Abstract
Background
Dexmedetomidine is increasingly used for surgical patients requiring general anaesthesia. However, its effectiveness on patient-centred outcomes remains uncertain. Our main objective was to evaluate the patient-centred effectiveness of intraoperative dexmedetomidine for adult patients requiring surgery under general anaesthesia.
Methods
We conducted a systematic search of MEDLINE, Embase, CENTRAL, Web of Science, and CINAHL from inception to October 2023. Randomised controlled trials (RCTs) comparing intraoperative use of dexmedetomidine with placebo, opioid, or usual care in adult patients requiring surgery under general anaesthesia were included. Study selection, data extraction, and risk of bias assessment were performed by two reviewers independently. We synthesised data using a random-effects Bayesian regression framework to derive effect estimates and the probability of a clinically important effect. For continuous outcomes, we pooled instruments with similar constructs using standardised mean differences (SMDs) and converted SMDs and credible intervals (CrIs) to their original scale when appropriate. We assessed the certainty of evidence using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) methodology. Our primary outcome was quality of recovery after surgery. To guide interpretation on the original scale, the Quality of Recovery-15 (QoR-15) instrument was used (range 0–150 points, minimally important difference [MID] of 6 points).
Results
We identified 49,069 citations, from which 44 RCTs involving 5904 participants were eligible. Intraoperative dexmedetomidine administration was associated with improvement in postoperative QoR-15 (mean difference 9, 95% CrI 4–14, n=21 RCTs, moderate certainty of evidence). We found 99% probability of any benefit and 88% probability of achieving the MID. There was a reduction in chronic pain incidence (odds ratio [OR] 0.42, 95% CrI 0.19–0.79, n=7 RCTs, low certainty of evidence). There was also increased risk of clinically significant hypotension (OR 1.98, 95% CrI 0.84–3.92, posterior probability of harm 94%, n=8 RCTs) and clinically significant bradycardia (OR 1.74, 95% CrI 0.93–3.34, posterior probability of harm 95%, n=10 RCTs), with very low certainty of evidence for both. There was limited evidence to inform other secondary patient-centred outcomes.
Conclusions
Compared with placebo or standard of care, intraoperative dexmedetomidine likely results in meaningful improvement in the quality of recovery and chronic pain after surgery. However, it might increase clinically important bradycardia and hypotension.
Systematic Review Protocol
PROSPERO (CRD42023439896).
Keywords: adult anaesthesia, chronic postsurgical pain, clinical pharmacology, dexmedetomidine, opioid minimisation strategies, pain management, patient-centred outcomes
Editor's key points.
-
•
Dexmedetomidine is a popular adjunct for intraoperative management of patients undergoing major surgery and was effective at improving pain scores and reducing opioid administration in previous systematic reviews. However, evidence to support improved patient-centred outcomes is lacking.
-
•
This systematic review team actively involved patient partners in development of their review. They used Bayesian modelling to directly estimate the probability of an effect of dexmedetomidine being credible based on prior knowledge and the observed data.
-
•
In their pooled analyses, intraoperative dexmedetomidine likely provided substantial benefit in terms of quality of recovery and potentially reduced the incidence of postoperative chronic pain. However, the credibility of evidence was moderate to low.
-
•
Future high-quality RCTs should include patient-centred outcomes and clinically important adverse events to confirm patient-centred effectiveness and safety of intraoperative dexmedetomidine.
Since its approval in 1999 for sedation of critically ill patients in the intensive care unit, dexmedetomidine, an alpha-2 adreneric receptor agonist, has been increasingly used as an off-label co-analgesic during surgery to complement general anaesthesia.1, 2, 3, 4, 5 Dexmedetomidine can reduce short-term opioid use6,7 and some adverse effects related to opioids (i.e. nausea and vomiting and respiratory depression). It is thus considered an opioid minimisation strategy.8, 9, 10, 11 However, adverse effects have been observed, and net clinical benefit for surgical patients remains uncertain.12,13
Evaluation of patient-centred outcome measures (i.e. outcomes important to patients and caregivers) can provide a holistic perspective on the overall effectiveness and impact of perioperative interventions. Patient-centred outcomes incorporate core domains that can drive evidence-based strategies to minimise opioid utilisation, including pain impact on function and daily living.14, 15, 16, 17, 18, 19, 20 Prominent perioperative national and international expert consensus statements21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31 such as the American Society for Enhanced Recovery and Perioperative Quality Initiative Joint Consensus Statement32,33 emphasised the importance of patient-centred outcomes such as the quality of recovery after surgery in the assessment of opioid minimisation strategies.34 Importantly, our recent scoping review of randomised controlled trials (RCTs) evaluating potential opioid minimisation strategies in which patient-centred outcomes were assessed indicated that dexmedetomidine was among the most promising pharmacologic strategies for intraoperative use.35
Previous systematic reviews and RCTs have evaluated the effectiveness of dexmedetomidine using unidimensional (e.g. VAS for pain intensity) or indirect (e.g. short-term opioid exposure) endpoints without direct patient-centred outcome assessment, providing results that were often inconsistent and of limited applicability.36, 37, 38 Similar limitations (i.e. lack of patient-centred outcomes) are also encountered in international guidelines39, 40, 41 that inform the use of opioids and opioid minimisation strategies.41 To address this knowledge gap, our objective was to estimate the magnitude and certainty of intraoperative dexmedetomidine's effect on patient-centred outcomes, such as the quality of recovery and chronic pain after surgery, among adult patients requiring surgery under general anaesthesia.
Methods
We performed a systematic review and Bayesian meta-analysis following the recommendations from the Cochrane Handbook for Systematic Reviews of Interventions.42 Our review is reported in accordance to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 statement and the WAMBS-checklist for Bayesian analysis.43, 44, 45 We registered our systematic review in PROSPERO (CRD42023439896) and published our protocol before data analyses.46 As detailed in our protocol46 and in a separate reflective article,47 we used a collaborative approach involving a multidisciplinary team, patient partners, and multiple partner organisations across all steps of this systematic review (Supplementary Table S1). Our patient engagement approach is reported according to the Guidance for Reporting Involvement of Patients and the Public (GRIPP2-2) (Supplementary Table S2).48
Information sources and search strategy
Our search strategy was used in our scoping review inclusive of all opioid minimisation strategies (Supplementary Appendix S1).35,37 We systematically searched MEDLINE, Embase, CENTRAL, Web of Science, and CINAHL from inception to October 17, 2023, for relevant key terms with no language or date restrictions. The search strategy was developed following the Peer Review of Electronic Search Strategies (PRESS) recommendations and was peer-reviewed independently by two information specialists.45
Study selection
We included peer-reviewed RCTs assessing the effectiveness of systemic dexmedetomidine compared with placebo, opioids, or usual standard of care. We focused on RCT evidence considering our study question focused on determining the effectiveness of an intervention and RCTs (conducted in a low risk of bias manner) offer the highest level of evidence to inform the use of an intervention.49 From our previous scoping review results, we observed that a substantial number of RCTs were potentially eligible and available for inclusion in the current systematic review.35 To be considered eligible, dexmedetomidine had to be initiated during the intraoperative period (i.e. day of surgery before the patient is in the PACU). RCTs were included if they reported at least one patient-centred outcome after the PACU phase of care.50 We defined and classified patient-centred outcomes based on the Standardised Endpoints in Perioperative Medicine initiative (StEP-COMPAC) recommendations, and examples can be found in Supplementary Table S3.50 In addition, we included long-term opioid use (including opioid substance use disorder or overdose), opioid-related adverse effects (multidimensional assessment), acute pain (multidimensional assessment <3 months), and postoperative chronic pain (≥3 months) as patient-centred outcomes.47 RCTs published in suspected predatory journals (i.e. not peer-reviewed) were excluded. A predatory journal was defined as an open-access journal that is not registered or in the Directory of Open Access Journals (DOAJ, https://doaj.org/) or in the Committee on Publication Ethics (COPE, https://publicationethics.org/).51,52
Data extraction
After removing duplicates in Zotero (https://www.zotero.org), we imported the citations into InsightScope, an online software tool designed to facilitate large reviews (https://insightscope.tech/).53 We performed study selection and data abstraction independently and in duplicate and resolved conflicts with a third senior reviewer when needed (MV, JBPL, RR, and HL).53 We used a standardised data extraction form, informed by our multidisciplinary team, which included the following: (1) study design characteristics, (2) study methods, (3) patient eligibility criteria, (4) surgery, (5) the type of anaesthesia and analgesia, (6) pain intensity associated with the procedure, and (7) the source of funding.46
Outcomes
Our primary outcome was postoperative quality of recovery, regardless of the measurement instrument used (see Supplementary Table S3 for examples).54 When multiple time points were available, we prioritised the 24-h time point or the closest time point available. If multiple quality of recovery instruments were reported, we prioritised the Quality of Recovery-15 (score between 0 and 150, minimally important difference [MID] ≥6 points)55 as this valid and reliable instrument56 is recommended by the StEP-COMPAC group (patient-centred and patient comfort consensus)50,57 and also highly ranked by our multidisciplinary team including patient partners.46,47
We assessed other patient-centred outcomes, such as well-being (i.e. other than quality of recovery such as sleep quality), patient function, health-related quality of life, life impact, acute pain (multidimensional assessment), chronic pain (clinically significant benefit defined as OR <0.70), opioid-related adverse events (multidimensional assessments) and chronic opioid use (see Supplementary Table S3 for examples).50,58
We assessed non-patient-centred outcomes including hospital and PACU lengths of stay, adverse events including delirium, non-fatal/fatal cardiac arrest, mortality, respiratory depression, and adverse events requiring intervention (i.e. bradycardia, hypotension, tachycardia, and hypertension).59,60
Risk of bias
We (MV, JBPL, RR, and HL) assessed the domain-specific and overall risk of bias in duplicate using the Cochrane Collaboration Risk of Bias (RoB 2.0) tool.61, 62, 63 The risk of bias assessment was outcome-oriented using our three outcome categories: quality of recovery, other patient-centred outcomes, and additional outcomes separately.61
Data synthesis and analyses
For each outcome measure reported, we conducted a random-effects Bayesian meta-analysis.64, 65, 66 Bayesian modelling directly estimates the probability of an effect (or a certain effect size) being credible based on a combination of prior knowledge and the observed data, in contrast to a frequentist approach that estimates the probability of the observed data (e.g. P-value-based null hypothesis testing). As such, a Bayesian statistical approach can facilitate translation of research findings into practice67, 68, 69, 70 and guidelines.71,72 We pooled instruments with similar constructs using standardised mean differences (SMDs) and their 95% credible intervals (CrIs).73 For our primary analysis, we assessed the effect of dexmedetomidine on the quality of recovery. We used two levels hierarchical models (i.e. participants nested within study variation and between study variation) to estimate the SMDs and corresponding 95% CrIs using linear regression (Supplementary Appendix S2). We also converted SMDs to their original scale (i.e. QoR-15) to facilitate clinical interpretation (i.e. multiplication of the pooled SMD by the QoR-15 mean SD across intervention groups of included RCTs [SD 10.87]).74 We calculated the probability of any benefit/harm using the highest density probability interval of the posterior distribution and the probability of achieving a mean difference (MD) as large or larger than the MID using the empirical cumulative distribution function (ECDF). The same approach was used for pooling secondary outcomes; modelling continuous data with mean differences (or SMDs if more than one instrument sharing similar constructs) and standard errors (i.e. linear regression) and binary data with odds ratio (logarithmic scale) and standard errors along with 95% CrIs. An SMD equal to or larger than 0.2 was considered clinically significant.42 We used weak informative prior distributions for effect size estimation of continuous and binary outcomes, and we conducted sensitivity analyses using non-informative priors (see Supplementary Appendix S2 for priors and model specifications). We estimated the heterogeneity distribution using tau (τ; prior distribution: half-Cauchy ∼0, 0.5).75, 76, 77 We interpreted τ as reasonable (between 0.1 and 0.5), fairly high (between 0.5 and 1.0), or fairly extreme (higher than 1.0).65,78 We assessed potential publication bias with funnel plots when more than 10 RCTs were included in a meta-analysis.
Meta-regressions
For each patient-centred outcome measure that was reported in 10 or more RCTs,42 we performed meta-regressions to explore sources of heterogeneity, namely, type of surgery (high risk of chronic pain vs not),79 population (older adults [≥65 yr] vs younger; chronic pain or opioid use at baseline), type of dexmedetomidine administration (bolus vs bolus and infusion vs infusion vs target-controlled infusion [i.e. incorporation of the drug pharmacokinetic profile in the infusion pump to estimate the desired plasma or effect site concentration]), the dosage of dexmedetomidine (concentration and duration), risk of bias of RCTs with available data (low, high, or some concerns), mean pain intensity in the control group (low vs moderate or severe [≥4 on a 10-point scale]), and type of funding (academic, industry, or academic and industry).80
Model diagnostics and code
Bayesian meta-analyses and posterior distribution results were calculated using the brms package in R version 4.3.2 (R Foundation for Statistical Computing, Vienna, Austria).66 We implemented our sampling procedure using the Markov chain Monte Carlo-based method, with an iteration phase of 6000 and a warm-up phase of 2000 iterations, followed by four chains as the initial approach.81 We assessed model convergence and validity using the Rhat values (<1.01 for adequate convergence) and potential scale reduction factor (PSFR) approach.82,83 We also assessed visual mixing of the chains (density plots), marginal posterior distribution of parameters (i.e. similarity between observed and predicated data), and autocorrelation using lag plots.
Certainty assessment
To determine confidence in the estimates, for each outcome, we assessed the certainty of evidence in duplicate using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) working group methodology.84
Results
Study characteristics and study selection
We identified 49,069 citations from our search, of which 46 RCTs involving 6024 participants (range 39–798 participants per RCT) were included in our systematic review. Some results could not be included in meta-analyses (reasons provided in Supplementary Table S4), and a total of 44 RCTs involving 5904 participants were included in our meta-analyses (Fig. 1).
Fig 1.
Flow diagram. RCT, randomised controlled trial.
Most RCTs were conducted in one centre (n=40; 3912 participants), and 13% were multicentre RCTs (n=6; 2112 participants). The mean age of participants was 56 (range: 27 -72) yr, and 51% were female (Supplementary Table S5). The most common types of surgery within RCTs were gastrointestinal surgery (24%, n=11; 742 participants), noncardiac thoracic surgery (17%, n=8; 726 participants), and obstetrics-gynaecological surgery (13%, n=6; 405 participants). The comparator was placebo in 91% of RCTs (n=42; 5774 participants), opioids in 7% (n=3; 186 participants), and usual care in 2% (n=1; 64 participants). Dexmedetomidine was administered as a bolus (i.e. single dose) in 17% of RCTs (n=8; 712 participants), an infusion in 35% of RCTs (n=16; 1963 participants), and using a combination of a bolus followed by an infusion in 48% of RCTs (n=22; 3349 participants). In 17% of RCTs (n=8; 1884 participants), dexmedetomidine administration was continued during the postoperative period. The median duration of infusion was 2.3 (interquartile range [IQR] 1.7–3.9) h, and the median dose of the bolus and intraoperative infusion were 0.5 (IQR 0.5–1.0) μg kg−1 and 0.5 (IQR 0.4–0.5) μg kg−1 h−1, respectively. No RCTs used a target-controlled infusion to administer the intervention or the control, and only five RCTs used a variable dose according to surgery duration or allowed dosing regimen adjustments according to the anaesthesiologist. Stratification for sex was performed in 15% of RCTs (n=7; 1436 participants). A summary of characteristics of included RCTs is provided in Supplementary Table S5. Results from RCTs that could not be pooled are summarised in Supplementary Table S4. Authors were contacted to provide detail on quantitative results of quality of recovery,85 sleep quality,86 quality of life,86 acute pain,87 and chronic pain,86 but no responses were obtained.
Risk of bias
For the 25 RCTs in which the primary outcome (quality of recovery) was reported, the overall risk of bias was considered low for 40% (n=10), unclear for 52% (n=13), and high for 8% (n=2). The overall risk of bias for other patient-centred outcomes (n=23) was low for 22% (n=5), unclear for 65% (n=15), and high for 13% (n=3). The overall risk of bias for the clinically important adverse events and lengths of stay was low for 19% (n=7), unclear for 68% (n=25), and high for 13% (n=5) (Supplementary Tables S6–S8 and Figs S1–S3).
Primary outcome
Quality of recovery
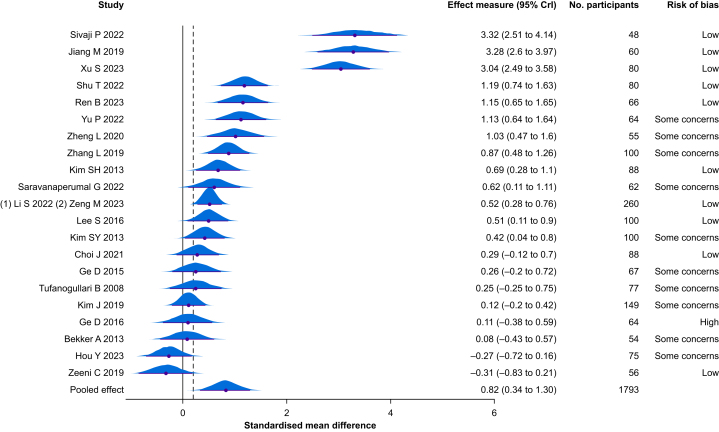
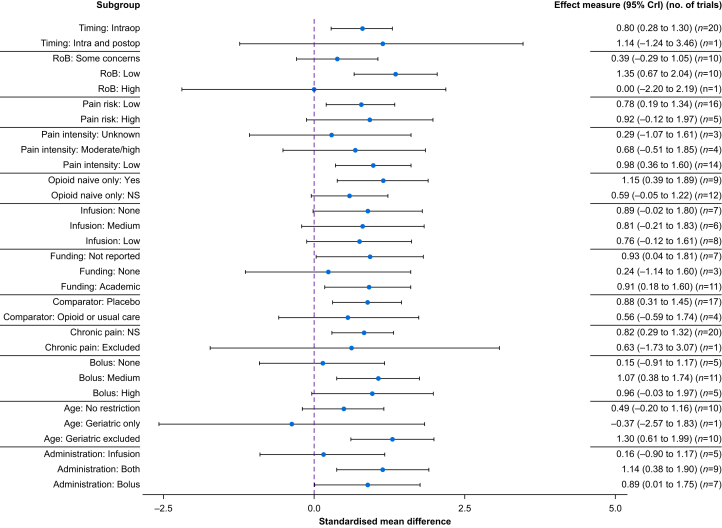
Among the 25 RCTs that assessed the quality of recovery, 21 RCTs (n=1793 participants) were pooled (i.e. outcome measure with the same or similar construct). The quality of recovery instruments pooled were the Quality of Recovery-9 (n=1), Quality of Recovery-15 (n=5), and Quality of Recovery-40 (n=15). The timing of evaluation was 24 h after surgery except for two RCTs that reported a later time point.85,88 The estimated mean increase in the quality of recovery was 0.82 SMD units (95% CrI 0.34–1.30, Tau=1.12). Results were similar when using a non-informative prior (SMD 0.86, 95% CrI 0.34–1.38, Tau=1.17) (Supplementary Table S9 and Appendix S4). Following conversion to the original scale (QoR-15), the mean increase in the quality of recovery was 9 points (95% CrI 4–14) (Table 1 and Fig. 2).74 The posterior probability of any benefit (i.e. probability that there is a difference between groups in favour of dexmedetomidine [MD >0]) from dexmedetomidine on the quality of recovery was 99%, and the probability of a clinically significant benefit (MD >6 units on the QoR-15 scale) was 88%.55 After removing three RCTs with extreme results (i.e. >3 times the pooled SMD), the posterior probability of a clinically significant benefit (MD >6 units on QoR-15 scale) was 81%. The certainty of evidence was moderate (Table 1). Based on forest plot visual inspection and meta-regression coefficients (Fig. 3), some factors may partly explain heterogeneity, such as the risk of bias and the type of population. RCTs at lower risk of bias reported larger effect sizes from dexmedetomidine, and there was an increased effect size when used among patients <65 yr old. A possible effect from the dosage regimen (the use of combined bolus and infusion were associated with increased effect size) was also noted. The duration of infusion (in hours) had no strong impact on the quality of recovery based on meta-regression (SMD 0.02, 95% CrI –0.07 to 0.10).
Table 1.
Summary estimates from Bayesian meta-analyses with the assessment of the certainty of the evidence. CI, confidence interval; CrI, credible interval; MD, mean difference; NA, not available; OR, odds ratio; RCT, randomized controlled trial; SMD, standardised mean difference. ∗Calculated with the empirical cumulative distribution function. †Conversion of pooled quality of recovery results in the QoR-15 unit by multiplying the pooled SMD by the QoR-15 mean standard deviation across intervention groups of included RCTs. Score ranged between 0 (very poor recovery) and 150 (excellent recovery). ‡Because of inconsistency. Not downgraded for risk of bias given results from meta-regression (RCTs at lower risk of bias reported larger effect sizes from dexmedetomidine). ¶Because of risk of bias and imprecision. §Score ranged between 0 and 100, with lower score indicating increased disability. ||Because of risk of bias, inconsistency, and imprecision. #Because of risk of bias and inconsistency.
| Outcome | Relative effect (95% CrI or CI) | Posterior probability of any benefit (%)∗ | Number of RCTs | Number of participants |
Heterogeneity using Tau (95% CrI) | Certainty of the evidence (GRADE) | |
|---|---|---|---|---|---|---|---|
| Dexmedetomidine | Comparator | ||||||
| Primary | |||||||
| Quality of recovery† | MD 9 (95% CrI 4 to 14) | 99 | 21 | 908 | 885 | 1.12 (0.81–1.56) | Moderate‡ |
| Secondary | |||||||
| Health-related quality of life (Short-Form score) | SMD 0.05 (95% CrI –0.61 to 0.72) | 46 | 2 | 209 | 219 | 0.40 (0.01–1.36) | Low¶ |
| Life impact (Barthel Index)§ | MD –5 (95% CI: –5 to 0) | NA | 1 | 55 | 55 | NA | Very low|| |
| Sleep quality (Numerical Rating Scale) | MD –0.6 (95% CrI –1.3 to 0.1) | 94 | 7 | 858 | 854 | 0.89 (0.47–1.60) | Very low|| |
| Chronic pain incidences | OR 0.42 (95% CrI 0.18 to 0.79) | 99 | 7 | 692 | 683 | 0.77 (0.23–1.60) | Low# |
| Mortality | OR 0.70 (95% CrI 0.37 to 1.21) | 93 | 10 | 1254 | 1261 | 0.36 (0.01–1.12) | Low¶ |
| Delirium | OR 0.62 (95% CrI 0.39 to 0.92) | 99 | 12 | 1457 | 1459 | 0.52 (0.23–0.95) | Very low|| |
| Bradycardia requiring an intervention | OR 1.74 (95% CrI 0.93 to 3.34) | 5 | 10 | 1106 | 888 | 0.62 (0.08–1.49) | Very low|| |
| Hypotension requiring an intervention | OR 1.98 (95% CrI 0.84 to 3.92) | 6 | 8 | 1026 | 808 | 0.91 (0.40–1.72) | Very low|| |
| Tachycardia requiring an intervention | OR 0.80 (95% CrI 0.25 to 2.37) | 80 | 2 | 195 | 196 | 0.65 (0.03–1.91) | Very low|| |
| Hypertension requiring an intervention | OR 0.81 (95% CrI 0.25 to 2.15) | 80 | 2 | 180 | 180 | 0.62 (0.02–1.90) | Very low|| |
Fig 2.
Forest plot for quality of recovery outcome measure. The black vertical line indicates a null value (standardised mean difference = 0), and the grey dashed vertical line indicates the threshold for minimally important difference. A value above zero (right direction) favours dexmedetomidine. A value below zero (left direction) favours control. CrI, credible interval.
Fig 3.
Forest plot showing meta-regression results for quality of recovery outcome measure. Blue dashed vertical line indicates a null value (standardised mean difference = 0). Values above zero (right direction) favours dexmedetomidine. Moderate/high pain intensity: mean pain intensity of 4 or more on a 10-point scale in the control group. Surgeries considered at high risk of chronic pain were as follows: mastectomy, thoracotomy, amputation, arthroplasty, Caesarean, cholecystectomy, craniotomy, hip replacement, inguinal hernia repair, spinal surgery, coronary artery bypass graft, trauma, and burn. CrI, credible interval; NS, not specified; RoB, risk of bias.
Other patient-centred outcomes
Life impact
There was little evidence that dexmedetomidine improved patient independence after surgery (MD –5, 95% confidence interval [CI] –5 to 0, n=1 RCT evaluated using the Barthel score).89 The certainty of evidence was very low (Table 1 and Supplementary Appendix S3).
Patient health-related quality of life
Pooled estimates suggested no strong benefit from dexmedetomidine on health-related quality of life after surgery (SMD 0.05, 95% CrI –0.61 to 0.72, Tau=0.40, n=2 RCTs). The posterior probability of any benefit was 46% (combining Short Form-8 [SF-8] and SF-12). The certainty of evidence was low (Table 1 and Supplementary Appendix S3).
Multidimensional acute pain
One RCT evaluated the effect of dexmedetomidine on DN4 (i.e. Neuropathic Pain Detection Questionnaire) and found no impact (i.e. absence of neuropathic pain in all intervention and control arms).87 No RCTs reported multidimensional acute pain assessment.
Well-being (sleep quality)
The posterior probability of any benefit from dexmedetomidine on sleep quality was 94% (MD –1 [Numerical Rating Scale 0–10, where lower is better], 95% CrI –1 to 0, τ=0.89, n=7 RCTs, low certainty of evidence) (Table 1 and Supplementary Appendix S3).
Postoperative function
No RCTs reported data on postoperative function.
Chronic pain
The posterior probability of any benefit (OR <1) on chronic pain incidence was 99%, and the posterior probability of clinically significant benefit (OR <0.70) was 95% (OR 0.42, 95% CrI 0.19–0.79, Tau=0.77, n=7 RCTs, low certainty of evidence) (Table 1 and Supplementary Appendix S3).
Other outcomes
The posterior probability of any harm on hypotension requiring an intervention was 94% (OR 1.98, 95% CrI 0.84–3.92, Tau=0.91, n=8 RCTs, very low certainty of evidence), and it was 95% for bradycardia requiring an intervention (OR 1.74, 95% CrI 0.93–3.34, Tau=0.62, n=10 RCTs, very low certainty of evidence). The posterior probability of any benefit on mortality was 93%, (OR 0.70, 95% CrI 0.37–1.21, Tau=0.36, n=10 RCTs, low certainty of evidence). The posterior probability of any benefit on hypertension requiring an intervention was 80% (OR 0.81, 95% CrI 0.25–2.15, Tau=0.62, n=2 RCTs, very low certainty of evidence), and the posterior probability of any benefit on tachycardia requiring an intervention was 80% (OR 0.80, 95% CrI 0.25–2.37, Tau=0.65, n=2 RCTs, very low certainty of evidence).
The posterior probability of any benefit on postoperative delirium was 99% (OR 0.62, 95% CrI 0.39–0.92, Tau=0.52, n=12 RCTs, very low certainty of evidence). There was no effect from dexmedetomidine on the odds of respiratory depression (OR 1.14, 95% CrI 0.43–2.48, Tau=0.51, n=10 RCTs, posterior probability of any benefit 47%). No RCTs reported results on chronic opioid use, fatal/non-fatal cardiac arrest, and opioid-related adverse effects (multidimensional assessment) outcome measures.
Length of PACU and hospital stay
The posterior probability for dexmedetomidine increasing PACU duration was 88% (MD 1 min, 95% CrI –1 to 2, Tau=1.8, n=16 RCTs), and the posterior probability of a reduction in hospital length of stay was 92% (MD−0.2 days, 95%CrI: −0.5 to 0.1, Tau=0.33, n=21 RCTs).
Publication biases
For all meta-analyses including more than 10 RCTs, we found no evidence of publication bias based on visual assessment of funnel plots (Supplementary Figs S4–S8).
Model diagnosis
All our models converged adequately with Rhat <1.01 (i.e. representative regions were explored by the chains) and adequate marginal posterior distributions (i.e. results from statistical models resembled the observed data). We also obtained good mixing of the Markov chains based on the trace plots, and there was no autocorrelation (i.e. dependency among Markov chain samples) except for the primary outcome model (i.e. quality of recovery) and for health-related quality of life that was resolved with increased thinning (i.e. subsampling each chain of simulation generated by the model) while providing similar effect estimates (thin=5). Density plots, trace plots, posterior check, and autocorrelation figures are provided in Supplementary Appendix S4. Results were similar whether a weak informative or non-informative prior was used (Supplementary Table S9).
Protocol deviation
All diagnostic tests (convergence, replication, and density) were satisfactory (Supplementary Appendix S4). Although we planned to explore the impact of the dose (continuous variable) of dexmedetomidine administration on our primary outcome (i.e. quality of recovery), we decided to categorise this independent variable given the overall limited range of doses used (i.e. low, 0–0.49 μg kg−1; medium, 0.5–0.99 μg kg−1; and high, ≥1 μg kg−1).
Discussion
In this systematic review and Bayesian meta-analysis of RCTs, we found that dexmedetomidine likely provides a meaningful improvement in the quality of recovery after surgery among adult patients undergoing surgery requiring general anaesthesia.55 We also found a high probability that dexmedetomidine initiated during surgery improves postoperative chronic pain, sleep quality, and delirium incidence that was, however, supported by low to very low certainty of evidence. Regarding safety, dexmedetomidine may increase the risk of clinically significant hypotension and bradycardia. However, mortality and hospital length of stay were reduced with the use of dexmedetomidine, indicating that bradycardia and hypotension episodes likely had minimal impact on patients. There were either few RCTs or none reporting the effect of dexmedetomidine on life impact, health-related quality of life, postoperative acute pain (multidimensional assessment), long-term opioid use, fatal/non-fatal cardiac arrest, clinically significant tachycardia, clinically significant hypertension, and opioid-related adverse effects outcome measures.
Our findings are consistent with previous systematic reviews and meta-analyses that demonstrated potential benefits from the intraoperative use of dexmedetomidne.36, 37, 38 For instance, previous systematic reviews showed that dexmedetomidine is associated with a substantial decrease in perioperative inflammation (interleukin-6 mean reduction on the first postoperative day 42, 95% CI –57 to –26 pg ml−1),90 a moderate reduction in opioid use (sufentanil equivalent) during the first 24 h after surgery (MD –14 μg, 95% CI –19 to –9 μg),6,7 and a moderate reduction in nausea and vomiting (OR 0.56, 95% CI 0.46–0.69).8,9 However, previous reviews also noted an increased risk of adverse events (bradycardia and hypotension) for which the clinical significance was uncertain.91 It was thus unclear whether the intraoperative use of dexmedetomidine could provide a net benefit for surgical patients given the absence of holistic multidimensional assessment. As suggested by multiple international societies,24 patients with lived experience have identified priorities23,24,92 and guidelines such as the American Society for Enhanced Recovery and Perioperative Quality Initiative Joint Consensus32 and The International Association for the Study of Pain,23 the evaluation of patient-centred outcomes, such as quality of recovery, should be used to address this knowledge gap and guide clinical practice. Given the clinically meaningful impact that we observed from dexmedetomidine on the quality of recovery, our findings suggest that dexmedetomidine likely provides a net benefit for surgical patients undergoing surgery. Further high-quality RCTs are required to confirm this finding and establish safety.
We also identified important knowledge gaps in patient-centred outcomes that should be further evaluated in high-quality RCTs. We found that although the target-controlled infusion method is increasingly used in perioperative practice to administer medications such as dexmedetomidine,93 no eligible RCTs used such mode of delivery. Most RCTs used a fixed dose of dexmedetomidine as opposed to a dosing regimen that could be altered by the health care provider or according to the duration of surgery, and comparators reported were almost exclusively placebos. These study characteristics highlight a lack of pragmatic RCTs, aimed at evaluating an intervention in a real-world setting that is similar to the one in which the intervention will be implemented, to inform the intraoperative use of dexmedetomidine.94 Future RCTs should thus incorporate pragmatic study design components to improve applicability of the findings and help guide practice in terms of optimal dosing regimen and mode of delivery.
Our systematic review has several strengths. First, we used an innovative co-creation approach, actively involving partner organisations and patient partners throughout the research process, to determine the magnitude of a potential net benefit for the patients.46,47 To enhance applicability of our findings, we evaluated the clinical significance of our findings using established MID thresholds of an established multidimensional patient-reported outcome measure (i.e. quality of recovery), and we evaluated the certainty of evidence.74 Second, we implemented a Bayesian regression framework to allow modelling of heterogeneity, enable efficient use of available data, and enhance the interpretability of our results.75 This probabilistic approach further facilitates knowledge translation to practice given its intuitive interpretation for the end users and decision-makers.67, 68, 69, 70, 71, 72 Third, we implemented the StEP-COMPAC consensus recommendations for the evaluation of perioperative patient-centred outcomes,50 which was previously identified as an important knowledge gap.35 Such an approach represents a pragmatic strategy to identify promising complementary agents to opioids in the context of the worldwide opioid crisis.95
Our review had limitations. Firstly, although patient-centred outcomes are extremely important to guide clinical decision-making during general anaesthesia, additional outcomes might be relevant to clinicians. Our systematic review does not include RCTs that did not report at least one patient-centred outcome; thus, our assessment of harms might not be exhaustive. Secondly, our systematic review does not compare dexmedetomidine with other opioid minimisation strategies.
Conclusions
Our pooled analyses demonstrated that intraoperative systemic dexmedetomidine likely provides substantial benefit on the quality of recovery after surgery and potentially reduces postoperative chronic pain incidence among adult patients. Future high-quality RCTs should include patient-centred outcomes and clinically important adverse events to confirm patient-centred effectiveness and safety.
Authors’ contributions
Conceptualisation and study question: MV, JBPL, MML, DM, SN, AFT, BH, FZ, MG, ML, AG, MB, IG, PP, HD, GM, JM, HM, DF
Design and methodology: MV, DF, DM, MML, AFT, BH, FZ, MG, ML, AG, KOH, MJ
Development of search strategy: MV, DF, MML, AFT, RS
Outcome prioritisation: MV, JBPL, MML, DM, SN, AFT, BH, FZ, MG, ML, AG, MB, IG, PP, HD, GM, JM, HM, DF
Drafting the manuscript: MV, JBPL, DF, MML, MSJ
Critical revision of the manuscript: MV, JBPL, MML, DM, SN, AFT, BH, FZ, MG, ML, AG, MB, IG, PP, HD, GM, JM, HM, DF, KOH, MSJ
Design data extraction form: MV, JBPL, MML, DM, SN, AFT, BH, FZ, MG, ML, AG, MB, IG, PP, HD, GM, JM, HM, DF
Development of statistical analytical plan: MV, DF, DM, BH, MSJ
Statistical analyses: MV, MSJ
Visualisation: MV, JBPL, ML, DM, DF, MSJ
Project administration: MV
Lead patient engagement activities: MV, SN
Lead knowledge user partnership activities: MV, DF, MML
Reviewed the content of the manuscript and approved the final version: MV, JBPL, MML, MSJ, DM, SN, AFT, BH, FZ, MG, ML, AG, MB, IG, PP, HD, GM, JM, HM, DF
Acknowledgements
Our partner organisations were SolvingPain (https://www.solvingpain.ca), Pain BC (https://painbc.ca), Health Canada (https://www.canada.ca/en/health-canada.html), Réseau Québécois de Recherche sur la Douleur (https://qprn.ca/fr/), Choosing Wisely (https://choosingwiselycanada.org), the Ontario SPOR SUPPORT unit (https://ossu.ca), the Canadian Anaesthesia Society (https://www.cas.ca/en/home), the Canadian Chronic Pain Network (https://cpn.mcmaster.ca), and the Canadian Perioperative Anaesthesia Clinical Trials (PACT) group (https://canadianpact.ca). They helped refine the research question, provided input on the design of the study, and contributed to the development of the dissemination plan.
Declaration of interest
IG has received consulting fees from GW Research, Eupraxia, Biogen, and Novaremed. The other authors have no conflict of interest to declare.
Funding
MV is supported by the Vanier Canada Graduate Scholarship Program from the CIHR (CGV-475607), and the Fonds de Recherche du Québec–Santé (FRQS)/Ministère de la Santé et des Services Sociaux du Québec (MSSS) Resident Physician Health Research Training Program (phase 2). MML is supported by University of Ottawa Junior Clinical Research Chair, Canadian Anesthesiologist's Career Scientist Award, and the Ottawa Hospital Anesthesia Alternate Funds Association. MSJ is supported by the Vanier Canada Graduate Scholarship program from the CIHR (CGV-186957). AFT is the chairholder of the Canada Research Chair in Critical Care Neurology and Trauma. MB is the recipient of salary support awards from the FRQS and the Strategy for Patient-Oriented Research-Québec.
Data availability statement
The list of included trials is included in Supplementary Table S4. Additional meta-data can be provided upon request to the corresponding author.
Handling Editor: Kate Leslie
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bja.2024.06.007.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Brown E.N., Pavone K.J., Naranjo M. Multimodal general anesthesia: theory and practice. Anesth Analg. 2018;127:1246–1258. doi: 10.1213/ANE.0000000000003668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weatherall M., Aantaa R., Conti G., et al. A multinational, drug utilization study to investigate the use of dexmedetomidine (Dexdor®) in clinical practice in the EU. Br J Clin Pharmacol. 2017;83:2066–2076. doi: 10.1111/bcp.13293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paranjpe J.S. Dexmedetomidine: expanding role in anesthesia. Med J Dr D Y Patil Univ. 2013;6:5–13. [Google Scholar]
- 4.Precedex Approval Letter. In: Administration USoAFaD, ed. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/nda/99/21-038_Precedex_Approv.pdf1999. [Accessed 30 June 2023].
- 5.Shukry M., Miller J.A. Update on dexmedetomidine: use in nonintubated patients requiring sedation for surgical procedures. Ther Clin Risk Manag. 2010;6:111–121. doi: 10.2147/tcrm.s5374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feng M., Chen X., Liu T., Zhang C., Wan L., Yao W. Dexmedetomidine and sufentanil combination versus sufentanil alone for postoperative intravenous patient-controlled analgesia: a systematic review and meta-analysis of randomized controlled trials. BMC Anesthesiol. 2019;19:81. doi: 10.1186/s12871-019-0756-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu Y., Liang F., Liu X., Shao X., Jiang N., Gan X. Dexmedetomidine reduces perioperative opioid consumption and postoperative pain intensity in neurosurgery: a meta-analysis. J Neurosurg Anesthesiol. 2018;30:146–155. doi: 10.1097/ANA.0000000000000403. [DOI] [PubMed] [Google Scholar]
- 8.Weihong Z., Jianli L., Na W., et al. Effect of dexmedetomidine on postoperative nausea and vomiting in patients under general anaesthesia: an updated meta-analysis of randomised controlled trials. BMJ Open. 2023;13 doi: 10.1136/bmjopen-2022-067102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jin S., Liang D.D., Chen C., Zhang M., Wang J. Dexmedetomidine prevent postoperative nausea and vomiting on patients during general anesthesia: a PRISMA-compliant meta analysis of randomized controlled trials. Medicine. 2017;96 doi: 10.1097/MD.0000000000005770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gerlach A.T., Dasta J.F. Dexmedetomidine: an updated review. Ann Pharmacother. 2007;41:245–252. doi: 10.1345/aph.1H314. [DOI] [PubMed] [Google Scholar]
- 11.Giovannitti JA Jr, Thoms S.M., Crawford J.J. Alpha-2 adrenergic receptor agonists: a review of current clinical applications. Anesth Prog. 2015;62:31–39. doi: 10.2344/0003-3006-62.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jessen Lundorf L., Korvenius Nedergaard H., Møller A.M. Perioperative dexmedetomidine for acute pain after abdominal surgery in adults. Cochrane Database Syst Rev. 2016;2 doi: 10.1002/14651858.CD010358.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Santa Cruz Mercado L.A., Liu R., Bharadwaj K.M., et al. Association of intraoperative opioid administration with postoperative pain and opioid use. JAMA Surg. 2023;158:854–864. doi: 10.1001/jamasurg.2023.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kalkman C.J., Kappen T.H. Patient-centered endpoints for perioperative outcomes research. Anesthesiology. 2015;122:481–483. doi: 10.1097/ALN.0000000000000587. [DOI] [PubMed] [Google Scholar]
- 15.Fønhus M.S., Dalsbø T.K., Johansen M., Fretheim A., Skirbekk H., Flottorp S.A. Patient-mediated interventions to improve professional practice. Cochrane Database Syst Rev. 2018;9 doi: 10.1002/14651858.CD012472.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meissner W., Coluzzi F., Fletcher D., et al. Improving the management of post-operative acute pain: priorities for change. Curr Med Res Opin. 2015;31:2131–2143. doi: 10.1185/03007995.2015.1092122. [DOI] [PubMed] [Google Scholar]
- 17.Wijeysundera D.N., Johnson S.R. How much better is good enough?: patient-reported outcomes, minimal clinically important differences, and patient acceptable symptom states in perioperative research. Anesthesiology. 2016;125:7–10. doi: 10.1097/ALN.0000000000001159. [DOI] [PubMed] [Google Scholar]
- 18.Weldring T., Smith S.M.S. Patient-reported outcomes (PROs) and patient-reported outcome measures (PROMs) Health Serv Insights. 2013;6:61–68. doi: 10.4137/HSI.S11093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perfetto E.M., Pomerantz P. Listening to the patient voice and learning from the patient experience. ASA Monitor. 2021;85:27–29. [Google Scholar]
- 20.Boney O., Moonesinghe S.R., Myles P.S., et al. Core Outcome Measures for Perioperative and Anaesthetic Care (COMPAC): a modified Delphi process to develop a core outcome set for trials in perioperative care and anaesthesia. Br J Anaesth. 2022;128:174–185. doi: 10.1016/j.bja.2021.09.027. [DOI] [PubMed] [Google Scholar]
- 21.Campbell F., Hudspith M., Choinière M., et al. Canadian Pain Task Force Report: March 2021. 2022. https://www.canada.ca/en/health-canada/corporate/about-health-canada/public-engagement/external-advisory-bodies/canadian-pain-task-force/report-2021.html Available from:
- 22.Choosing Wisely Canada Opioid wisely. 2021. https://choosingwiselycanada.org/campaign/opioid-wisely/ Available from:
- 23.International Association for the Study of Pain Making sense of pain assessment after surgery. 2022. https://www.iasp-pain.org/publications/relief-news/article/making-sense-of-pain-assessment-after-surgery/ Available from:
- 24.Mariano E.R., Dickerson D.M., Szokol J.W., et al. A multisociety organizational consensus process to define guiding principles for acute perioperative pain management. Reg Anesth Pain Med. 2022;47:118–127. doi: 10.1136/rapm-2021-103083. [DOI] [PubMed] [Google Scholar]
- 25.Levy N., Quinlan J., El-Boghdadly K., et al. An international multidisciplinary consensus statement on the prevention of opioid-related harm in adult surgical patients. Anaesthesia. 2021;76:520–536. doi: 10.1111/anae.15262. [DOI] [PubMed] [Google Scholar]
- 26.McKeen D.M., Banfield J.C., McIsaac D.I., et al. Top ten priorities for anesthesia and perioperative research: a report from the Canadian Anesthesia Research Priority Setting Partnership. Can J Anaesth. 2020;67:641–654. doi: 10.1007/s12630-020-01607-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gewandter J.S., Smith S.M., Dworkin R.H., et al. Research approaches for evaluating opioid sparing in clinical trials of acute and chronic pain treatments: Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials recommendations. Pain. 2021;162:2669–2681. doi: 10.1097/j.pain.0000000000002283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aslakson R.A., Weiss M. Patient-centered outcomes research—opportunities for novel, innovative, and transformative partnerships with patients and their families. JAMA Surg. 2016;151:945–946. doi: 10.1001/jamasurg.2016.1321. [DOI] [PubMed] [Google Scholar]
- 29.Pogatzki-Zahn E.M., Liedgens H., Hummelshoj L., et al. Developing consensus on core outcome domains for assessing effectiveness in perioperative pain management: results of the PROMPT/IMI-PainCare Delphi Meeting. Pain. 2021;162:2717–2726. doi: 10.1097/j.pain.0000000000002254. [DOI] [PubMed] [Google Scholar]
- 30.Srivastava D., Hill S., Carty S., et al. Surgery and opioids: evidence-based expert consensus guidelines on the perioperative use of opioids in the United Kingdom. Br J Anaesth. 2021;126:1208–1216. doi: 10.1016/j.bja.2021.02.030. [DOI] [PubMed] [Google Scholar]
- 31.van den Heuvel S.A., van Boekel R.L., Cox F.J., et al. Perioperative pain management models in four European countries: a narrative review of differences, similarities and future directions. Eur J Anaesthesiol. 2024;41:188–198. doi: 10.1097/EJA.0000000000001919. [DOI] [PubMed] [Google Scholar]
- 32.Abola R.E., Bennett-Guerrero E., Kent M.L., et al. American society for enhanced recovery and perioperative quality initiative joint consensus statement on patient-reported outcomes in an enhanced recovery pathway. Anesth Analg. 2018;126:1874–1882. doi: 10.1213/ANE.0000000000002758. [DOI] [PubMed] [Google Scholar]
- 33.Wu C.L., King A.B., Geiger T.M., et al. American society for enhanced recovery and perioperative quality initiative joint consensus statement on perioperative opioid minimization in opioid-naïve patients. Anesth Analg. 2019;129:567–577. doi: 10.1213/ANE.0000000000004194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Frank L., Basch E., Selby J.V., Patient-Centered Outcomes Research Institute The PCORI perspective on patient-centered outcomes research. JAMA. 2014;312:1513–1514. doi: 10.1001/jama.2014.11100. [DOI] [PubMed] [Google Scholar]
- 35.Verret M., Lam N.H., Lalu M., et al. Intraoperative pharmacologic opioid minimisation strategies and patient-centred outcomes after surgery: a scoping review. Br J Anaesth. 2024;32:758–770. doi: 10.1016/j.bja.2024.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miao M., Xu Y., Li B., Chang E., Zhang L., Zhang J. Intravenous administration of dexmedetomidine and quality of recovery after elective surgery in adult patients: a meta-analysis of randomized controlled trials. J Clin Anesth. 2020;65 doi: 10.1016/j.jclinane.2020.109849. [DOI] [PubMed] [Google Scholar]
- 37.Verret M., Lam N.H., Fergusson D.A., et al. Intraoperative pharmacologic opioid minimisation strategies and patient-centred outcomes after surgery: a scoping review protocol. BMJ Open. 2023;13 doi: 10.1136/bmjopen-2022-070748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baamer R.M., Iqbal A., Lobo D.N., Knaggs R.D., Levy N.A., Toh L.S. The utility of unidimensional and functional pain assessment tools in adult postoperative patients: a systematic review. Br J Anaesth. 2022;128:874–888. doi: 10.1016/j.bja.2021.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Laserna A., Rubinger D.A., Barahona-Correa J.E., et al. Levels of evidence supporting the North American and European perioperative care guidelines for Anesthesiologists between 2010 and 2020: a systematic review. Anesthesiology. 2021;135:31–56. doi: 10.1097/ALN.0000000000003808. [DOI] [PubMed] [Google Scholar]
- 40.Mai H.T., Croxford D., Kendall M.C., De Oliveira G. An appraisal of published clinical guidelines in anesthesiology practice using the AGREE II instrument. Can J Anaesth. 2021;68(7):1038–1044. doi: 10.1007/s12630-021-01973-9. [DOI] [PubMed] [Google Scholar]
- 41.Cao L., Yao L., He W., et al. Methodological quality in guidelines for enhanced recovery after surgery was suboptimal. J Clin Epidemiol. 2022;152:151–163. doi: 10.1016/j.jclinepi.2022.09.016. [DOI] [PubMed] [Google Scholar]
- 42.Higgins J.P.T., Thomas J., Chandler J., et al., editors. 2021. Cochrane Handbook for Systematic Reviews of Interventions, version 6.2 (updated February 2021)https://training.cochrane.org/handbook Available from: [Google Scholar]
- 43.Depaoli S., van de Schoot R. Improving transparency and replication in Bayesian statistics: the WAMBS-checklist. Psychol Methods. 2017;22:240–261. doi: 10.1037/met0000065. [DOI] [PubMed] [Google Scholar]
- 44.Page M.J., McKenzie J.E., Bossuyt P.M., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. J Clin Epidemiol. 2021;134:178–189. doi: 10.1016/j.jclinepi.2021.03.001. [DOI] [PubMed] [Google Scholar]
- 45.McGowan J., Sampson M., Salzwedel D.M., Cogo E., Foerster V., Lefebvre C. PRESS Peer Review of Electronic Search Strategies: 2015 guideline statement. J Clin Epidemiol. 2016;75:40–46. doi: 10.1016/j.jclinepi.2016.01.021. [DOI] [PubMed] [Google Scholar]
- 46.Verret M., Le J.B.P., Lalu M.M., et al. Effectiveness of dexmedetomidine during surgery under general anaesthesia on patient-centred outcomes: a systematic review and Bayesian meta-analysis protocol. BMJ Open. 2024;14 doi: 10.1136/bmjopen-2023-080012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Verret M., Fergusson D.A., Nicholls S.G., et al. Engaging patients in anesthesiology research: a rewarding frontier. Can J Anaesth. 2023;70:817–823. doi: 10.1007/s12630-023-02432-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Staniszewska S., Brett J., Simera I., et al. GRIPP2 reporting checklists: tools to improve reporting of patient and public involvement in research. BMJ. 2017;358:j3453. doi: 10.1136/bmj.j3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Burns P.B., Rohrich R.J., Chung K.C. The levels of evidence and their role in evidence-based medicine. Plast Reconstr Surg. 2011;128:305–310. doi: 10.1097/PRS.0b013e318219c171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moonesinghe S.R., Jackson A.I.R., Boney O., et al. Systematic review and consensus definitions for the Standardised Endpoints in Perioperative Medicine initiative: patient-centred outcomes. Br J Anaesth. 2019;123:664–670. doi: 10.1016/j.bja.2019.07.020. [DOI] [PubMed] [Google Scholar]
- 51.Cobey K.D., Lalu M.M., Skidmore B., Ahmadzai N., Grudniewicz A., Moher D. What is a predatory journal? A scoping review. F1000Res. 2018;7:1001. doi: 10.12688/f1000research.15256.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cukier S., Helal L., Rice D.B., et al. Checklists to detect potential predatory biomedical journals: a systematic review. BMC Med. 2020;18:104. doi: 10.1186/s12916-020-01566-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nama N., Barrowman N., O'Hearn K., Sampson M., Zemek R., McNally J.D. Quality control for crowdsourcing citation screening: the importance of assessment number and qualification set size. J Clin Epidemiol. 2020;122:160–162. doi: 10.1016/j.jclinepi.2020.02.009. [DOI] [PubMed] [Google Scholar]
- 54.Allvin R., Berg K., Idvall E., Nilsson U. Postoperative recovery: a concept analysis. J Adv Nurs. 2007;57:552–558. doi: 10.1111/j.1365-2648.2006.04156.x. [DOI] [PubMed] [Google Scholar]
- 55.Myles P.S., Myles D.B. An updated minimal clinically important difference for the QoR-15 Scale. Anesthesiology. 2021;135:934–935. doi: 10.1097/ALN.0000000000003977. [DOI] [PubMed] [Google Scholar]
- 56.Myles P.S., Shulman M.A., Reilly J., Kasza J., Romero L. Measurement of quality of recovery after surgery using the 15-item quality of recovery scale: a systematic review and meta-analysis. Br J Anaesth. 2022;128:1029–1039. doi: 10.1016/j.bja.2022.03.009. [DOI] [PubMed] [Google Scholar]
- 57.Myles P.S., Boney O., Botti M., et al. Systematic review and consensus definitions for the Standardised Endpoints in Perioperative Medicine (StEP) initiative: patient comfort. Br J Anaesth. 2018;120:705–711. doi: 10.1016/j.bja.2017.12.037. [DOI] [PubMed] [Google Scholar]
- 58.Williams ACdC., Craig K.D. Updating the definition of pain. Pain. 2016;157:2420–2423. doi: 10.1097/j.pain.0000000000000613. [DOI] [PubMed] [Google Scholar]
- 59.Jammer I., Wickboldt N., Sander M., et al. Standards for definitions and use of outcome measures for clinical effectiveness research in perioperative medicine: European Perioperative Clinical Outcome (EPCO) definitions: a statement from the ESA-ESICM joint taskforce on perioperative outcome measures. Eur J Anaesthesiol. 2015;32:88–105. doi: 10.1097/EJA.0000000000000118. [DOI] [PubMed] [Google Scholar]
- 60.Mukherjee K., Schubl S.D., Tominaga G., et al. Non-surgical management and analgesia strategies for older adults with multiple rib fractures: a systematic review, meta-analysis, and practice management guideline from the Eastern Association for the Surgery of Trauma and the Chest Wall Injury Society. J Trauma Acute Care Surg. 2023;94:398–407. doi: 10.1097/TA.0000000000003830. [DOI] [PubMed] [Google Scholar]
- 61.Sterne J.A.C., Savović J., Page M.J., et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366 doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 62.Minozzi S., Gonzalez-Lorenzo M., Cinquini M., et al. Adherence of systematic reviews to Cochrane RoB2 guidance was frequently poor: a meta epidemiological study. J Clin Epidemiol. 2022;152:47–55. doi: 10.1016/j.jclinepi.2022.09.003. [DOI] [PubMed] [Google Scholar]
- 63.Minozzi S., Dwan K., Borrelli F., Filippini G. Reliability of the revised Cochrane risk-of-bias tool for randomised trials (RoB2) improved with the use of implementation instruction. J Clin Epidemiol. 2022;141:99–105. doi: 10.1016/j.jclinepi.2021.09.021. [DOI] [PubMed] [Google Scholar]
- 64.Harrer M., Cuijpers P., Furukawa T.A., Ebert D.D. 1st Edn. Chapman & Hall/CRC Press; Boca Raton, FL, and London: 2021. Doing Meta-Analysis With R: A Hands-On Guide. [Google Scholar]
- 65.Röver C. Bayesian random-effects meta-analysis using the bayesmeta R package. J Stat Softw. 2020;93:1–51. [Google Scholar]
- 66.Bürkner P.-C. brms: an R package for Bayesian multilevel models using Stan. J Stat Softw. 2017;80:1–28. [Google Scholar]
- 67.Lammers D., Richman J., Holcomb J.B., Jansen J.O. Use of Bayesian statistics to reanalyze data from the pragmatic randomized optimal platelet and plasma ratios trial. JAMA Netw Open. 2023;6 doi: 10.1001/jamanetworkopen.2023.0421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ruberg S.J., Beckers F., Hemmings R., et al. Application of Bayesian approaches in drug development: starting a virtuous cycle. Nat Rev Drug Discov. 2023;22:235–250. doi: 10.1038/s41573-023-00638-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Millili J.J., Philiponis V.S., Nusbaum M. Predicting surgical outcome using Bayesian analysis. J Surg Res. 1998;77:45–49. doi: 10.1006/jsre.1998.5333. [DOI] [PubMed] [Google Scholar]
- 70.Quintana M., Viele K., Lewis R.J. In: JAMA Guide to Statistics and Methods. Livingston E.H., Lewis R.J., editors. McGraw-Hill Education; New York, NY: 2019. Bayesian analysis: using prior information to interpret the results of clinical trials. [DOI] [PubMed] [Google Scholar]
- 71.Bittl J.A., He Y. Vol. 10. Circ Cardiovasc Qual Outcomes; 2017. (Bayesian analysis: a practical approach to interpret clinical trials and create clinical practice guidelines). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ferreira D., Barthoulot M., Pottecher J., Torp K.D., Diemunsch P., Meyer N. Theory and practical use of Bayesian methods in interpreting clinical trial data: a narrative review. Br J Anaesth. 2020;125:201–207. doi: 10.1016/j.bja.2020.04.092. [DOI] [PubMed] [Google Scholar]
- 73.Bowyer A., Jakobsson J., Ljungqvist O., Royse C. A review of the scope and measurement of postoperative quality of recovery. Anaesthesia. 2014;69:1266–1278. doi: 10.1111/anae.12730. [DOI] [PubMed] [Google Scholar]
- 74.Johnston B.C., Patrick D.L., Thorlund K., et al. Patient-reported outcomes in meta-analyses-part 2: methods for improving interpretability for decision-makers. Health Qual Life Outcomes. 2013;11:211. doi: 10.1186/1477-7525-11-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gronau Q.F., Heck D.W., Berkhout S.W., Haaf J.M., Wagenmakers E.-J. A primer on Bayesian model-averaged meta-analysis. Adv Methods Pract Psychol Sci. 2021;4 doi: 10.1177/25152459211031256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rhodes K.M., Turner R.M., Higgins J.P. Predictive distributions were developed for the extent of heterogeneity in meta-analyses of continuous outcome data. J Clin Epidemiol. 2015;68:52–60. doi: 10.1016/j.jclinepi.2014.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Turner R.M., Davey J., Clarke M.J., Thompson S.G., Higgins J.P. Predicting the extent of heterogeneity in meta-analysis, using empirical data from the Cochrane Database of Systematic Reviews. Int J Epidemiol. 2012;41:818–827. doi: 10.1093/ije/dys041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Röver C., Bender R., Dias S., et al. On weakly informative prior distributions for the heterogeneity parameter in Bayesian random-effects meta-analysis. Res Synth Methods. 2021;12:448–474. doi: 10.1002/jrsm.1475. [DOI] [PubMed] [Google Scholar]
- 79.Glare P., Aubrey K.R., Myles P.S. Transition from acute to chronic pain after surgery. Lancet. 2019;393:1537–1546. doi: 10.1016/S0140-6736(19)30352-6. [DOI] [PubMed] [Google Scholar]
- 80.Berlin J.A., Longnecker M.P., Greenland S. Meta-analysis of epidemiologic dose-response data. Epidemiology. 1993;4:218–228. doi: 10.1097/00001648-199305000-00005. [DOI] [PubMed] [Google Scholar]
- 81.Doubilet P., Begg C.B., Weinstein M.C., Braun P., McNeil B.J. Probabilistic sensitivity analysis using Monte Carlo simulation. A practical approach. Med Decis Making. 1985;5:157–177. doi: 10.1177/0272989X8500500205. [DOI] [PubMed] [Google Scholar]
- 82.Reis D.J., Kaizer A.M., Kinney A.R., et al. A practical guide to random-effects Bayesian meta-analyses with application to the psychological trauma and suicide literature. Psychol Trauma. 2023;15:121–130. doi: 10.1037/tra0001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.R Core Team R. R: a language and environment for statistical computing. R Foundation for Statistical Computing; Vienna: 2013. [Google Scholar]
- 84.Schünemann H., Brożek J., Guyatt G., Oxman A., editors. Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach. 2013. https://gdt.gradepro.org/app/handbook/handbook Published. [Google Scholar]
- 85.Efe Mercanoglu E., Girgin Kelebek N., Turker G., et al. Comparison of the effect of ketamine and dexmedetomidine combined with total intravenous anesthesia in laparoscopic cholecystectomy procedures: a prospective randomized controlled study. Int J Clin Pract. 2022;2022 doi: 10.1155/2022/1878705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.van Norden J., Spies C.D., Borchers F., et al. The effect of peri-operative dexmedetomidine on the incidence of postoperative delirium in cardiac and non-cardiac surgical patients: a randomised, double-blind placebo-controlled trial. Anaesthesia. 2021;76:1342–1351. doi: 10.1111/anae.15469. [DOI] [PubMed] [Google Scholar]
- 87.Andjelković L., Novak-Jankovič V., Požar-Lukanovič N., Bosnić Z., Spindler-Vesel A. Influence of dexmedetomidine and lidocaine on perioperative opioid consumption in laparoscopic intestine resection: a randomized controlled clinical trial. J Int Med Res. 2018;46:5143–5154. doi: 10.1177/0300060518792456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zheng L., Zhao J., Zheng L., Jing S., Wang X. Effect of dexmedetomidine on perioperative stress response and immune function in patients with tumors. Technol Cancer Res Treat. 2020;19 doi: 10.1177/1533033820977542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shan X-s, Hu L-k, Wang Y., et al. Effect of perioperative dexmedetomidine on delayed graft function following a donation-after-cardiac-death kidney transplant: a randomized clinical trial. JAMA Netw Open. 2022;5 doi: 10.1001/jamanetworkopen.2022.15217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Li B., Li Y., Tian S., et al. Anti-inflammatory effects of perioperative dexmedetomidine administered as an adjunct to general anesthesia: a meta-analysis. Sci Rep. 2015;5 doi: 10.1038/srep12342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Duncan D., Sankar A., Beattie W.S., Wijeysundera D.N. Alpha-2 adrenergic agonists for the prevention of cardiac complications among adults undergoing surgery. Cochrane Database Syst Rev. 2018;3 doi: 10.1002/14651858.CD004126.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.van Dijk J.F.M., Vervoort S.C.J.M., van Wijck A.J.M., Kalkman C.J., Schuurmans M.J. Postoperative patients’ perspectives on rating pain: a qualitative study. Int J Nurs Stud. 2016;53:260–269. doi: 10.1016/j.ijnurstu.2015.08.007. [DOI] [PubMed] [Google Scholar]
- 93.Short T.G., Campbell D., Egan T.D. Increasing the utility of target-controlled infusions: one model to rule them all. Br J Anaesth. 2018;120:887–890. doi: 10.1016/j.bja.2018.02.012. [DOI] [PubMed] [Google Scholar]
- 94.Loudon K., Treweek S., Sullivan F., Donnan P., Thorpe K.E., Zwarenstein M. The PRECIS-2 tool: designing trials that are fit for purpose. BMJ. 2015;350:h2147. doi: 10.1136/bmj.h2147. [DOI] [PubMed] [Google Scholar]
- 95.Kharasch E.D., Clark J.D., Adams J.M. Opioids and public health: the prescription opioid ecosystem and need for improved management. Anesthesiology. 2022;136:10–30. doi: 10.1097/ALN.0000000000004065. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The list of included trials is included in Supplementary Table S4. Additional meta-data can be provided upon request to the corresponding author.