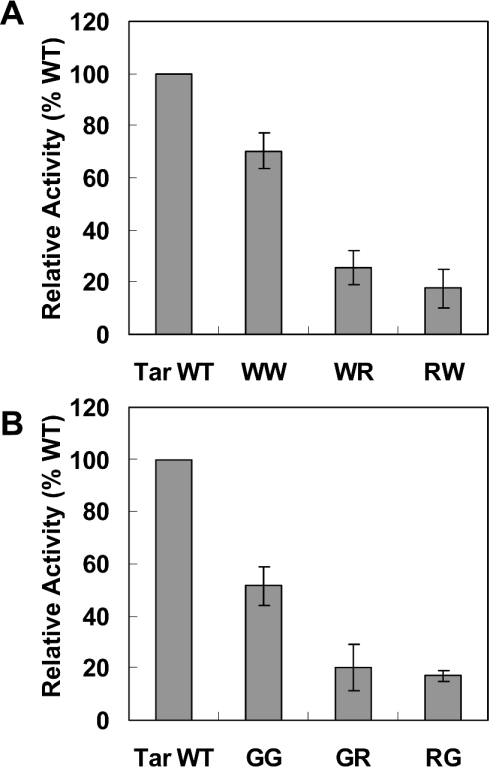
Figure 2. Arginine mutations of the Tar-1 WT TM domain.
Cells expressing a ToxR-TM–MalE chimaera were examined for dimerization activity (normalized relative to the WT Tar-1 TM domain activity). All values are the average of at least three independent assays. Error bars represent the estimated S.D. The exact sequences are shown in Table 1.
Arginine mutations within non-native dimerization motifs (WxxW and GxxG)
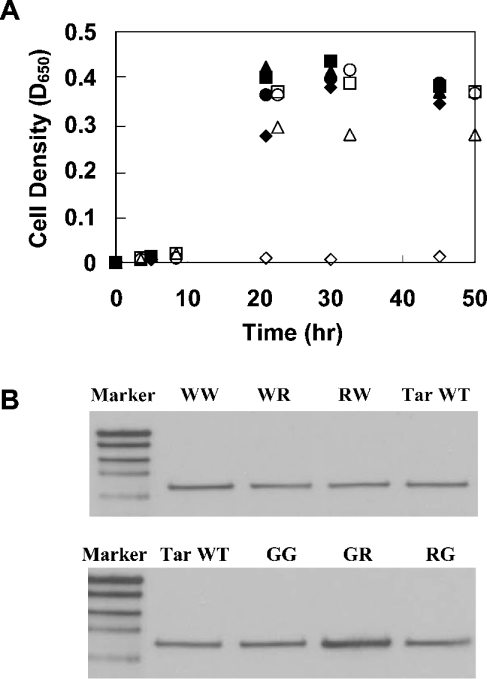
To broaden our understanding regarding the disruptive effect of arginine on TM domain self-assembly, we constructed two Tar-1 mutants that showed considerable ability to form dimers. One mutant contained two tryptophan residues instead of the two original polar residues (W/W). This construct preserved ≈75% of the dimerization activity of the WT Tar-1 construct. The dimerization of this TM domain is probably mediated by ‘stacking’ interactions between the four aromatic residues [27,28] instead of the interhelical hydrogen bonds that drive the WT TM–TM interaction. In the second construct, the two original polar residues were replaced by two glycines. Together with the native glycine at position 26, a GxxxG dimerization motif was created. Previous studies demonstrated the involvement of the GxxxG motif in the non-covalent association of TM segments [17–19]. As shown in Figure 3, the G/G construct restored ≈50% of the WT activity. The data revealed that replacement of one of the tryptophan or glycine residues by arginine resulted in a marked reduction in the association level compared with the parental dimerization systems (Figure 3). Correct integration of the different ToxR-TM–MalE chimaeric proteins into the membrane was confirmed by MalE complementation (Figure 4A) and protease-accessibility assay (results not shown). Furthermore, the expression levels of the chimaeric proteins compared with that of the WT were either similar or slightly higher, as observed from the Western blot (Figure 4B). These results exclude the possibility that the low dimerization ability of the constructs is the consequence of reduced expression levels of the chimaeric protein. Thus we conclude that the introduction of arginine residues into non-polar motifs within a TM domain can disrupt TM–TM interaction.
Figure 3. Arginine mutations of the Tar-1 W/W and G/G TM domains.
Cells expressing a ToxR-TM–MalE chimaera were examined for dimerization activity. The details are as described in the legend to Figure 2.
Figure 4. Membrane insertion and chimaeric protein expression of arginine mutants.
(A) Correct integration of the ToxR-TM–MalE chimaeric proteins was examined as described in the legend to Figure 1(A). All constructs showed similar growth curves, except the negative control with the deleted TM domain (ΔTM), indicating proper membrane integration. ◆, Tar-1 WT; ▲, G/G; ●, R/G; ■, G/R; ○, W/W; △, R/W; □, W/R; and ◇, ΔTM. (B) Comparison of the ToxR-TM–MalE chimaeric proteins' expression levels (65 kDa). The details are as described in the legend to Figure 1(B).
Peptide synthesis
Peptides representing the Tar-1 WT TM domain, the E/E mutant TM domain and the R/R mutant TM domain were synthesized. These peptides were examined for their ability to disrupt the proper dimerization of a tightly dimerized, negatively charged construct ToxR (E/E) [14]. The sequences and designations of the peptides are given in Table 2.
Table 2. Peptide designations and sequences.
WT amino acids that were changed are shown in bold and underlined. The C-termini of the peptides were amidated (-NH2).
| Peptide designation | Amino acid sequence |
|---|---|
| Tar-1 WT | KKKMVLGVFALLQLISGSLKK-NH2 |
| Tar-1 EE mutant | KKKMVLGVFALLELIEGSLKK-NH2 |
| Tar-1 RR mutant | KKKMVLGVFALLRLIRGSLKK-NH2 |
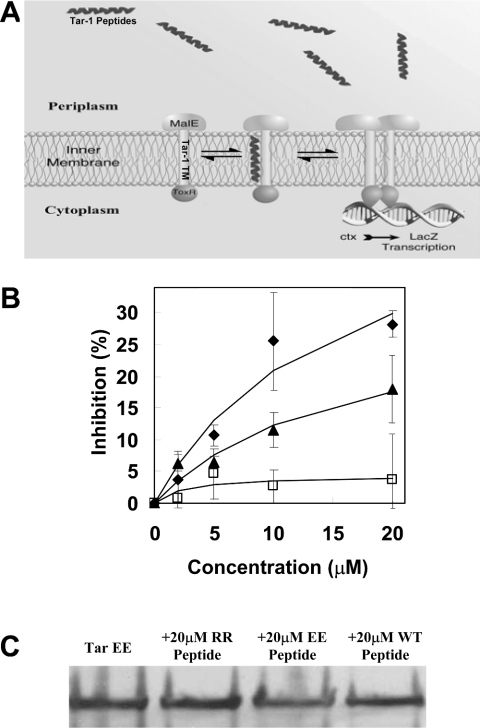
Heterodimerization between the E/E TM domain and the Tar-1 peptides
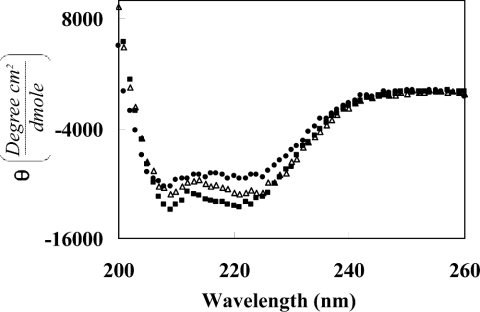
Many membrane proteins that relate to human diseases contain mutations that involve non-polar to strong polar amino acid substitutions within the TM domains [4]. In a previous study [14], we presented a mechanism that possibly explains this phenomenon based on the dimerization properties of a mutated construct, which contains two glutamic acid residues in its TM sequence. This construct (E/E) showed marked dimerization activity compared with the Tar-1 WT TM domain, suggesting that mutations to strong polar residues can lead to uncontrolled association, and therefore to protein malfunction. Here, we examined the ability of exogenous peptides to directly disrupt the high dimerization of the E/E construct by using the ToxR system (Figure 5A). The TM–TM interactions were monitored by observing the activity of β-galactosidase in the presence of different concentrations of exogenous Tar-1 WT, E/E and R/R peptides. Figure 5(B) shows that the Tar-1 E/E peptide has only marginal inhibition ability, suggesting that both the TM–TM interactions of the E/E construct and the assembly of the E/E peptides are relatively strong, and tend to be in their dimer form. Thus the ability of the E/E peptides to interact and compete with the TM domain of the construct is limited. The Tar-1 R/R peptide, however, showed greater inhibition ability compared with the E/E peptide (Figure 5B). The TM domains that contained an arginine residue showed diminished dimerization activity; therefore we believe that the R/R peptides are mostly in their monomeric form. This enables easier interaction between the R/R peptide and the E/E TM domains, which is probably mediated by salt-bridge interactions. The ability of the Tar-1 WT peptide to hetero-associate with the E/E TM domain was found to be only partial compared with the inhibition induced by the Tar-1 R/R peptides. The secondary structures of the Tar-1 WT, E/E and R/R peptides, determined by CD spectroscopy in a micellar environment (1% SDS), demonstrated similar, but not identical, structures for all of them (Figure 6).
Figure 5. Tar-1 hetero-oligomerization.
(A) Schematic illustration of the ToxR hetero-oligomerization system. The association of the Tar-1 TM domains activates ToxR, which only then can bind the ctx promoter and initiate the lacZ transcription process. Hetero-association of the exogenous peptides with the ToxR-Tar-1 TM domain prevents the activation of ToxR by shifting the equilibrium towards monomeric ToxR, thus reducing lacZ transcription and hence its signal. (B) Inhibition of the dimerization level in the presence of the Tar-1 WT, Tar-1 E/E and Tar-1 R/R peptides. The dose–response of β-gal inhibition as a function of exogenous peptide concentration: ▲, Tar-1 WT; ◆, Tar-1 R/R; and □, Tar-1 E/E. The results were normalized relative to the WT Tar-1 TM domain activity. All values are the average of at least three independent assays. Error bars represent the estimated S.D. (C) Comparison of the Tar-1 E/E chimaera expression levels in the presence of 20 μM of each peptide. The details are as described in the legend to Figure 1. The peptides had no significant effect on the expression of the Tar-1 E/E chimaeric protein.
Figure 6. CD spectra of the peptides in SDS micelles.
Far-UV CD spectra of W/T (●), E/E (△) and R/R (■) Tar-1 TM domain peptides in 1% SDS. Spectra were measured on an Aviv spectropolarimeter at 0.2 nm intervals with a 10 s average time, using a 0.1 cm light path.
Localization of the Tar-1 peptides
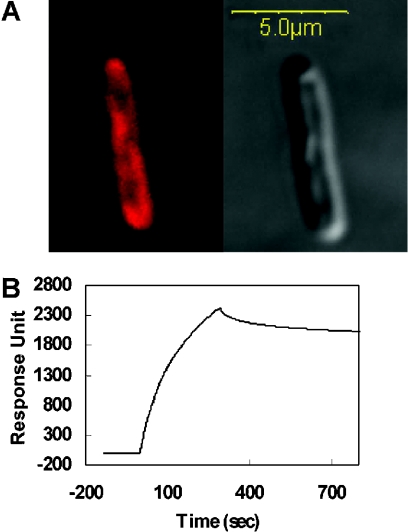
The bacterial localization of the Tar-1 WT, E/E and R/R peptides was examined by confocal fluorescence microscopy of E. coli-bound labelled peptides. As shown in Figure 7(A), the peptides were mainly localized to the bacterial membrane. We estimate that at least part of the peptide population binds to the inner membrane, since we observed labelled regions that appear to be inner membrane invaginations into the cytoplasm. However, it is impossible to conclusively distinguish between the inner and the outer membrane. In order to determine whether the Tar-1 WT, E/E and R/R peptides are integrated into the membranes, we used a BIAcore biosensor. The data revealed that all three peptides bind almost irreversibly to the lipid bilayers, as demonstrated by the sensogram shown in Figure 7(B) for Tar-1 WT. Similar sensograms were obtained for the other two peptides. Note that the peptides did not wash out after NaOH treatment.
Figure 7. Localization of the Tar-1 peptides to the bacterial membrane.
(A) Partitioning of rhodamine-labelled Tar-1 R/R to the inner membrane of E. coli was determined by confocal laser-scanning microscopy. A similar result was observed for the Tar-1 WT and Tar-1 E/E peptides. The Tar-1 WT peptide was previously shown to be localized to the bacterial membrane. The fluorescence image is on the upper-left-hand side and the transmission light image is on the upper-right-hand side. Most of the peptide is localized on the plasma membrane, although some peptide also penetrates into the cytoplasm. Confocal images were obtained using an Olympus IX70 FV500 confocal laser-scanning microscope. There were only minute background fluorescence intensities observed in the control bacteria. (B) A sensogram of the binding between Tar-1 WT peptide (10 μM) and PE/PG (7:3, w/w) bilayers.
DISCUSSION
Approx. 50% of membrane proteins that are associated with human genetic diseases contain mutations of non-polar to polar amino acids in their TM domains [4]. In the present study, we have examined the effect of positively charged residues on TM–TM interaction and recognition by utilizing the Tar receptor N-terminal TM domain (Tar-1), which served as a model system for TM dimerization. Our data have revealed that introducing an arginine residue to Tar-1 significantly reduces its ability to self-associate. Taken together, our results provide a mechanistic explanation for the involvement of positively charged residues in protein malfunction.
When discussing the effect of positively charged residues within the membrane environment, it is important to bear in mind that the core of the biological membrane has a low dielectric constant. This imposes altered physical and chemical properties on to all polar amino acids [29,30]. As a result, arginine, which contains three polar atoms, can either stabilize dimerization by forming hydrogen bonds or shift the equilibrium towards monomers by inducing repulsion. Alternatively, or in addition to repulsion, reduced dimerization can be due to the change of the interacting helical surfaces that results from a reorganization of the two helices with respect to each other, which cannot be measured using the ToxR system. With regard to the repulsion forces, our results indicate that all arginine-containing mutants are mostly in their monomeric form, implying that the repulsion between the polar atoms is stronger than their potential to form hydrogen bonds. Similar observations regarding the repulsion effects of polar residues within the membrane environment were previously demonstrated for the CD3γδ [31]. A monomeric TM domain can ‘mask’ arginine polar atoms either by creating intramolecular interactions with the peptide backbone or by extending the large arginine side chain towards the phospholipid head groups.
The large size of the arginine side chain probably serves as an additional factor that contributes to the disruptive nature of this amino acid, since it creates steric hindrance and prevents close packing of the two helices. It is most probable that arginine has a disruptive effect on dimerization only when it is localized to the interacting surfaces of the helices, as was previously shown by the use of synthetic peptides [7]. The inability of the R/R construct to insert into the membrane implies that the presence of two arginine residues within the TM domain reduces the total hydrophobicity under the threshold level required for membrane insertion. Thus we assume that the arginine residue is at least partially charged within the membrane's milieu; therefore two arginine residues within a single TM domain prevent the insertion of the ToxR-TM–MalE chimaeric protein into the membrane. Nevertheless, the insertion of a chimaeric protein that contains only one arginine residue in its TM domain was still possible (see Figures 2 and 4).
The dissociation of TM–TM dimerization in the presence of an arginine residue correlates with our assumption that the arginine residues contain charged or strong polar atoms. Thus the repulsion forces induced by arginine residues of the corresponding TM domains prevent their close interaction. The E/E Tar-1 mutant, however, was correctly integrated into the membrane of E. coli, although it also contains two strong polar residues [14]. This suggests that the polar glutamate residue is not charged within the membrane environment under these conditions, and therefore two glutamic acid residues are able to create interhelical hydrogen bonds that eventually stabilize the dimeric form. However, as indicated above, since the dimerization measured by the ToxR system is sensitive to the position of the interacting surface [8,32], we cannot exclude the possibility that the decreased dimerization level of the arginine-containing constructs results from alteration of the relative orientation of the two interacting helices. This could also explain the partial dimerization ability of the G/G construct, which contains a GxxxG motif.
Previous studies showed that a defective phenotype of the Tar receptor containing an Ala19→Lys mutation within its Tar-1 can be suppressed by an additional mutation of a negatively charged residue at several positions, either at Tar-1 or Tar-2 [16,33]. Considering these results, we examined the ability of exogenous TM peptide that contains positively charged residues to interact with the TM domain that contains negatively charged residues, and to inhibit their dimerization. For this purpose, we compared the ability of three different peptides to perform a dominant-negative effect on the dimerization of the E/E construct. Our results revealed that the R/R peptide was the most potent inhibitor, since it probably interacts with the negatively charged residues through salt-bridge interactions. This observation is very significant in light of the extensive involvement of non-polar-to-polar mutations in constitutive dimerization, and hence in the appearance of diseases [4,7]. The CD results showed that all three peptides adopt similar but not identical structures, and therefore the possibility that slight differences in peptide structure may play a role in their ability to inhibit the dimerization activity of the E/E construct cannot be ruled out.
Overall, this study broadens our knowledge concerning the effect of positively charged residues on TM–TM interactions in vivo, and its possible linkage to genetic diseases. On the basis of dimerization activity of the nine different ToxR constructs, we propose that the polar atoms of arginine cause repulsion between the monomers when located on the interacting surfaces of the helices. Alternatively, or in addition, they cause reorganization of the two helices with respect to each other, thus preventing the proper structural arrangement of a protein. In addition, the results of the inhibitory activity of the peptides provide a possible therapeutic approach whose aim is to inhibit uncontrolled dimerization caused by mutations to strong polar residues within the TM domain.
Acknowledgments
We thank Dr D. Langosch of Heidelberg University, Germany, who provided the ToxR-GPA and A16 plasmids, as well as the FHK12 and PD28 E. coli strains. We thank V. Kiss from the Weizmann Institute for his technical assistance in the microscopy studies, and D. Gerber for his comments and a critical reading of the manuscript. This study was supported by The Dr Josef Cohn Minerva Center for Biomembrane Research.
References
- 1.Ding P. Z., Wilson T. H. The effect of modifications of the charged residues in the transmembrane helices on the transport activity of the melibiose carrier of Escherichia coli. Biochem. Biophys. Res. Commun. 2001;285:348–354. doi: 10.1006/bbrc.2001.5200. [DOI] [PubMed] [Google Scholar]
- 2.Burnay M., Crambert G., Kharoubi-Hess S., Geering K., Horisberger J. D. Electrogenicity of Na,K- and H,K-ATPase activity and presence of a positively charged amino acid in the fifth transmembrane segment. J. Biol. Chem. 2003;278:19237–19244. doi: 10.1074/jbc.M300946200. [DOI] [PubMed] [Google Scholar]
- 3.Xu Y., Kakhniashvili D. A., Gremse D. A., Wood D. O., Mayor J. A., Walters D. E., Kaplan R. S. The yeast mitochondrial citrate transport protein. Probing the roles of cysteines, Arg181, and Arg189 in transporter function. J. Biol. Chem. 2000;275:7117–7124. doi: 10.1074/jbc.275.10.7117. [DOI] [PubMed] [Google Scholar]
- 4.Partridge A. W., Melnyk R. A., Deber C. M. Polar residues in membrane domains of proteins: molecular basis for helix-helix association in a mutant CFTR transmembrane segment. Biochemistry. 2002;41:3647–3653. doi: 10.1021/bi0120502. [DOI] [PubMed] [Google Scholar]
- 5.Partridge A. W., Therien A. G., Deber C. M. Missense mutations in transmembrane domains of proteins: phenotypic propensity of polar residues for human disease. Proteins. 2004;54:648–656. doi: 10.1002/prot.10611. [DOI] [PubMed] [Google Scholar]
- 6.Meij I. C., Koenderink J. B., van Bokhoven H., Assink K. F., Groenestege W. T., de Pont J. J., Bindels R. J., Monnens L. A., van den Heuvel L. P., Knoers N. V. Dominant isolated renal magnesium loss is caused by misrouting of the Na+,K+-ATPase gamma-subunit. Nat. Genet. 2000;26:265–266. doi: 10.1038/81543. [DOI] [PubMed] [Google Scholar]
- 7.Therien A. G., Deber C. M. Oligomerization of a peptide derived from the transmembrane region of the sodium pump gamma subunit: effect of the pathological mutation G41R. J. Mol. Biol. 2002;322:583–590. doi: 10.1016/s0022-2836(02)00781-7. [DOI] [PubMed] [Google Scholar]
- 8.Langosch D., Brosig B., Kolmar H., Fritz H. J. Dimerisation of the glycophorin A transmembrane segment in membranes probed with the ToxR transcription activator. J. Mol. Biol. 1996;263:525–530. doi: 10.1006/jmbi.1996.0595. [DOI] [PubMed] [Google Scholar]
- 9.Scott W. G., Milligan D. L., Milburn M. V., Prive G. G., Yeh J., Koshland D. E., Jr, Kim S. H. Refined structures of the ligand-binding domain of the aspartate receptor from Salmonella typhimurium. J. Mol. Biol. 1993;232:555–573. doi: 10.1006/jmbi.1993.1411. [DOI] [PubMed] [Google Scholar]
- 10.Milburn M. V., Prive G. G., Milligan D. L., Scott W. G., Yeh J., Jancarik J., Koshland D. E., Jr, Kim S. H. Three-dimensional structures of the ligand-binding domain of the bacterial aspartate receptor with and without a ligand. Science. 1991;254:1342–1347. doi: 10.1126/science.1660187. [DOI] [PubMed] [Google Scholar]
- 11.Kim K. K., Yokota H., Kim S. H. Four-helical-bundle structure of the cytoplasmic domain of a serine chemotaxis receptor. Nature (London) 1999;400:787–792. doi: 10.1038/23512. [DOI] [PubMed] [Google Scholar]
- 12.Gerstein M., Chothia C. Perspectives: signal transduction. Proteins in motion. Science. 1999;285:1682–1683. doi: 10.1126/science.285.5434.1682. [DOI] [PubMed] [Google Scholar]
- 13.Pakula A. A., Simon M. I. Determination of transmembrane protein structure by disulfide cross-linking: the Escherichia coli Tar receptor. Proc. Natl. Acad. Sci. U.S.A. 1992;89:4144–4148. doi: 10.1073/pnas.89.9.4144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sal-Man N., Gerber D., Shai Y. The composition rather than position of polar residues (QxxS) drives aspartate receptor transmembrane domain dimerization in vivo. Biochemistry. 2004;43:2309–2313. doi: 10.1021/bi0356294. [DOI] [PubMed] [Google Scholar]
- 15.Melnyk R. A., Partridge A. W., Deber C. M. Retention of native-like oligomerization states in transmembrane segment peptides: application to the Escherichia coli aspartate receptor. Biochemistry. 2001;40:11106–11113. doi: 10.1021/bi010642e. [DOI] [PubMed] [Google Scholar]
- 16.Oosawa K., Simon M. Analysis of mutations in the transmembrane region of the aspartate chemoreceptor in Escherichia coli. Proc. Natl. Acad. Sci. U.S.A. 1986;83:6930–6934. doi: 10.1073/pnas.83.18.6930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lemmon M. A., Flanagan J. M., Hunt J. F., Adair B. D., Bormann B. J., Dempsey C. E., Engelman D. M. Glycophorin A dimerization is driven by specific interactions between transmembrane alpha-helices. J. Biol. Chem. 1992;267:7683–7689. [PubMed] [Google Scholar]
- 18.Lemmon M. A., Treutlein H. R., Adams P. D., Brunger A. T., Engelman D. M. A dimerization motif for transmembrane alpha-helices. Nat. Struct. Biol. 1994;1:157–163. doi: 10.1038/nsb0394-157. [DOI] [PubMed] [Google Scholar]
- 19.Gerber D., Sal-Man N., Shai Y. Two motifs within a transmembrane domain, one for homodimerization and the other for heterodimerization. J. Biol. Chem. 2004;279:21177–21182. doi: 10.1074/jbc.M400847200. [DOI] [PubMed] [Google Scholar]
- 20.Brosig B., Langosch D. The dimerization motif of the glycophorin A transmembrane segment in membranes: importance of glycine residues. Protein Sci. 1998;7:1052–1056. doi: 10.1002/pro.5560070423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Han X., Tamm L. K. A host-guest system to study structure–function relationships of membrane fusion peptides. Proc. Natl. Acad. Sci. U.S.A. 2000;97:13097–13102. doi: 10.1073/pnas.230212097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gerber D., Shai Y. In vivo detection of hetero-association of glycophorin-A and its mutants within the membrane. J. Biol. Chem. 2001;276:31229–31232. doi: 10.1074/jbc.M101889200. [DOI] [PubMed] [Google Scholar]
- 23.Eckert D. M., Malashkevich V. N., Hong L. H., Carr P. A., Kim P. S. Inhibiting HIV-1 entry: discovery of D-peptide inhibitors that target the gp41 coiled-coil pocket. Cell. 1999;99:103–115. doi: 10.1016/s0092-8674(00)80066-5. [DOI] [PubMed] [Google Scholar]
- 24.Wu C. S., Ikeda K., Yang J. T. Ordered conformation of polypeptides and proteins in acidic dodecyl sulfate solution. Biochemistry. 1981;20:566–570. doi: 10.1021/bi00506a019. [DOI] [PubMed] [Google Scholar]
- 25.Greenfield N., Fasman G. D. Computed circular dichroism spectra for the evaluation of protein conformation. Biochemistry. 1969;8:4108–4116. doi: 10.1021/bi00838a031. [DOI] [PubMed] [Google Scholar]
- 26.Mendrola J. M., Berger M. B., King M. C., Lemmon M. A. The single transmembrane domains of ErbB receptors self-associate in cell membranes. J. Biol. Chem. 2002;277:4704–4712. doi: 10.1074/jbc.M108681200. [DOI] [PubMed] [Google Scholar]
- 27.McGaughey G. B., Gagne M., Rappe A. K. pi-Stacking interactions. Alive and well in proteins. J. Biol. Chem. 1998;273:15458–15463. doi: 10.1074/jbc.273.25.15458. [DOI] [PubMed] [Google Scholar]
- 28.Gazit E. A possible role for pi-stacking in the self-assembly of amyloid fibrils. FASEB J. 2002;16:77–83. doi: 10.1096/fj.01-0442hyp. [DOI] [PubMed] [Google Scholar]
- 29.Gratkowski H., Lear J. D., DeGrado W. F. Polar side chains drive the association of model transmembrane peptides. Proc. Natl. Acad. Sci. U.S.A. 2001;98:880–885. doi: 10.1073/pnas.98.3.880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou F. X., Merianos H. J., Brunger A. T., Engelman D. M. Polar residues drive association of polyleucine transmembrane helices. Proc. Natl. Acad. Sci. U.S.A. 2001;98:2250–2255. doi: 10.1073/pnas.041593698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Manolios N., Letourneur F., Bonifacino J. S., Klausner R. D. Pairwise, cooperative and inhibitory interactions describe the assembly and probable structure of the T-cell antigen receptor. EMBO J. 1991;10:1643–1651. doi: 10.1002/j.1460-2075.1991.tb07687.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ruan W., Lindner E., Langosch D. The interface of a membrane-spanning leucine zipper mapped by asparagine-scanning mutagenesis. Protein Sci. 2004;13:555–559. doi: 10.1110/ps.03357404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Umemura T., Tatsuno I., Shibasaki M., Homma M., Kawagishi I. Intersubunit interaction between transmembrane helices of the bacterial aspartate chemoreceptor homodimer. J. Biol. Chem. 1998;273:30110–30115. doi: 10.1074/jbc.273.46.30110. [DOI] [PubMed] [Google Scholar]