Abstract
Vascular relaxation to GTN (nitroglycerin) and other antianginal nitrovasodilators requires bioactivation of the drugs to NO or a related activator of sGC (soluble guanylate cyclase). Conversion of GTN into 1,2-GDN (1,2-glycerol dinitrate) and nitrite by mitochondrial ALDH2 (aldehyde dehydrogenase 2) may be an essential pathway of GTN bioactivation in blood vessels. In the present study, we characterized the profile of GTN biotransformation by purified human liver ALDH2 and rat liver mitochondria, and we used purified sGC as a sensitive detector of GTN bioactivity to examine whether ALDH2-catalysed nitrite formation is linked to sGC activation. In the presence of mitochondria, GTN activated sGC with an EC50 (half-maximally effective concentration) of 3.77±0.83 μM. The selective ALDH2 inhibitor, daidzin (0.1 mM), increased the EC50 of GTN to 7.47±0.93 μM. Lack of effect of the mitochondrial poisons, rotenone and myxothiazol, suggested that nitrite reduction by components of the respiratory chain is not essential to sGC activation. However, since co-incubation of sGC with purified ALDH2 led to significant stimulation of cGMP formation by GTN that was completely inhibited by 0.1 mM daidzin and NO scavengers, ALDH2 may convert GTN directly into NO or a related species. Studies with rat aortic rings suggested that ALDH2 contributes to GTN bioactivation and showed that maximal relaxation to GTN occurred at cGMP levels that were only 3.4% of the maximal levels obtained with NO. Comparison of sGC activation in the presence of mitochondria with cGMP accumulation in rat aorta revealed a slightly higher potency of GTN to activate sGC in vitro compared with blood vessels. Our results suggest that ALDH2 catalyses the mitochondrial bioactivation of GTN by the formation of a reactive NO-related intermediate that activates sGC. In addition, the previous conflicting notion of the existence of a high-affinity GTN-metabolizing pathway operating in intact blood vessels but not in tissue homogenates is explained.
Keywords: aldehyde dehydrogenase, glycerol dinitrate, mitochondria, nitric oxide, nitroglycerin, soluble guanylate cyclase
Abbreviations: ALDH, aldehyde dehydrogenase; DEA/NO, 2,2-diethyl-1-nitroso-oxyhydrazine; DTT, dithiothreitol; EC50, half-maximally effective concentration; FMN, flavin adenine mononucleotide; GDN, glycerol dinitrate; GTN, glycerol trinitrate (or nitroglycerin); ODQ, 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one; sGC, soluble guanylate cyclase; TEA, triethanolamine
INTRODUCTION
GTN (nitroglycerin) and other antianginal organic nitrates cause cGMP-dependent vasodilation on bioactivation to NO (nitric oxide) or a related activator of sGC (soluble guanylate cyclase) in vascular smooth muscles [1]. The precise pathway of GTN bioactivation is unknown, but the reaction appears to be associated with specific biotransformation to 1,2-GDN (1,2-glycerol dinitrate) and nitrite [2]. Because of the thiol dependence of GTN action [3], it has been suggested that nitrite reacts with cellular thiols to form S-nitrosothiols, which activate sGC through the release of NO [4]. However, nitrite is a poor vasodilator, and at least one study provided compelling evidence against the nitrite/S-nitrosothiol hypothesis [5]. Several enzymes, in particular cytochrome P450 [6–8] and glutathione S-transferase [9–11], were shown to catalyse the bioactivation of GTN, but later studies have questioned the essential role of these enzymes [12,13].
Recently, Chen et al. [14] have reported on the bioactivation of GTN by ALDH2 (aldehyde dehydrogenase). They showed that this enzyme catalyses the formation of 1,2-GDN and nitrite from low concentrations of GTN. The reaction required DTT (dithiothreitol) or 2-mercaptoethanol as reductants and was stimulated by the ALDH cofactor NAD+. To demonstrate the involvement of ALDH2-catalysed GTN metabolism in bioactivation of the nitrate, the authors [14] studied the effects of established (albeit nonspecific) inhibitors (chloral hydrate and cyanamide) as well as those of the natural substrate, acetaldehyde, in experiments with isolated blood vessels and GTN-infused rats. They found that all three inhibitors attenuated the relaxation of isolated rat aorta to GTN, as evident from rightward shifts of the concentration–response curves and the completely inhibited tissue cGMP accumulation. In addition, the decrease in blood pressure was less pronounced when rats were pretreated with ALDH inhibitors before GTN infusion. The compounds had no effect on direct vasorelaxation by sodium nitroprusside, indicating that they did not interfere with signalling downstream of NO formation. These results were confirmed by recent studies of Zhang et al. [15] as well as Bennett and co-workers [16]. The latter showed that non-selective ALDH inhibitors partially inhibit vascular relaxation to GTN, but since these inhibitors were equally effective in nitrate-tolerant animals, the authors concluded that inactivation of ALDH2 does not explain nitrate tolerance and attributed inhibition of GTN relaxation by these compounds to non-specific effects. On the other hand, Sydow et al. [17] provided opposing evidence indicating that ALDH2 plays a central role in GTN tolerance and cross-tolerance.
These previous studies have raised a number of important questions [18]. Besides the controversial role of ALDH2 in nitrate tolerance, the gap between ALDH2-catalysed nitrite formation and sGC activation needs to be addressed. Chen et al. [14] speculated that nitrite could be reduced to NO by components of the respiratory chain, as proposed by Kozlov et al. [19]. Although the opposite reaction, i.e. oxidation of NO to nitrite by cytochrome c oxidase, is well established [20–22], there is no convincing experimental evidence for efficient and rapid reduction of nitrite by mitochondria under physiological conditions, and no supporting data were provided by Chen et al. [14].
The present study was designed to elucidate the link between GTN metabolism and sGC activation and to probe the role of ALDH2 in GTN bioactivation in different biological systems. Purified sGC was used as a highly sensitive detector for the bioactivation of GTN. This approach had the additional advantage of circumventing the current controversy about the identity of the GTN metabolite responsible for sGC activation [23–25]. The selective ALDH2 inhibitor, daidzin [26,27], was used to the test for the involvement of ALDH2 in the bioactivation of GTN by subcellular fractions (mitochondria, microsomes), cultured RFL-6 cells and rat aortic rings.
MATERIALS AND METHODS
Materials
Bovine lung sGC was purified as described in [28]. Human liver ALDH2 was expressed in Escherichia coli and purified as described in [29]. RFL-6 cells were obtained from A.T.C.C. (CCL-192). Antibiotics and fetal calf serum were from PAA Laboratories (Linz, Austria). Daidzin was synthesized as described in [27] and dissolved in a buffer (solubility limit, 160 μM). [2-14C]GTN (50–60 mCi/mmol) was obtained from American Radiolabeled Chemicals, supplied by Humos Diagnostica (Maria Enzersdorf, Austria). [α-32P]GTP (400 Ci/mmol) was obtained from Amersham Biosciences (Vienna, Austria). Solutions of unlabelled GTN (4.4 mM) were obtained from G. Pohl-Boskamp (Hohenlockstedt, Germany). 1,2- and 1,3-GDN were from LGC Promochem (Wesel, Germany), chloral hydrate was from Fluka Chemie (Vienna, Austria) and all other chemicals were from Sigma (Vienna, Austria).
Isolation of rat liver mitochondria and microsomes
Rat liver mitochondria and microsomes were isolated by a standard method [30]. All steps, including centrifugation, were performed at 4 °C. Fresh rat livers were homogenized in 10× solution of 0.25 mM sucrose and 10 mM Tris/HCl (pH 7.4), with a Potter–Elvehjem-type homogenizer. The homogenates were centrifuged for 10 min at 600 g, and the white lipid layer floating on top was removed. Mitochondria were sedimented by centrifugation of the supernatants for 15 min at 6500 g. The resulting supernatants were used for the preparation of microsomes, the pellets were washed twice by resuspending in half of the original volume of Tris/HCl-buffered sucrose and centrifugation was performed for 5 min at 15000 g. The isolated mitochondria were suspended in Tris/HCl-buffered sucrose to 10 mg of protein/ml and either kept on ice until use (freshly isolated) or stored for up to 4 weeks at −70 °C. We observed no significant differences between freshly isolated and frozen mitochondria in terms of O2 consumption or GTN biotransformation/bioactivation.
To obtain microsomes, supernatants from the 6500 g centrifugation step were centrifuged for 10 min at 12000 g to sediment light mitochondria, which were discarded. The supernatants were centrifuged for 1 h at 100000 g. The resulting pellets were resuspended in the original volume of 0.15 mM KCl and centrifuged for 30 min at 100000 g to sediment the microsomes. The pellets were suspended in Tris/HCl-buffered sucrose to approx. 20 mg of protein/ml and kept on ice until use or stored for up to 4 weeks at −70 °C.
Activity of the marker enzyme, glutamate dehydrogenase, was measured to judge the purity of mitochondrial preparations and to test for mitochondrial contamination of microsomes. Various amounts of protein were incubated for 3 min at ambient temperature in 35 mM TEA (triethanolamine)/HCl buffer (pH 7.4), containing 3.88 mM α-ketoglutarate, 137 μM NADPH, 0.83 mM ADP and 100 mM ammonium acetate. NADPH oxidation was continuously monitored as the decrease in absorbance at 340 nm. The mitochondrial preparations exhibited glutamate dehydrogenase activities between 0.8 and 2.0 μmol·min−1·mg−1; enzyme activity was not detected in microsomes. There was no apparent correlation between the glutamate dehydrogenase activity of mitochondria and any of the results of the present study.
Determination of GTN biotransformation by radio-TLC
Radiolabelled GTN (2 μM unless indicated otherwise) was incubated with isolated rat liver mitochondria, rat liver microsomes or purified ALDH2 at 37 °C for 10 min in a total volume of 200 μl of 50 mM phosphate buffer (pH 7.4), containing 2 mM GSH and test compounds as indicated. Except for the experiment shown in Figure 2(A), DTT (2 mM) was always present for reductive re-activation of ALDH2 [14,31]. Products were extracted twice with 1 ml of diethyl ether, followed by evaporation of the solvent under a gentle stream of nitrogen. The residues were dissolved in 50 μl of ethanol and transferred on to a silica-gel TLC plate. A solution containing non-radioactive 1,2-GDN, 1,3-GDN and GTN standards was applied on to the same plate. Water-saturated toluene/ethyl acetate (10:1, v/v) was used as the solvent. Separated products were localized by spraying the marker-band with a solution of diphenyl amine (1 g in 100 ml of methanol) and after exposure to UV light, the corresponding radioactive spots were scraped off and radioactivity was determined by liquid-scintillation counting. Blank levels were determined in the absence of added protein. The sum of radioactivity (corrected for blanks) associated with the three isolated spots (GTN and 1,2- and 1,3-GDN) was taken as 100% (i.e. the amount of GTN per tube) and used to calculate the relative amounts of each product, which were transformed into absolute values through the known specific radioactivity of [14C]GTN. Results are expressed as amount of product (pmol or nmol) obtained per min per mg of protein. When required, commercially available [14C]GTN was purified by TLC using the method described for product analysis.
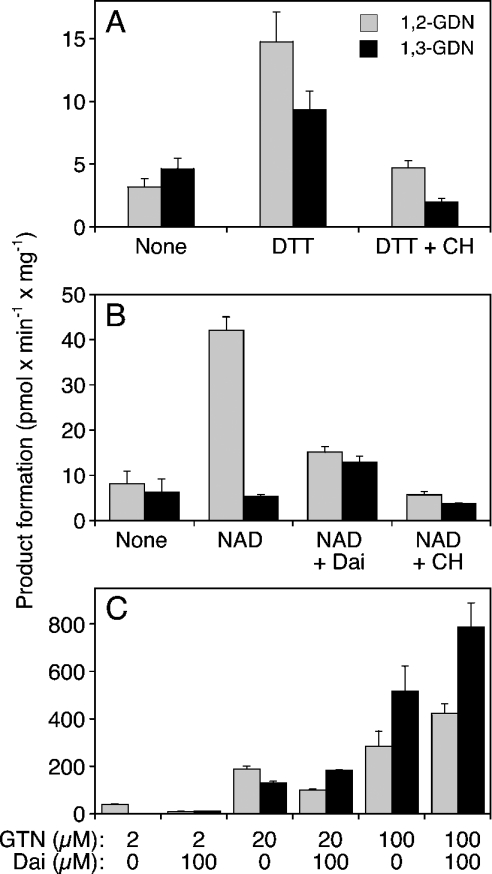
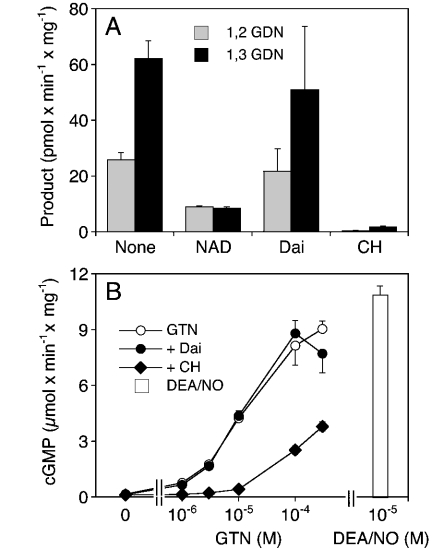
Figure 2. Biotransformation of GTN by rat liver mitochondria.
Isolated rat liver mitochondria (0.3 mg of protein) were incubated for 10 min at 37 °C in the presence of [14C]GTN (at 2 μM in A and B and at the indicated concentrations in C), 2 mM GSH and additives as indicated: DTT (2 mM), 1 mM chloral hydrate (CH), 1 mM NAD+ (NAD), daidzin (Dai; 10 μM in B, 100 μM in C). (A) Effects of DTT and chloral hydrate on the formation of 1,2- and 1,3-GDN. (B) Effects of NAD+ and chloral hydrate in the presence of 2 mM DTT. (C) Effects of 0.1 mM daidzin on the formation of 1,2- and 1,3-GDN from 2, 20 and 100 μM GTN. Products were extracted and analysed by TLC as described in the Materials and methods section. Data are the means±S.E.M. for 3–4 experiments.
Determination of oxygen consumption
Freshly isolated mitochondria (1.7–2 mg) were suspended in 1.8 ml of 50 mM Tris/HCl buffer (pH 7.4), containing 0.2 M sucrose, 3 mM MgCl2, 0.5 mM EGTA and 0.5 mM EDTA. Oxygen consumption was measured with a Clark-type electrode (ISO2, World Precision Instruments, Berlin, Germany) in a thermostatically controlled glass vial sealed with a rubber septum. After equilibration (∼1 min), solutions of the test compounds (5–10 μl) were injected through the septum and O2 consumption was monitored under constant stirring. Two-point calibration of the sensor was performed in air-saturated water at 37 °C (6.9 p.p.m.; 0.216 mM O2) in an argon atmosphere (zero O2 content).
Determination of sGC activity
Purified bovine lung sGC (50–100 ng) was incubated at 37 °C for 10 min, in a final volume of 0.2 ml, with various concentrations of GTN [or with 10 μM DEA/NO (2,2-diethyl-1-nitroso-oxyhydrazine) for maximal sGC activation] in the absence and presence of rat liver mitochondria, rat liver microsomes (0.3 mg of protein each) or purified ALDH2 (5 μg). Assay mixtures contained 50 mM TEA/HCl (pH 7.4), [α-32P]GTP (0.5 mM, ∼150000 c.p.m.), 3 mM MgCl2, 5 mM phosphocreatine, 152 m-units/ml creatine kinase, 1 mM cGMP, 2 mM GSH and 2 mM DTT. Reactions were terminated by the addition of 450 μl of zinc acetate (120 mM) and 450 μl of sodium bicarbonate (120 mM), followed by separation of [32P]cGMP was as described in [32]. Blank values were determined in the absence of sGC.
Culture of RFL-6 cells and determination of intracellular cGMP
RFL-6 cells were cultured in 24-well plastic plates in Ham's F-12 medium (37 °C, 5% CO2), containing 20% (v/v) heat-inactivated fetal calf serum, 100 units/ml penicillin, 0.1 mg/ml streptomycin and 1.25 μg/ml amphotericin B. Confluent monolayers (∼2×105 cells/dish) were washed and preincubated for 15 min at 37 °C in 50 mM Tris buffer (pH 7.4), containing 100 mM NaCl, 5 mM KCl, 1 mM MgCl2, 3 mM CaCl2 and 1 mM 3-isobutyl-1-methylxanthine in the presence of the ALDH inhibitors daidzin, chloral hydrate, cyanamide and acetaldehyde or the sGC inhibitor ODQ (1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one). GTN was added and cells were incubated for 10 min in the absence and presence of haemoglobin, FMN (flavin adenine mononucleotide) or hypoxanthine/xanthine oxidase. Finally, cells were lysed with 0.01 M HCl, and cGMP was determined in the supernatants by RIA as described in [33].
Rat aortic ring experiments
Female (∼250 g) or male rats (∼340 g) were anaesthetized, and the aortic arch removed, cleaned and cut into small rings of approx. 3 mm width. Rings were suspended in 5-ml organ baths containing oxygenated Krebs–Henseleit buffer (118 mM NaCl/4.7 mM KCl/1.2 mM MgSO4/2.5 mM CaCl2/1.2 mM KH2PO4/25 mM NaHCO3/10 mM glucose; pH 7.4) [50], equilibrated for 90 min at 2 g tension and precontracted with a combination of phenylephrine (10– 40 μM) and U 4661980 (10–40 nM), giving a mean tension of 5.5±0.1 g (n=26 rings) after approx. 40 min. GTN was added in a non-cumulative manner at 1–10 μM. The potency of daidzin to inhibit relaxation of GTN was tested at 0.3 mM by adding the drug to the organ bath, 5 min after the initiation of precontraction. In some tissues, daidzin slightly decreased contraction, which was offset by adding U 46619, resulting in a contraction, which was displayed as a stable plateau in all cases. After the last GTN concentration, papaverine (2.3 μM final concentration) was added to obtain maximal relaxation.
Tissue cGMP was determined essentially as described previously [34]. Briefly, tissues were equilibrated and precontracted, followed by the addition of vehicle or GTN at 0.01, 0.1, 1.4 or 20 μM (control curve). These concentrations represent the EC15 and EC50, a submaximally active and a supra-maximally active concentration respectively (cf. Figure 8A). To determine the cGMP response to GTN in the presence of daidzin (0.3 mM), roughly equi-effective and thus higher GTN concentrations were used, namely 0.07, 0.9, 10 and 100 μM respectively. Tissues were freeze-clamped for 2 min (determined in pilot studies) after the addition of agonist in liquid nitrogen and stored at −70 °C pending analysis by RIA. One ring (wet weight, 7–10 mg) was used per determination, except for baseline, for which four rings were necessary for each single cGMP analysis.
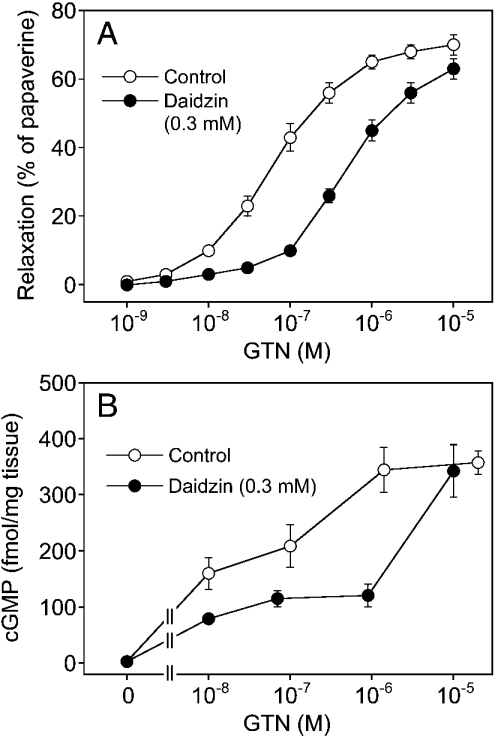
Figure 8. GTN-induced vascular relaxation and cGMP accumulation.
(A) Vascular relaxant effect of GTN. Aortic rings were contracted, followed by addition of increasing concentrations of GTN in the absence (○, control) or presence of 0.3 mM daidzin (●). (B) cGMP levels in response to different GTN concentrations in the absence and presence of 0.3 mM daidzin. Data are the means±S.E.M. for 13 tissues (A) or 8 tissues (B).
RESULTS
Biotransformation of GTN by purified ALDH2
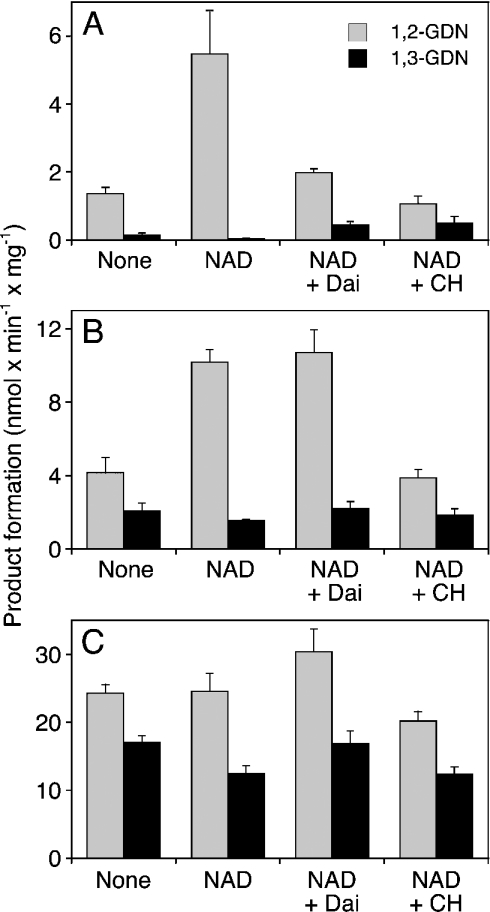
Figure 1(A) shows that purified ALDH2 converted 2 μM GTN into 1,2- and 1,3-GDN at the rate of 1.37±0.18 and 0.14±0.07 nmol·min−1·mg−1 respectively. The presence of the cofactor NAD+ (1 mM) led to a 4-fold increase in the rate of 1,2-GDN formation (5.47±1.28 nmol·min−1·mg−1), whereas 1,3-GDN formation was very low under these conditions (0.04±0.02 nmol·min−1·mg−1). The selective ALDH inhibitor, daidzin (10 μM), led to pronounced inhibition of 1,2-GDN formation that was accompanied by a 10-fold increase in formation of 1,3-GDN. The non-selective inhibitor, chloral hydrate, had a similar effect. At higher GTN concentrations (20 and 100 μM; see Figures 1B and 1C respectively), the rates of product formation increased (24.3±1.29 nmol·min−1·mg−1 at 0.1 mM GTN), but selectivity of denitration was lost. In addition, all agents tested were less effective at high GTN concentrations. This was particularly apparent with daidzin (10 μM), which did not inhibit 1,2-GDN formation when the GTN concentration was increased to 20 μM. NAD+ (1 mM) increased 1,2-GDN formation by 2.5-fold at 20 μM GTN but was ineffective at 0.1 mM GTN.
Figure 1. Biotransformation of GTN by purified ALDH2.
Purified human liver ALDH2 (2 μg) was incubated for 10 min at 37 °C in the presence of 2 μM (A), 20 μM (B) or 100 μM (C) [14C]GTN, 2 mM GSH, 2 mM DTT and additives as indicated: 1 mM NAD+ (NAD), 10 μM daidzin (Dai) and 1 mM chloral hydrate (CH). 1,2- and 1,3-GDN were extracted and analysed by TLC as described in the Materials and methods section. Data are the means±S.E.M. for 3–4 experiments.
Biotransformation of GTN by isolated mitochondria
Similar experiments were performed with isolated rat liver mitochondria to test for the contribution of ALDH2 to mitochondrial GTN biotransformation. As shown in Figure 2(A), mitochondria metabolized 2 μM GTN to approximately equal amounts of 1,2- and 1,3-GDN at the rate of approx. 4 pmol·min−1·mg−1. Although these experiments were performed with 2 mM GSH, the rates of product formation were markedly increased by the additional presence of 2 mM DTT (from 3.22±0.62 to 14.8±2.37 pmol 1,2-GDN min−1·mg−1 and from 4.65±0.85 to 9.37±1.47 pmol 1,3-GDN min−1·mg−1). Chloral hydrate (1 mM) decreased the rates of product formation with a slightly more pronounced effect on 1,3-GDN formation. On the basis of these results, all further experiments were performed in the presence of 2 mM DTT. As observed with pure ALDH2, NAD+ (1 mM) led to a pronounced, approx. 4-fold increase in the rate of 1,2-GDN formation (42.1±3.03 pmol·min−1·mg−1; Figure 2B). Formation of 1,3-GDN was not affected by the nucleotide. Daidzin (10 μM) inhibited 1,2-GDN formation to approx. 36% of control but led to an approx. 2-fold stimulation of 1,3-GDN formation. Chloral hydrate (1 mM) markedly inhibited the formation of 1,2-GDN (residual activity, approx. 14% of control) and slightly decreased the rate of 1,3-GDN formation (from 5.47±0.42 to 3.75±0.19 pmol·min−1·mg−1; Figure 2B). As found with purified ALDH2, higher concentrations of GTN resulted in loss of selective denitration. At 0.1 mM GTN, the rates of 1,2- and 1,3-GDN formation were 0.29±0.06 and 0.52±0.11 nmol·min−1·mg−1 respectively.
Similar to our observations with purified ALDH2, the effectiveness of NAD+, chloral hydrate and daidzin decreased with increasing GTN concentration. The GTN-competitive effect of daidzin is illustrated in Figure 2(C). In the presence of 2 μM GTN, 0.1 mM daidzin inhibited the formation of 1,2-GDN from 40.2±3.36 to 10.44±0.51 pmol·min−1·mg−1, whereas formation of the 1,3-isomer was increased from 2.60±0.33 to 11.8±2.26 pmol·min−1·mg−1. The effect of daidzin was much less pronounced at 20 μM GTN (54% residual 1,2-GDN formation). At 0.1 mM GTN, daidzin did not inhibit but slightly increased the rates of product formation.
Bioactivation of GTN by mitochondria, microsomes and purified ALDH2
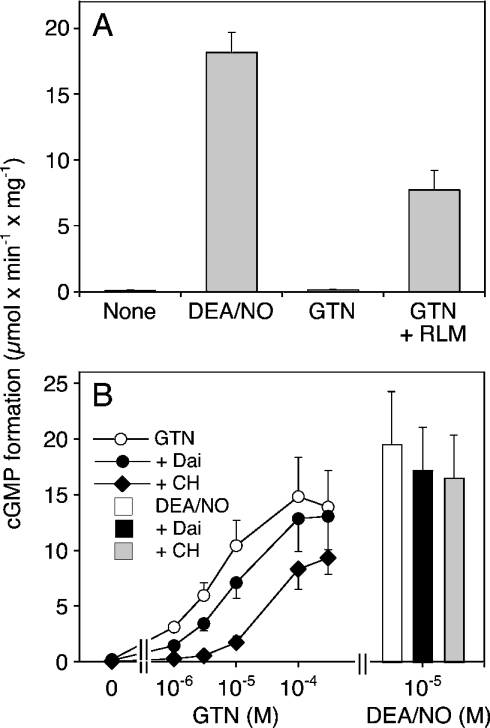
Mitochondrial bioactivation of GTN was studied by co-incubation of rat liver mitochondria with purified bovine lung sGC under various conditions. As shown in Figure 3(A), 10 μM DEA/NO increased the rate of cGMP formation by purified sGC from 0.12±0.02 to 18.1±1.52 μmol·min−1·mg−1, corresponding to a 150-fold enzyme stimulation by NO. Under these conditions, GTN (0.3 mM) had no significant effect on basal sGC activity (0.14±0.02 μmol·min−1·mg−1), confirming that GSH (unlike L-cysteine) does not support non-enzymic bioactivation of GTN. In the presence of rat liver mitochondria, 0.3 mM GTN led to a pronounced, approx. 65-fold activation of sGC. The GTN concentration–response shown in Figure 3(B) revealed an EC50 of 3.7±0.83 μM, with a maximal effect corresponding to 70–80% of the maximum obtained with 10 μM DEA/NO. Chloral hydrate (1 mM) significantly attenuated the effect of GTN, both in terms of maximal sGC activation and EC50 (∼30 μM), but had no significant effect on sGC activation by DEA/NO. In the presence of daidzin, the EC50 value for GTN was increased by approx. 2-fold (7.47±0.93 μM). As expected from the biotransformation data, the effect of the inhibitor was apparent only at GTN concentrations below 10 μM. At 1 μM GTN, 0.1 mM daidzin decreased the rate of cGMP formation from 3.14±0.30 to 1.46±0.22 μmol· min−1·mg−1, corresponding to approx. 50% inhibition.
Figure 3. Activation of sGC by GTN in the presence of rat liver mitochondria.
Purified bovine lung sGC (50–100 ng) was incubated at 37 °C for 10 min in the presence of [α-32P]GTP (0.5 mM, ∼150000 c.p.m.), 3 mM MgCl2, 5 mM phosphocreatine, 152 m-units/ml creatine kinase, 1 mM cGMP, 2 mM GSH and 2 mM DTT. (A) Effects of DEA/NO (10 μM), GTN (0.3 mM) and GTN in the presence of isolated rat liver mitochondria (0.3 mg of protein). (B) Effect of GTN (1–300 μM) in the presence of 0.1 mM daidzin (+Dai) and 1 mM chloral hydrate (+CH). The bars show cGMP formation stimulated by 10 μM DEA/NO in the absence and presence of 0.1 mM daidzin (+Dai) and 1 mM chloral hydrate (+CH). Data are the means±S.E.M. for three experiments.
Similar experiments were performed with rat liver microsomes. As shown in Figure 4(A), the pattern of microsomal GTN biotransformation was clearly different from that observed with mitochondria. Microsomes converted GTN primarily into 1,3-GDN (62.1±6.53 versus 25.9±2.63 pmol 1,2-GDN min−1·mg−1), NAD+ inhibited the formation of both products (∼9 pmol·min−1·mg−1) and daidzin had no effect. In contrast with the selective ALDH2 inhibitor daidzin, chloral hydrate almost completely blocked GTN metabolism catalysed by microsomes. As shown in Figure 4(B), GTN activated sGC in the presence of rat liver microsomes with an EC50 of 11±0.5 μM; the effect of the nitrate was sensitive to chloral hydrate, but daidzin had no significant effect at any of the GTN concentrations tested (1–300 μM).
Figure 4. Metabolism and bioactivation of GTN by rat liver microsomes.
(A) Metabolism of GTN in the presence of rat liver microsomes (0.3 mg of protein). Experimental conditions and abbreviations are as in Figure 2(B). Data are the means±S.E.M. for 3–4 experiments. (B) Activation of sGC by GTN in the presence of rat liver microsomes (0.3 mg of protein). Experimental conditions and abbreviations are as in Figure 3(B). Data are the means±S.E.M. for three experiments.
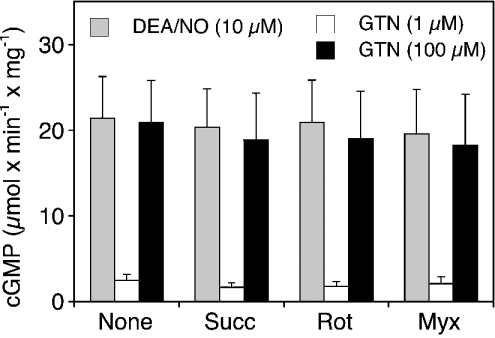
It has been reported that mitochondria exhibit nitrite reductase activity that is stimulated by respiratory substrates and inhibited by mitochondrial poisons [19]. To account for the possibility that respiratory rate is critical for the reduction of GTN-derived nitrite, we studied mitochondrial GTN bioactivation in the presence of the complex II substrate succinate and two inhibitors of respiration, rotenone and myxothiazol. Basal O2 consumption of isolated mitochondria was 11.2±0.9 nmol·min−1·mg−1. Respiratory rate was increased by approx. 7-fold by succinate (10 mM) and almost completely inhibited by rotenone (2.9±0.3 nmol·min−1·mg−1; 5 μM) and myxothiazol (1.1±0.5 nmol·min−1·mg−1; 5 μM). As shown in Figure 5, none of these agents had any effect on activation of sGC by low (1 μM) or high (100 μM) GTN. Similarly, a combination of glutamate and malate (2.5 mM each) [19] had no effect (results not shown).
Figure 5. Effects of succinate, rotenone and myxothiazol on GTN bioactivation.
Purified sGC was incubated at 37 °C for 10 min as described in the Materials and methods section in the presence of rat liver mitochondria (0.3 mg of protein). Succinate (10 mM), rotenone and myxothiazol (5 μM each) were present as indicated. The enzyme was stimulated by either DEA/NO (10 μM) or GTN (1 μM, 0.1 mM). Data are the means±S.E.M. for three experiments.
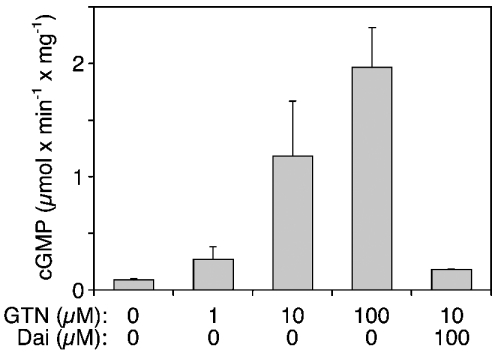
Since these results provided no evidence for efficient nitrite reductase activity of mitochondria, we studied whether purified ALDH2 alone is sufficient to catalyse GTN bioactivation. As shown in Figure 6, GTN significantly activated sGC in the presence of 5 μg of purified ALDH2. Formation of cGMP was stimulated 3-, 13- and 21-fold by 1, 10 and 100 μM GTN respectively, and the effect of 10 μM GTN was completely inhibited by 0.1 mM daidzin. The effect was also blocked by the haemsite sGC inhibitor ODQ (30 μM), the NO scavenger haemoglobin (20 μM) and the superoxide-generating compound FMN (0.1 mM; results not shown).
Figure 6. Activation of sGC in the presence of purified ALDH2.
Purified sGC was incubated at 37 °C for 10 min as described in the Materials and methods section in the presence of 5 μg of purified human liver ALDH2 and GTN (1, 10 and 100 μM). +Dai, daidzin (0.1 mM). Data are the means±S.E.M. for three experiments.
Bioactivation of GTN by cultured RFL-6 cells
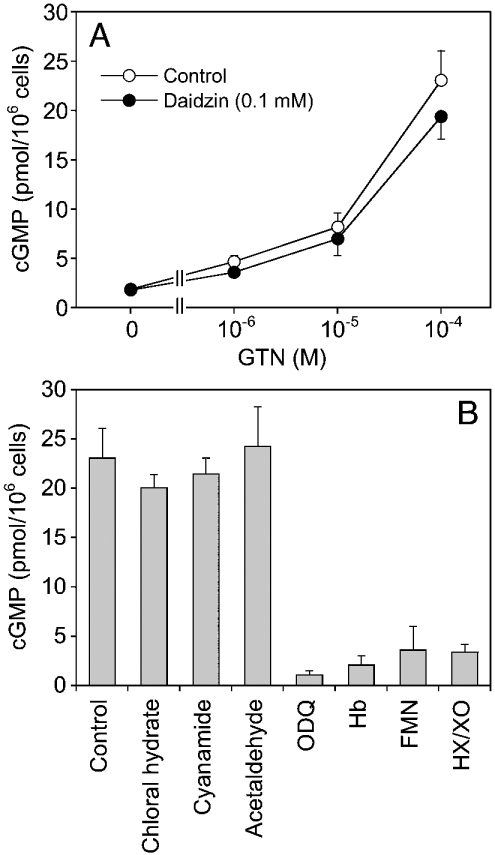
It has been shown previously that metabolism of GTN by RFL-6 cells is accompanied by accumulation of intracellular cGMP [35]. On the basis of results obtained with several inhibitors, Schröder [35] proposed that GTN bioactivation by these cells was catalysed by cytochrome P450 enzymes. In the present study, there was no effect of daidzin (0.1 mM) and chloral hydrate (1 mM) on GTN (2 μM) metabolism (results not shown). Nevertheless, the cells showed a significant albeit moderate accumulation of cGMP in response to GTN (Figure 7A). At 0.1 mM of the organic nitrate, intracellular cGMP levels were approx. 20 pmol/106 cells, corresponding to 3.6% of the maximal levels obtained with 1 μM DEA/NO (551±29 pmol/106 cells). Daidzin (0.1 mM) had no significant effect at any of the GTN concentrations tested (Figure 7A). As shown in Figure 7(B), cGMP accumulation was not affected by the ALDH inhibitors chloral hydrate, cyanamide or acetaldehyde (1 mM each), but fully blocked by ODQ, haemoglobin, FMN (0.1 mM each) and hypoxanthine (1 mM)/xanthine oxidase (10 m-units/ml).
Figure 7. Accumulation of cGMP in cultured RFL-6 cells in response to GTN.
Cells were incubated with the indicated concentrations of GTN for 10 min followed by determination of intracellular cGMP by RIA. ALDH inhibitors and ODQ were added 15 min before the addition of GTN. Haemoglobin, FMN and hypoxanthine/xanthine oxidase were added together with GTN. (A) GTN concentration–response in the absence and presence of 0.1 mM daidzin. (B) cGMP levels triggered by 0.1 mM GTN in the presence of the ALDH inhibitors chloral hydrate, cyanamide and acetaldehyde (1 mM each), the haem-site sGC inhibitor ODQ (0.1 mM), the NO scavenger haemoglobin (Hb; 0.1 mM), the superoxide-generating agent FMN (0.1 mM) and the superoxide-generating system hypoxanthine (HX; 1 mM)/xanthine oxidase (XO; 10 m-units/ml). Data are the means±S.E.M. for three experiments.
GTN bioactivation by rat aortic rings
Experiments with rat aortic rings suggested that ALDH2 is involved in vascular GTN bioactivation. As shown in Figure 8(A), daidzin (0.3 mM) caused a rightward shift of the GTN concentration–response curve [EC50=96±22 and 449±65 nM (P<0.05; n=13) in the absence and presence of daidzin respectively]. Also, the blood vessels showed a pronounced accumulation of cGMP in response to GTN that was significantly attenuated by 0.3 mM daidzin (Figure 8B). Basal cGMP levels (3.2±0.24 fmol/mg of wet weight) were increased by 100-fold to 345±40 fmol/mg of wet weight on incubation with 1.4 μM GTN. Intriguingly, this was only 3.4% of the maximal levels observed on incubation of the rings with 0.1 mM DEA/NO (10132±838 fmol/mg of wet weight). In response to a supra-maximal GTN concentration (0.1 mM), cGMP levels were 2501±386 fmol/mg of wet weight, corresponding to approx. 25% of the DEA/NO maximum. Note that, in the presence of mitochondria, 1 μM GTN activated purified sGC to approx. 16% of the maximal enzyme activity obtained with DEA/NO. Comparison of sGC activation and vascular cGMP accumulation in response to GTN versus that triggered by maximal stimulation with DEA/NO is summarized in Table 1.
Table 1. Effects of DEA/NO and GTN on the activity of purified sGC in the presence of mitochondria and on accumulation of tissue cGMP in rat aortic rings.
| sGC activity | cGMP content in aortic rings | |||
|---|---|---|---|---|
| Stimulation | (μmol·min−1·mg−1)* | Percentage of max. | (fmol/mg of wet weight) | Percentage of max. |
| DEA/NO maximum† | 19.5±4.75 | 100 | 10130±840 | 100 |
| Basal | 0.23±0.03 | 1.2 | 3.2±0.24 | 0.03 |
| GTN (1–1.4 μM)‡ | 3.1±0.30 | 16.1 | 345±40 | 3.4 |
| GTN (0.1 mM) | 14.8±3.56 | 75.9 | 2500±386 | 24.7 |
* Data are taken from Figure 3(B).
† sGC activity in response to 10 μM DEA/NO (n=3) and aortic cGMP content in response to 0.1 mM DEA/NO (n=8) respectively.
‡ sGC activity in response to 1 μM GTN (n=3) and aortic cGMP content in response to 1.4 μM GTN (n=6–8) respectively.
DISCUSSION
Our results show that purified human liver ALDH2 efficiently and specifically metabolizes GTN to 1,2-GDN. Stimulation by NAD+, inhibition by chloral hydrate, loss of specific 1,2-GDN formation at higher GTN concentrations and a maximal activity of approx. 25 nmol·min−1·mg−1 agree well with results published previously for the bovine liver enzyme [14]. The isoflavone daidzin from Radix puerariae, which has been described as a potent ALDH2 inhibitor that selectively inhibits the enzyme both in vitro [26] and in vivo [36], inhibited the biotransformation of GTN by purified ALDH2 in a GTN-competitive manner, indicating that denitration may result from the intrinsic esterase activity of the enzyme [31,37].
The profile of GTN biotransformation, i.e. stimulation by NAD+, inhibition by daidzin and loss of specificity at higher GTN concentrations, identifies ALDH2 as a major GTN-metabolizing enzyme in mitochondria but not in microsomes. However, ALDH2-catalysed biotransformation of GTN in mitochondria was not efficiently linked to sGC activation. Assuming quantitative reduction of GTN-derived nitrite to NO by mitochondria, the rates of 1,2-GDN formation should have given rise to a maximal accumulation of approx. 1 μM NO in the presence of 2 μM GTN. Considering that the affinity of NO binding to sGC is in the range of 1–10 nM [38], maximal sGC activation should have occurred in the presence of 2 μM GTN even if only approx. 1% of GTN-derived nitrite was converted into NO. This was apparently not the case under our experimental conditions. Conceivably, efficient reduction of mitochondrial nitrite may require specific conditions provided by the cellular environment in vivo but not in isolated mitochondria. The involvement of components of the respiratory chain would be an obvious possibility. Kozlov et al. [19] observed a slow nitrite reduction by the ubiquinone cycle in the presence of respiratory substrates that was sensitive to mitochondrial poisons [19]. However, in our experiments designed to detect both potentiation and inhibition of GTN-triggered sGC activation, several agents modulating respiration had no effect on the rates of cGMP formation in the presence of low or high GTN. Our results do not necessarily contradict those of Kozlov et al. [19]. The spin-trapping technique used in their study was based on scavenging of NO for 2 h, resulting in quantitative detection of total NO accumulating during this rather long period of time. Furthermore, in the absence of a trapping agent, low amounts of nitrite-derived NO may be efficiently scavenged by superoxide or mitochondrial haem proteins competing for NO binding to sGC. Thus the mismatch between the rate of GTN metabolism and sGC activation argues strongly against a link between 1,2-GDN formation and cGMP synthesis. This conclusion is also supported by results obtained with DTT, since the thiol compound significantly increased ALDH2-catalysed 1,2-GDN formation (Figure 2) without affecting GTN-triggered sGC activation (results not shown). Unfortunately, the ALDH cofactor NAD+ had a pronounced effect on basal sGC activity (results not shown) precluding reliable interpretation of its effect on mitochondrial GTN bioactivation.
The GTN-competitive actions of daidzin and its relatively low water solubility limited the usefulness of this drug to test for the contribution of ALDH2 to sGC activation at GTN concentrations above 10 μM. Nevertheless, daidzin significantly inhibited sGC activation at low GTN concentrations (1–3 μM), indicating that ALDH2 indeed contributed to, albeit not exclusively mediated, the observed bioactivation of GTN in mitochondria. The selectivity of daidzin is supported by its lack of effect in the presence of microsomes not containing ALDH2. In contrast with daidzin, chloral hydrate markedly inhibited GTN bioactivation by both mitochondria and microsomes. Inhibition of the bioactivation of GTN by microsomes lacking the potential for specifically forming 1,2-GDN might suggest pleiotropic effects of chloral hydrate. However, the drug had no effect on GTN metabolism and bioactivation in RFL-6 cells, reportedly catalysed by cytochrome P450 [35], and did not interfere with NO-mediated activation of sGC. Chloral hydrate is a well-established non-selective, substrate-competitive ALDH inhibitor [39–42] and the 11 human ALDH isoforms cloned so far exhibit rather broad substrate specificity [43]. Accordingly, inhibition by chloral hydrate of microsomal GTN metabolism and bioactivation may suggest the involvement of another ALDH isoform, e.g. fatty ALDH10 (aldehyde dehydrogenase 10), which is present in liver microsomes [44].
Unexpectedly, GTN activated sGC in the presence of purified ALDH2 (Figure 6). This effect is unlikely to be due to non-enzymic formation of NO from GTN-derived nitrite, since more than 1 mM authentic nitrite was required to produce comparable sGC activation (results not shown). Inhibition by daidzin suggests that interaction of GTN with the substrate-binding site of ALDH2 is essential to bioactivation. The mechanism behind GTN-triggered sGC activation in the presence of ALDH2 is unclear. It has been suggested that GTN bioactivity does not involve formation of free NO [23–25]. Since the expected amounts of NO release were below the dectection limit of available analytical methods (e.g. NO chemiluminescence), we addressed this issue indirectly, using established agents known to interfere with NO-mediated sGC activation. Inhibition of GTN-triggered cGMP formation by ODQ indicates an important role for sGC-bound haem [45], and sensitivity to the NO scavenger haemoglobin as well as to superoxide (generated from illuminated FMN; see [46,47]) provides strong circumstantial evidence for the involvement of free NO. On the basis of these results, we conclude that ALDH2 is capable of converting GTN directly into the sGC activator NO. The relatively moderate sGC activation suggests that formation of NO is not the main pathway of ALDH2-catalysed GTN metabolism, at least under our experimental conditions. Nevertheless, the 1,2-GDN data in Figures 1 and 2 show that similar amounts of ALDH2 were present in the experiments with pure enzyme (5 μg) and isolated mitochondria (0.3 mg of protein corresponding to approx. 4 μg of ALDH2), indicating that conversion of GTN into NO or a related sGC-activating species may fully account for the daidzin-sensitive component of mitochondrial GTN bioactivation. Work is underway in our laboratory to characterize the interaction of GTN with ALDH2.
In the presence of mitochondria, GTN activated sGC with an EC50 of approx. 4 μM. This value is clearly below the in vitro potency of the organic nitrate reported previously (EC50∼30 μM; see e.g. [8]). Nevertheless, relaxation of isolated rat aorta occurred at approx. 40 times lower GTN concentrations. This apparent loss of GTN potency on disruption of intact blood vessels has been pointed out in numerous previous studies and led to the search for a high-affinity ‘GTN receptor’ in vascular smooth muscles that might lose its function in the course of tissue homogenization. However, our results suggest that the apparent discrepancy between in vitro and in vivo potency of GTN arises from unjustified comparison of functional relaxation data with measurements of cGMP formation expressed as ‘fold-stimulation’. In agreement with numerous earlier studies, we found that maximal relaxation of rat aortic rings to GTN was accompanied by an approx. 100-fold increase in tissue cGMP. However, the respective cGMP levels were only 3.4% of the maximal levels obtained with DEA/NO, and a supra-maximal (in terms of relaxation) concentration of GTN (0.1 mM) led to a further increase in tissue cGMP that was almost 1000-fold above the basal level. These results indicate that half-maximal stimulation of sGC in intact rat aorta occurs with an EC50>10 μM GTN, i.e. with a potency similar or even lower than that observed in tissue homogenates. Expression of data in terms of ‘fold-increase’ of cGMP levels or ‘fold-activation’ of sGC is misleading since it critically depends on the respective basal values. Basal tissue cGMP levels are affected by numerous intracellular processes, in particular phosphodiesterase hydrolysis, and are not comparable with the basal activity of isolated sGC, which markedly varies depending on isolation techniques and incubation conditions. Thus purified sGC is typically activated 100–200-fold by NO [28,48,49], whereas aortic cGMP levels were increased 3200-fold in response to 0.1 mM DEA/NO. It appears essential, therefore, to express the efficiency of a potential sGC activator relative to the maximal capacity of the system for a reliable comparison of in vitro data on sGC activation with cGMP levels measured in intact blood vessels or other tissues.
In conclusion, our results demonstrate that ALDH2 contributes to GTN bioactivation in blood vessels and rat liver mitochondria but is not the exclusive mammalian enzyme catalysing this process. Secondly, the comparison of mitochondrial GTN metabolism with sGC activation provided no evidence for a link between 1,2-GDN formation and cGMP synthesis. Finally, we showed that purified human liver ALDH2 converts GTN into an NO-like species that activates sGC. Further studies are necessary to understand the mechanism of this reaction and to examine whether microsomal ALDH or other mammalian isoenzymes exhibit a similar catalytic activity.
Acknowledgments
The excellent technical assistance of B. Heiling, A. Spiess, G. Wölkart, M. Rehn and D. J. Li is gratefully acknowledged. This work was supported by the Fonds zur Förderung der Wissenschaftlichen Forschung in Austria (K. S., B. M.), the Deutsche Forschungs-gemeinschaft (D. K.) and Endowment for Research in Human Biology (W. M. K.).
References
- 1.Torfgard K. E., Ahlner J. Mechanisms of action of nitrates. Cardiovasc. Drugs Ther. 1994;8:701–717. doi: 10.1007/BF00877117. [DOI] [PubMed] [Google Scholar]
- 2.Bennett B. M., McDonald B. J., Nigam R., Simon W. C. Biotransformation of organic nitrates and vascular smooth muscle cell function. Trends Pharmacol. Sci. 1994;15:245–249. doi: 10.1016/0165-6147(94)90319-0. [DOI] [PubMed] [Google Scholar]
- 3.Needleman P. Organic nitrate metabolism. Annu. Rev. Pharmacol. Toxicol. 1976;16:81–93. doi: 10.1146/annurev.pa.16.040176.000501. [DOI] [PubMed] [Google Scholar]
- 4.Ignarro L. J., Gruetter C. A. Requirement of thiols for activation of coronary arterial guanylate cyclase by glyceryl trinitrate and sodium nitrite: possible involvement of S-nitrosothiols. Biochim. Biophys. Acta. 1980;631:221–231. doi: 10.1016/0304-4165(80)90297-4. [DOI] [PubMed] [Google Scholar]
- 5.Romanin C., Kukovetz W. R. Guanylate cyclase activation by organic nitrates is not mediated via nitrite. J. Mol. Cell. Cardiol. 1988;20:389–396. doi: 10.1016/s0022-2828(88)80130-5. [DOI] [PubMed] [Google Scholar]
- 6.Servent D., Delaforge M., Ducrocq C., Mansuy D., Lenfant M. Nitric oxide formation during microsomal hepatic denitration of glyceryl trinitrate: involvement of cytochrome P-450. Biochem. Biophys. Res. Commun. 1989;163:1210–1216. doi: 10.1016/0006-291x(89)91106-6. [DOI] [PubMed] [Google Scholar]
- 7.Schröder H., Schrör K. Inhibitors of cytochrome P-450 reduce cyclic GMP stimulation by glyceryl trinitrate in LLC-PK1 kidney epithelial cells. Naunyn-Schmiedeberg's Arch. Pharmacol. 1990;342:616–618. doi: 10.1007/BF00169054. [DOI] [PubMed] [Google Scholar]
- 8.Bennett B. M., Mcdonald B. J., St James M. J. Hepatic cytochrome-P-450-mediated activation of rat aortic guanylyl cyclase by glyceryl trinitrate. J. Pharmacol. Exp. Ther. 1992;261:716–723. [PubMed] [Google Scholar]
- 9.Yeates R. A., Schmid M., Leitold M. Antagonism of glycerol trinitrate activity by an inhibitor of glutathione S-transferase. Biochem. Pharmacol. 1989;38:1749–1753. doi: 10.1016/0006-2952(89)90408-5. [DOI] [PubMed] [Google Scholar]
- 10.Tsuchida S., Maki T., Sato K. Purification and characterization of glutathione transferases with an activity toward nitroglycerin from human aorta and heart. Multiplicity of the human class Mu forms. J. Biol. Chem. 1990;265:7150–7155. [PubMed] [Google Scholar]
- 11.Nigam R., Anderson D. J., Lee S. F., Bennett B. M. Isoform-specific biotransformation of glyceryl trinitrate by rat aortic glutathione S-transferases. J. Pharmacol. Exp. Ther. 1996;279:1527–1534. [PubMed] [Google Scholar]
- 12.Liu Z. G., Brien J. F., Marks G. S., McLaughlin B. E., Nakatsu K. Lack of evidence for the involvement of cytochrome P-450 or other hemoproteins in metabolic activation of glyceryl trinitrate in rabbit aorta. J. Pharmacol. Exp. Ther. 1993;264:1432–1439. [PubMed] [Google Scholar]
- 13.Kurz M. A., Boyer T. D., Whalen R., Peterson T. E., Harrison D. G. Nitroglycerin metabolism in vascular tissue: role of glutathione S-transferases and relationship between NO and NO2-formation. Biochem. J. 1993;292:545–550. doi: 10.1042/bj2920545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen Z. Q., Zhang J., Stamler J. S. Identification of the enzymatic mechanism of nitroglycerin bioactivation. Proc. Natl. Acad. Sci. U.S.A. 2002;99:8306–8311. doi: 10.1073/pnas.122225199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang J., Chen Z. P., Cobb F. R., Stamler J. S. Role of mitochondrial aldehyde dehydrogenase in nitroglycerin-induced vasodilation. Circulation. 2004;110:750–755. doi: 10.1161/01.CIR.0000138105.17864.6B. [DOI] [PubMed] [Google Scholar]
- 16.DiFabio J., Yanbin J., Vasiliou V., Thatcher R. J., Bennett B. M. Role of mitochondrial aldehyde dehydrogenase in nitrate tolerance. Mol. Pharmacol. 2003;64:1109–1116. doi: 10.1124/mol.64.5.1109. [DOI] [PubMed] [Google Scholar]
- 17.Sydow K., Daiber A., Oelze M., Chen Z. P., August M., Wendt M., Ullrich V., Mülsch A., Schulz E., Keaney J. F., et al. Central role of mitochondrial aldehyde dehydrogenase and reactive oxygen species in nitroglycerin tolerance. J. Clin. Invest. 2004;113:482–489. doi: 10.1172/JCI19267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mayer B. Bioactivation of nitroglycerin – a new piece in the puzzle. Angew. Chem. Int. Edn. 2003;42:388–391. doi: 10.1002/anie.200390124. [DOI] [PubMed] [Google Scholar]
- 19.Kozlov A. V., Staniek K., Nohl H. Nitrite reductase activity is a novel function of mammalian mitochondria. FEBS Lett. 1999;454:127–130. doi: 10.1016/s0014-5793(99)00788-7. [DOI] [PubMed] [Google Scholar]
- 20.Torres J., Cooper C. E., Wilson M. T. A common mechanism for the interaction of nitric oxide with the oxidized binuclear centre and oxygen intermediates of cytochrome c oxidase. J. Biol. Chem. 1998;273:8756–8766. doi: 10.1074/jbc.273.15.8756. [DOI] [PubMed] [Google Scholar]
- 21.Torres J., Sharpe M. A., Rosquist A., Cooper C. E., Wilson M. T. Cytochrome c oxidase rapidly metabolises nitric oxide to nitrite. FEBS Lett. 2000;475:263–266. doi: 10.1016/s0014-5793(00)01682-3. [DOI] [PubMed] [Google Scholar]
- 22.Cooper C. E. Nitric oxide and cytochrome oxidase: substrate, inhibitor or effector? Trends Biochem. Sci. 2002;27:33–39. doi: 10.1016/s0968-0004(01)02035-7. [DOI] [PubMed] [Google Scholar]
- 23.Artz J. D., Toader V., Zavorin S. I., Bennett B. M., Thatcher G. R. J. In vitro activation of soluble guanylyl cyclase and nitric oxide release: a comparison of NO donors and NO mimetics. Biochemistry. 2001;40:9256–9264. doi: 10.1021/bi002885x. [DOI] [PubMed] [Google Scholar]
- 24.Artz J. D., Schmidt B., McCracken J. L., Marletta M. A. Effects of nitroglycerin on soluble guanylate cyclase – implications for nitrate tolerance. J. Biol. Chem. 2002;277:18253–18256. doi: 10.1074/jbc.C200170200. [DOI] [PubMed] [Google Scholar]
- 25.Kleschyov A. L., Oelze M., Daiber A., Huang Y., Mollnau H., Schulz E., Sydow K., Fichtlscherer B., Mülsch A., Münzel T. Does nitric oxide mediate the vasodilator activity of nitroglycerin? Circulation. 2003;93:104–112. doi: 10.1161/01.RES.0000100067.62876.50. [DOI] [PubMed] [Google Scholar]
- 26.Keung W. M., Vallee B. L. Daidzin: a potent, selective inhibitor of human mitochondrial aldehyde dehydrogenase. Proc. Natl. Acad. Sci. U.S.A. 1993;90:1247–1251. doi: 10.1073/pnas.90.4.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Keung W. M., Vallee B. L. Daidzin and daidzein suppress free-choice ethanol intake by Syrian golden hamsters. Proc. Natl. Acad. Sci. U.S.A. 1993;90:10008–10012. doi: 10.1073/pnas.90.21.10008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Humbert P., Niroomand F., Fischer G., Mayer B., Koesling D., Hinsch K.-D., Gausepohl H., Frank R., Schultz G., Böhme E. Purification of soluble guanylyl cyclase from bovine lung by a new immunoaffinity chromatographic method. Eur. J. Biochem. 1990;190:273–278. doi: 10.1111/j.1432-1033.1990.tb15572.x. [DOI] [PubMed] [Google Scholar]
- 29.Zheng C. F., Wang T. T., Weiner H. Cloning and expression of the full-length cDNAS encoding human liver class 1 and class 2 aldehyde dehydrogenase. Alcohol Clin. Exp. Res. 1993;17:828–831. doi: 10.1111/j.1530-0277.1993.tb00849.x. [DOI] [PubMed] [Google Scholar]
- 30.Schenkman J. B., Jansson I. Subfractionation of different tissues for analysis of cytochrome P-450 content. In: Maines M. D., Costa L. G., Reed D. J., Sassa S., Sipes I. G., editors. Current Protocols in Toxicology. New York: John Wiley & Sons; 1999. pp. 4.1.9–4.1.11. [Google Scholar]
- 31.Mukerjee N., Pietruszko R. Inactivation of human aldehyde dehydrogenase by iosorbide dinitrate. J. Biol. Chem. 1994;269:21664–21669. [PubMed] [Google Scholar]
- 32.Schultz G., Böhme E. Guanylate cyclase. GTP pyrophosphate-lyase (cyclizing), E.C.4.6.1.2. In: Bergmeyer H. U., Bergmeyer J., Grassl M., editors. Methods of Enzymatic Analysis. Germany: Verlag Chemie, Weinheim; 1984. pp. 379–389. [Google Scholar]
- 33.Schmidt K., Schrammel A., Koesling D., Mayer B. Molecular mechanisms involved in the synergistic activation of soluble guanylyl cyclase by YC-1 and nitric oxide in endothelial cells. Mol. Pharmacol. 2001;59:220–224. doi: 10.1124/mol.59.2.220. [DOI] [PubMed] [Google Scholar]
- 34.Brunner F., Schmidt K., Nielsen E. B., Mayer B. Novel guanylyl cyclase inhibitor potently inhibits cyclic GMP accumulation in endothelial cells and relaxation of bovine pulmonary artery. J. Pharmacol. Exp. Ther. 1996;277:48–53. [PubMed] [Google Scholar]
- 35.Schröder H. Cytochrome-P-450 mediates bioactivation of organic nitrates. J. Pharmacol. Exp. Ther. 1992;262:298–302. [PubMed] [Google Scholar]
- 36.Keung W. M., Klyosov A. A., Vallee B. L. Daidzin inhibits mitochondrial aldehyde dehydrogenase and suppresses ethanol intake of Syrian golden hamsters. Proc. Natl. Acad. Sci. U.S.A. 1997;94:1675–1679. doi: 10.1073/pnas.94.5.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sidhu R. S., Blair A. H. Human liver aldehyde dehydrogenase. Esterase activity. J. Biol. Chem. 1975;250:7894–7898. [PubMed] [Google Scholar]
- 38.Bellamy T. C., Wood J., Garthwaite J. On the activation of soluble guanylyl cyclase by nitric oxide. Proc. Natl. Acad. Sci. U.S.A. 2002;99:507–510. doi: 10.1073/pnas.012368499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Feldman R. I., Weiner H. Horse liver aldehyde dehydrogenase. I. Purification and characterization. J. Biol. Chem. 1972;247:260–266. [PubMed] [Google Scholar]
- 40.Eckfeldt J., Mope L., Takio K., Yonetami T. Horse liver aldehyde dehydrogenase. Purification and characterization of two isozymes. J. Biol. Chem. 1976;251:236–240. [PubMed] [Google Scholar]
- 41.MacGibbon A. K., Haylock S. J., Buckley P. D., Blackwell L. F. Kinetic studies on the esterase activity of cytoplasmic sheep liver aldehyde dehydrogenase. Biochem. J. 1978;171:533–538. doi: 10.1042/bj1710533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Senior D. J., Tsai C. S. Purification and characterization of aldehyde dehydrogenase from rat liver mitochondria. Arch. Biochem. Biophys. 1988;262:211–220. doi: 10.1016/0003-9861(88)90183-x. [DOI] [PubMed] [Google Scholar]
- 43.Yoshida A., Rzhetsky A., Hsu L. C., Chang C. Human aldehyde dehydrogenase gene family. Eur. J. Biochem. 1998;251:549–557. doi: 10.1046/j.1432-1327.1998.2510549.x. [DOI] [PubMed] [Google Scholar]
- 44.Kelson T. L., Secor McVoy J. R., Rizzo W. B. Human liver fatty aldehyde dehydrogenase: microsomal localization, purification, and biochemical characterization. Biochim. Biophys. Acta. 1997;1335:99–110. doi: 10.1016/s0304-4165(96)00126-2. [DOI] [PubMed] [Google Scholar]
- 45.Schrammel A., Behrends S., Schmidt K., Koesling D., Mayer B. Characterization of 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one as a heme-site inhibitor of nitric oxide-sensitive guanylyl cyclase. Mol. Pharmacol. 1996;50:1–5. [PubMed] [Google Scholar]
- 46.Pfeiffer S., Schmidt K., Mayer B. Dityrosine formation outcompetes tyrosine nitration at low steady-state concentrations of peroxynitrite – implications for tyrosine modification by nitric oxide/superoxide in vivo. J. Biol. Chem. 2000;275:6346–6352. doi: 10.1074/jbc.275.9.6346. [DOI] [PubMed] [Google Scholar]
- 47.Schrammel A., Gorren A. C. F., Schmidt K., Pfeiffer S., Mayer B. S-nitrosation of glutathione by nitric oxide, peroxynitrite, and •NO/O2•−. Free Radical Biol. Med. 2003;34:1078–1088. doi: 10.1016/s0891-5849(03)00038-8. [DOI] [PubMed] [Google Scholar]
- 48.Gerzer R., Böhme E., Hofmann F., Schultz G. Soluble guanylate cyclase purified from bovine lung contains heme and copper. FEBS Lett. 1981;132:71–74. doi: 10.1016/0014-5793(81)80429-2. [DOI] [PubMed] [Google Scholar]
- 49.Stone J. R., Marletta M. A. Soluble guanylate cyclase from bovine lung – activation with nitric oxide and carbon monoxide and spectral characterization of the ferrous and ferric states. Biochemistry. 1994;33:5636–5640. doi: 10.1021/bi00184a036. [DOI] [PubMed] [Google Scholar]
- 50.Krebs H. A., Henseleit K. Untersuchungen über die Harnstoffbildung im Tierkörper. Hoppe-Seyler's Z. Physiol. Chem. 1932;210:33–36. [Google Scholar]