Abstract
Background
Identifying lipidomic markers of diet quality is needed to inform the development of biomarkers of diet, and to understand the mechanisms driving the diet- coronary heart disease (CHD) association.
Objectives
This study aimed to identify lipidomic markers of diet quality and examine whether these lipids are associated with incident CHD.
Methods
Using liquid chromatography–mass spectrometry, we measured 1542 lipid species from 1694 American Indian adults (aged 18–75 years, 62% female) in the Strong Heart Family Study. Participants were followed up for development of CHD through 2020. Information on the past year diet was collected using the Block Food Frequency Questionnaire, and diet quality was assessed using the Alternative Healthy Eating Index-2010 (AHEI). Mixed-effects linear regression was used to identify individual lipids cross-sectionally associated with AHEI. In prospective analysis, Cox frailty model was used to estimate the hazard ratio (HR) of each AHEI-related lipid for incident CHD. All models were adjusted for age, sex, center, education, body mass index, smoking, alcohol drinking, level of physical activity, energy intake, diabetes, hypertension, and use of lipid-lowering drugs. Multiple testing was controlled at a false discovery rate of <0.05.
Results
Among 1542 lipid species measured, 71 lipid species (23 known), including acylcarnitine, cholesterol esters, glycerophospholipids, sphingomyelins and triacylglycerols, were associated with AHEI. Most of the identified lipids were associated with consumption of ω-3 (n–3) fatty acids. In total, 147 participants developed CHD during a mean follow-up of 17.8 years. Among the diet-related lipids, 10 lipids [5 known: cholesterol ester (CE)(22:5)B, phosphatidylcholine (PC)(p-14:0/22:1)/PC(o-14:0/22:1), PC(p-38:3)/PC(o-38:4)B, phosphatidylethanolamine (PE)(p-18:0/20:4)/PE(o-18:0/20:4), and sphingomyelin (d36:2)A] were associated with incident CHD. On average, each standard deviation increase in the baseline level of these 5 lipids was associated with 17%–23% increased risk of CHD (from HR: 1.17; 95% CI: 1, 1.36; to HR: 1.23; 95% CI: 1.05, 1.43).
Conclusions
In this study, lipidomic markers of diet quality in American Indian adults are found. Some diet-related lipids are associated with risk of CHD beyond established risk factors.
Keywords: lipidomics, diet quality, Alternative Healthy Eating Index, coronary heart disease, American Indian adults, Strong Heart Study
Introduction
Diet is a key modifiable factor affecting the development of coronary heart disease (CHD) [1,2], a leading cause of death worldwide [3]. In addition to specific food groups or nutrients, diet quality is an overall measure of a diet that reflects the interactive and synergistic effects of various dietary components. Accumulated evidence shows that high diet quality, assessed by the Alternative Healthy Eating Index-2010 (AHEI), is associated with reduced risks of CHD [4,5] and other cardiovascular diseases (CVDs) [[4], [5], [6]]. It is known that lipid metabolism plays a major role in the complex diet–cardiovascular health relation [7], but a deeper understanding of the lipid pathways is needed.
The human lipidome is highly dynamic and influenced by both genetic and environmental factors, including diet [8]. High-throughput untargeted lipidomics offers new opportunities to develop novel biomarkers of dietary exposure and to improve our understanding of the mechanisms underlying the diet–CHD relation. Using this technology, recent research has linked lipidomic profiles with diet quality or adherence to healthy dietary patterns [[9], [10], [11], [12]]. However, they were limited by small sample size or not examining the significance of these biomarkers regarding subsequent disease outcomes [10,11]. Furthermore, previous studies have a low coverage of the lipidome and largely focus on relatively homogenous populations, and thus, more research is needed to validate these markers in diverse populations such as American Indian individuals who have relatively unique food environments and a disproportionately higher prevalence of poor diet quality [13]. To this end, based on data from 1694 American Indian participants in the Strong Heart Family Study (SHFS), we aimed to identify lipidomic markers of habitual diet quality assessed by AHEI and to examine whether these markers are independently associated with CHD risk.
Methods
Study sample
The SHFS is a multicenter, family-based prospective study designed to identify genetic, metabolic, and behavioral factors for CVD and diabetes, and their risk factors in American Indian individuals. In brief, 2786 Tribal members (aged 14 years or older) residing in Arizona, North Dakota, South Dakota, and Oklahoma were recruited and examined in 2001–2003 (baseline) and followed up for CVD events. Detailed descriptions of the SHFS protocols for data collection have been reported previously [14,15]. All participants received a personal interview and a physical examination at each visit, during which fasting blood samples were collected for laboratory tests. This analysis included participants with complete information for diet, lipidomics, and clinical data. In particular, 1983 participants had plasma lipidomic data and were free of overt CVD at baseline. After excluding participants with missing information for diet, covariates, or follow-up and those with unrealistic energy intake (ie, <600 or >6000 kcal/d among females and <600 or >8000 kcal/d among males), 1694 participants were included in the final data analyses (Supplemental Figure 1).
All participants provided informed consent. The SHFS protocols were approved by the institutional review boards of the participating institutions and the American Indian tribes.
Assessments of diet quality
An interview-administered Block 119-item Food Frequency Questionnaire with a supplemental American Indian foods questionnaire was administered to SHFS participants at baseline to assess usual dietary intake over the past year. Methods on the dietary data collection in SHFS have been described previously [16].
Diet quality was assessed by the AHEI using the abovementioned dietary data. A detailed description of the AHEI has been described elsewhere [4]. In brief, the AHEI total score ranges from 0 to 110, with each component of the 11 food groups that comprise the score (ie, fruits, vegetables, whole grains, sugar-sweetened beverages and fruit juice, nuts and legumes, polyunsaturated fatty acids [PUFAs], long-chain ω-3 [n–6] fatty acids, transfats, red and/or processed meats, sodium, and alcohol) ranging from 0 to 10. A higher AHEI score indicates a better diet quality, which is categorized by higher consumption of the 6 healthy components (ie, fruits, vegetables, whole grains, nuts and legumes, PUFAs, and ω-3 fatty acids) and lower consumption of the 5 unhealthy components (ie, sugar-sweetened beverages and fruit juice, transfats, red/processed meats, sodium, and alcohol).
Ascertainment of incident CHD
Participants were followed up for the development of CHD through December 31, 2020. The primary outcomes were fatal and nonfatal CHD. Nonfatal CHD events included definite nonfatal myocardial infarction or definite nonfatal CHD. Fatal CHD was defined as definite fatal myocardial infarction, definite fatal CHD, or sudden death due to CHD. Detailed criteria and definitions have been described previously [17,18]. In brief, incident CHD events were assessed by annual mortality and morbidity surveillance reviews of hospitalization and death records and self-report (with follow-up medical record review) at the SHFS follow-up study examination. Time to event was defined as the time from the earliest study examination to the earliest of the following: date of incident CHD, death, or loss to follow-up. Medical records were abstracted, and CHD death and events were ascertained and confirmed by the mortality and morbidity review committees (ie, physician reviewers who evaluated available medical records for confirmation of cardiovascular events) using specific criteria.
Assessments of covariates
Sociodemographic information, lifestyle factors (cigarette smoking, and alcohol use), medical history, family history of illnesses, and use of prescription medications (eg, use of antihypertensive, diabetic, and/or lipid-lowering drugs) were collected using structured questionnaires at the baseline examination [14,15]. For cigarette smoking, participants were classified as current, former, or never-smokers. Similarly, alcohol use was categorized as current, former, or never-drinkers. To collect physical activity data, participants were asked to wear Accusplit AE120 pedometers for 7 consecutive days, except while bathing or swimming; the mean number of steps per day was calculated by averaging the total number of steps recorded each day during the 7-day period. Anthropometric measures including height, weight, waist circumference, and fasting blood tests were obtained during a brief physical examination and laboratory workup at each visit. Body mass index (BMI) was calculated as body weight in kilograms divided by the square of height in meters. Fasting glucose was measured by standard laboratory methods [14]. Type 2 diabetes (T2D) was defined as a fasting plasma glucose of ≥126 mg/dL or use of hypoglycemic drugs. Hypertension was defined as measured systolic blood pressure of ≥140 mm Hg, diastolic blood pressure of ≥90 mm Hg, or use of antihypertensive medications.
Lipidomic data acquisition, processing, and normalization
Methods for lipidomic data acquisition, processing, and normalization in the SHFS have been described previously [19]. In brief, relative abundance of molecular lipid species in fasting plasma samples was quantified by untargeted liquid chromatography–mass spectrometry. After preprocessing and quality control, we obtained 1542 lipids (518 known and 1024 unknown) in 1983 participants at baseline.
Statistical analysis
Supplemental Figure 1 illustrates the procedures for participant selection and statistical analyses. Statistical analyses were performed using R version 4.1.1 (R Foundation for Statistical Computing). All continuous variables, including lipids and AHEI scores, were standardized to zero mean and unit variance before the analyses.
To identify individual lipid species associated with AHEI total score at baseline, we constructed mixed-effects linear regression models. In the model, relative abundance of each lipid (continuous) was the independent variable, and the AHEI total score (continuous) was the dependent variable. Covariates included age (years), sex (male/female), study center (Arizona, Oklahoma, or Dakotas), education (years), BMI (in kg/m2), smoking (never/former/current), physical activity (steps/d), total energy intake (kcal/d), lipid-lowering medication use (yes/no), T2D (yes/no), and hypertension (yes/no). Alcohol use was not included as a covariate in the model because it is a component of the AHEI score. The model included random effects to account for relatedness between family members. Multiple testing was controlled by false discovery rate using the Storey q value method [20,21], wherein statistical significance is set at a q value of <0.05 level.
To further investigate the relationship between the identified lipids and the individual AHEI components, we constructed mixed-effects linear regression models. In this model, each lipid was the independent variable, and an AHEI component score (ie, fruits, vegetables, whole grains, sugar-sweetened beverages and fruit juice, nuts and legumes, PUFAs, ω-3 fatty acids, trans fats, red/processed meats, sodium, and alcohol) was the dependent variable. The model adjusted for the same covariates as described earlier.
To examine whether the AHEI-related lipids were associated with incident CHD, we built Cox frailty models, adjusting for all covariates included in the lipids-AHEI association analyses plus alcohol use (never/former/current). The frailty term was used in this study to account for the family relatedness among participants. The assumptions for Cox proportional hazards model were checked using the Schoenfeld residuals.
Additional analysis
To examine whether baseline age, diabetes status or hypertension status modifies the association between lipid metabolism and AHEI total score, we included an interaction term, ie, age (years) × lipids, diabetes status (yes/no) × lipids, or hypertension status (yes/no) × lipids, in the above-described mixed-effects linear regression models. The model adjusted for the same covariates included in the lipids–AHEI association analyses.
To examine whether the AHEI total score (both continuously and in quintiles) was associated with incident CHD or 10-year CHD risk score [22], we constructed Cox frailty model or mixed-effects linear regression, respectively. Before the analyses, we standardized all continuous variables to zero mean and unit variance and regressed out the baseline age from the AHEI total score. Three models were fitted: model 1 was crude model; model 2 was adjusted for sex, center, and education; and model 3 was further adjusted for BMI, smoking, physical activity, total energy intake, use of lipid-lowering medication, T2D, and hypertension.
Results
Characteristics of study participants
Baseline characteristics of study participants are summarized in Table 1. Among the 1694 individuals included in this analyses, 62% were females, and the mean age was 40.4 years (range: 18–75 years) at baseline. The mean AHEI score was 44.5 (range: 18.8–77.6). Participants with a lower AHEI score were younger, less educated, more likely to report current smoking, and less likely to present with diabetes or hypertension than participants with a higher AHEI score.
TABLE 1.
Baseline Characteristics of the Participants by Quintiles of Alternative Healthy Eating Index (AHEI)
| Characteristics1 | Total (N = 1694) | AHEI quintiles |
||||
|---|---|---|---|---|---|---|
| Q1 (n = 339) | Q2 (n = 339) | Q3 (n = 339) | Q4 (n = 339) | Q5 (n = 338) | ||
| Age (y) | 40.4 ± 13.9 | 35.2 ± 12.4 | 38.4 ± 13.7 | 41.8 ± 13.7 | 42.4 ± 13.1 | 44.4 ± 14.5 |
| Female | 1054 (62.2) | 188 (55.5) | 217 (64.0) | 216 (63.7) | 218 (64.3) | 215 (63.6) |
| Education (y) | 12.6 ± 2.1 | 12.1 ± 2.0 | 12.4 ± 2.1 | 12.7 ± 2.2 | 12.7 ± 2.1 | 13.0 ± 2.1 |
| BMI ± kg/m2) | 31.8 ± 7.4 | 31.1 ± 8.2 | 32.4 ± 6.8 | 32.1 ± 7.7 | 31.5 ± 7.1 | 32.0 ± 7.4 |
| Smoking status | ||||||
| Never | 630 (37.2) | 127 (37.5) | 121 (35.7) | 124 (36.6) | 128 (37.8) | 130 (38.5) |
| Former | 397 (23.4) | 63 (18.6) | 80 (23.6) | 85 (25.1) | 71 (20.9) | 98 (29.0) |
| Current | 667 (39.4) | 149 (44.0) | 138 (40.7) | 130 (38.3) | 140 (41.3) | 110 (32.5) |
| Drinking status | ||||||
| Never | 135 (8.0) | 19 (5.6) | 28 (8.3) | 38 (11.2) | 19 (5.6) | 31 (9.2) |
| Former | 503 (29.7) | 91 (26.8) | 121 (35.7) | 98 (28.9) | 93 (27.4) | 100 (29.6) |
| Current | 1056 (62.3) | 229 (67.6) | 190 (56.0) | 203 (59.9) | 227 (67.0) | 207 (61.2) |
| Physical activity (steps/d) | 5772.5 ± 3784.9 | 6082.2 ± 3722.8 | 5710.1 ± 3748.1 | 5538.7 ± 3554.7 | 5562.8 ± 3208.6 | 5969.2 ± 4554.2 |
| Total energy intake (kcal/d) | 2445.3 ± 1308.4 | 2495.5 ± 1060.9 | 2309.0 ± 1123.1 | 2289.6 ± 1251.1 | 2417.3 ± 1372.5 | 2716.1 ± 1620.7 |
| SBP (mm Hg) | 122.6 ± 15.4 | 119.8 ± 13.7 | 122.7 ± 15.9 | 123.1 ± 15.5 | 123.5 ± 15.9 | 124.0 ± 15.6 |
| DBP (mm Hg) | 77.4 ± 10.6 | 77.0 ± 10.3 | 77.3 ± 10.5 | 77.5 ± 10.7 | 77.3 ± 11.0 | 77.9 ± 10.8 |
| Lipid-lowering medicine use | 61 (3.6) | 7 (2.1) | 14 (4.1) | 11 (3.2) | 10 (2.9) | 19 (5.6) |
| Diabetes | 311 (18.4) | 38 (11.2) | 62 (18.3) | 68 (20.1) | 74 (21.8) | 69 (20.4) |
| Hypertension | 506 (29.9) | 77 (22.7) | 90 (26.5) | 106 (31.3) | 114 (33.6) | 119 (35.2) |
| AHEI score | 44.5 ± 8.9 | 32.8 ± 3.4 | 39.2 ± 1.4 | 43.9 ± 1.3 | 49.0 ± 1.6 | 57.5 ± 5.1 |
Abbreviations: BMI, body mass index; DBP, diastolic blood pressure; SBP, systolic blood pressure.
Continuous variables were expressed as mean ± standard deviation and categorical variables as n (%).
Fasting plasma lipid species associated with AHEI
A total of 298 lipid species (114 known) were associated with AHEI at P < 0.05. After adjusting for multiple testing, 71 lipids (23 known) were significantly associated with AHEI total score at a q value of <0.05 (Figure 1). Among the 23 known lipids, 13 lipids, including 5 phosphatidylcholines (PCs), 3 phosphatidylethanolamines (PEs), 1 phosphatidylinositol, and 4 triacylglycerols (TAGs), were positively associated with AHEI total score (β: 0.07–0.09). Meanwhile, 10 lipids, including acylcarnitine (AC) (18:0), 3 cholesterol esters (CEs), 3 PCs, 1 PE, and 2 sphingomyelins (SMs), were negatively associated with AHEI total scores (β: −0.07 to −0.11). (Figure 2, Supplemental Table 1).
FIGURE 1.
Manhattan plot depicting the association of individual lipid species with AHEI total score (N = 1694). x-axis: lipid classes; y-axis: −log10 P. Different colors represent different lipid categories. The dashed line represents significance level at q = 0.05 or P = 0.05. The letter A or B in the name of lipids represents isomers.
FIGURE 2.
Baseline plasma lipid species associated with AHEI total score (q < 0.05) and incident CHD (N = 1694). β coefficients of baseline lipids associated with baseline AHEI total score were obtained by mixed-effects linear regression models, adjusting for age, sex, study center, education, BMI, smoking, physical activity, total energy intake, lipid-lowering medication use, type 2 diabetes, and hypertension at baseline. Hazard ratios of baseline lipids associated with incident CHD were obtained by Cox frailty models, adjusting for all aforementioned covariates, in addition to alcohol use. Bolded lipids were significantly associated with incident CHD (P < 0.05). The letter A or B in the name of lipids indicates isomers. Abbreviations: AC, acylcarnitine; AHEI, Alternative Healthy Eating Index; CE, cholesterol ester; CHD, coronary heart disease; HR, hazard ratio; PC, phosphatidylcholine; PE, phosphatidylethanolamine; PI, phosphatidylinositol; SD, standard deviation; SM, sphingomyelin; TAG, triacylglycerol.
AHEI-related lipids associated with AHEI components
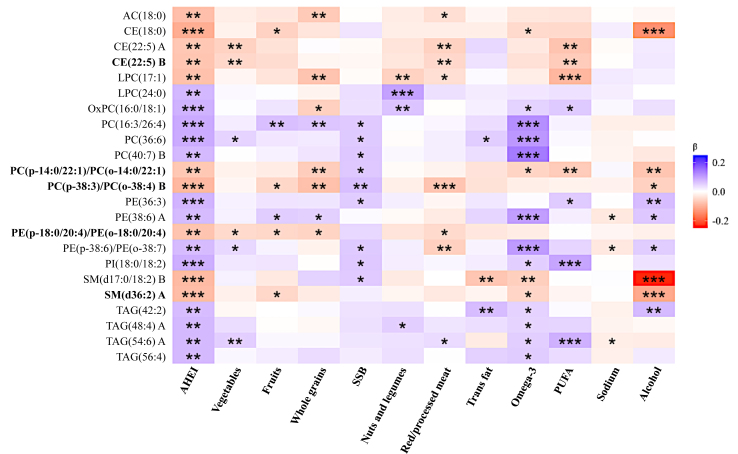
Figure 3 shows the associations between AHEI-related lipids and individual AHEI components. Most of the identified lipid species were associated with ω-3 fatty acids (15 of the 23 known lipids, including 11 showing positive associations), sugar-sweetened beverages (9 of the 23), and alcohol intake (9 of the 23). Notably, CE(22:5) A and CE(22:5) B were negatively associated with some individual AHEI component scores, such as vegetable, PUFAs, and red/processed meats (ie, they were related to a lower intake of vegetable and PUFAs but a higher intake of red/processed meats); Two SMs [ie, SM(d17:0/18:2) B and SM(d36:2) A] were negatively associated with individual component scores for alcohol consumption and ω-3 fatty acid (ie, they were related to suboptimal levels of alcohol consumption and a lower intake of ω-3); and the 4 TAGs [ie, TAG(42:2), TAG(48:4)A, TAG(54:6)A, and TAG(56:4)] were positively associated with the component score of the ω-3 fatty acids (ie, they were related to a higher intake of ω-3).
FIGURE 3.
Associations between AHEI-related lipids and AHEI component scores (N = 1694). Color codes are based on the regression coefficients obtained from the mixed-effects linear regression model, adjusting for age, sex, study center, education, BMI, smoking, physical activity, total energy intake, lipid-lowering medication use, T2D, and hypertension. Bolded lipids were significantly associated with incident CHD (P < 0.05). A higher AHEI component score indicates better diet quality, which is categorized by higher consumption of the 6 healthy components (ie, fruits, vegetables, whole grains, nuts and legumes, PUFAs and ω-3 fatty acids) and lower consumption of the 5 unhealthy components (ie, sugar-sweetened beverages and fruit juice, trans fats, red/processed meats, sodium, and alcohol). ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001. Abbreviations: AC, acylcarnitine; AHEI, Alternative Healthy Eating Index; CE, cholesterol ester; CHD, coronary heart disease; PC, phosphatidylcholine; PE, phosphatidylethanolamine; PI, phosphatidylinositol; PUFA, polyunsaturated fatty acid; SM, sphingomyelin; SSB, sugar-sweetened beverages; TAG, triacylglycerol.
AHEI-related lipids associated with incident CHD
In total, 147 participants developed CHD during a mean follow-up of 17.8 years. Of the 71 AHEI-related lipids, baseline levels of 10 lipids (5 known) were significantly associated with an increased risk of CHD at P < 0.05, after adjusting for age, sex, center, education, BMI, smoking, alcohol drinking, level of physical activity, energy intake, diabetes, prevalent hypertension, and use of lipid-lowering drugs at baseline (Figure 2, Supplemental Table 1). In particular, higher baseline concentrations of all 5 known lipids were associated with a greater risk of CHD, including CE(22:5) B (HR: 1.22; 95% CI: 1.06, 1.42, per standard deviation increase in baseline level), PC(p-14:0/22:1)/PC(o-14:0/22:1) (HR: 1.23; 95% CI: 1.05, 1.43), PC(p-38:3)/PC(o-38:4) (HR: 1.17; 95% CI: 1, 1.36), PE(p-18:0/20:4)/PE(o-18:0/20:4) (HR: 1.18; 95% CI: 1.01, 1.39), and SM(d36:2) A (HR: 1.19; 95% CI: 1.01, 1.39). These lipids consistently showed a negative association with AHEI scores.
Results from additional analyses
As shown in Supplemental Table 2, after including an interaction term (lipids × age, lipids × diabetes status, or lipids × hypertension status) in the model, the observed associations of lipids with AHEI total score remained largely unchanged (all interaction P > 0.05 after multiple testing correction).
Supplemental Table 3 lists the associations of AHEI total score with incident CHD and 10-year CHD risk score [22]. It shows that AHEI scores were inversely associated with risk of CHD (HR: 0.51; 95% CI: 0.30, 0.89; Q3 vs. Q1) in the full adjustment model. In addition, AHEI scores were inversely associated with 10-year CHD risk score both continuously (β: −0.006; 95% CI: −0.011, −0.002) and in quantiles (β: −0.16; 95% CI: −0.27, −0.04; Q5 vs. Q1), after adjusting for all covariates.
Discussion
In this large cohort of American Indian individuals, we identified 23 known lipid species associated with habitual diet quality assessed by AHEI, including AC, CEs, glycerophospholipids, SMs, and TAGs. Among these lipids, 5 lipid species were independently associated with a higher risk of CHD and lower diet quality [ie, CE(22:5) B, PC(p-14:0/22:1)/PC(o-14:0/22:1), PC(p-38:3)/PC(o-38:4), PE(p-18:0/20:4)/PE(o-18:0/20:4), and SM(d36:2) A]. About half of the diet-related lipid species were positively associated with consumption of ω-3 fatty acids. These findings may inform the lipidomic markers of habitual diet quality and provide insights into our understanding of the relationship among diet, lipid metabolism, and CVD.
We found that higher levels of several ACs and CEs were associated with lower diet quality. ACs are esters of carnitine and fatty acids that play an essential role in fatty acid metabolism [23]. Previous studies have reported that higher levels of ACs were associated with red and processed meat intake [24,25]. Our analyses support these findings. For instance, a higher level of AC (18:0) was associated with poorer diet quality and a higher intake of red/processed meats [25,26]. In addition, several CEs were also inversely associated with diet quality. These findings corroborate previous studies reporting an inverse association of CE(18:0) with AHEI [27] or Mediterranean diet adherence [28]. On the contrary, some studies reported that CE(22:5) was positively associated with AHEI [27,29] and other healthy diet quality indices [29,30], whereas we found that CE(22:5) may reflect a higher intake of red/processed meat and/or lower vegetable and PUFA intakes, and was associated with a higher risk of CHD among American Indian individuals. CEs serve as a storage form of cholesterol and facilitate its transport in blood. The close link between serum CE concentrations and dietary fatty acid intake [31,32] may explain the observed associations of CE(22:5) with these dietary components in our study. Notably, CE(22:5) B was also associated with an increased risk of CVD and all-cause mortality in American Indian individuals [33]. Despite the different directions of association with diet quality, which possibly arise from population differences, CE(22:5) was linked to healthy diet adherence in many studies and needs to be further studied.
Some SMs were also negatively associated with diet quality, including 1 lipid [ie, SM(d36:2) A], which was also associated with an increased risk of CHD. Higher plasma SM concentration has been shown to be an independent risk factor for CHD [34,35] and promote atherosclerosis [36], whereas SM species with a very long-chain saturated fatty acid were associated with a reduced risk of CVD among individuals with T2D [37]. These conditions were consistently associated with unhealthy diets [1]. Furthermore, evidence showed that SM(d36:2) was associated with markers of inflammation in mice [38,39]. This study showed that SM(d36:2) A was associated with unhealthy alcohol use and lower consumption of fruits and ω-3 fatty acids, which are recognized as important proinflammatory or anti-inflammatory dietary factors [[40], [41], [42]]. It is possible that SM(d36:2) A reflects inflammation level that is attributable to these dietary factors, thereby contributing to CHD risk.
Our findings demonstrated that several glycerophospholipids [eg, LPC(24:0), PC(36:6), and PE(36:3)] are associated with diet quality. These findings appeared to be in agreement with previous evidence suggesting positive associations of LPC(24:0) with AHEI [43] or Mediterranean diet adherence [26]. However, our findings are discordant with a study showing an inverse association of PE(36:3) with AHEI [27]. These glycerophospholipids are mostly related to consumption of sugar-sweetened beverages and ω-3 fatty acids. We also observed novel associations, including an increased risk of CHD associated with 3 AHEI-related lipids [ie, PC(p-14:0/22:1)/PC(o-14:0/22:1), PC(p-38:3)/PC(o-38:4) B, and PE(p-18:0/20:4)/PE(o-18:0/20:4)]. Previous studies indicated that disturbed glycerophospholipid metabolism is a major altered metabolic pathway [44,45] in the development of CHD. Taken together, these results may suggest disturbed lipid metabolism involved in the association of dietary intake with CHD.
In line with previous studies reporting a beneficial role of dietary PUFAs, particularly ω-3 fatty acids, in the prevention of CVD [46,47], we also found that many of the AHEI-related lipid species were associated with ω-3 intakes in American Indian individuals, underscoring the importance of beneficial dietary fats in maintaining a healthy diet. Notably, the 4 unsaturated long-chain TAGs [ie, TAG(42:2), TAG(48:4)A, TAG(54:6)A, and TAG(56:4)] were positively associated with ω-3 intakes and the AHEI total score. This appears to be in agreement with previous studies reporting that multiple long-chain TAGs were positively associated with AHEI or other healthy diet adherence [10,29] and the ω-3 component of AHEI [10,27]. Our findings suggest that specific unsaturated long-chain TAGs may serve as potential markers of high diet quality, possibly by reflecting higher consumption of beneficial ω-3 fatty acids.
Our study has several strengths. We used an untargeted lipidomic platform that measured >1500 individual lipid species across 15 lipid classes in a large sample of community-dwelling individuals. Another strength is the prospective cohort design enabling us to examine the significance of the identified biomarkers regarding incident disease outcomes. Furthermore, the comprehensive assessment of sociodemographic, lifestyle, and clinical factors allows us to extensively adjust for potential confounders. Of note, our additional analyses showed that age, diabetes, or hypertension status may modify the association between lipid metabolism and AHEI (Supplemental Table 2), despite that the interactions in association did not survive multiple testing correction. Some limitations should also be mentioned. First, although the comprehensive lipidomic panel provides high coverage of the plasma lipidome, many lipids detected are unknown compounds and isomeric lipids cannot be distinguished either. Additional experiments are needed to further characterize these unknown compounds in future research. Second, because our results were derived from American Indian individuals who have a relatively unique food environment and less healthy diet, it is unclear how well our findings could be generalized to other racial/ethnic groups. In addition, we observed that participants with lower diet quality were relatively younger and less likely to present with diabetes or hypertension than those with higher diet quality. This was also reported in other studies [30,43]. It is possible that older individuals with diabetes and/or hypertension tend to be more health conscious. An external cohort with a comparable study design and data collection methods including lipidomic data is warranted to replicate our findings. Furthermore, habitual dietary intake was assessed only once (baseline), thus the change in diet quality cannot be evaluated. Nonetheless, the SHFS participants are known to have relatively consistent dietary intake over time [48]. Finally, similar to all other large-scale epidemiological studies, the observational nature of the SHFS limited our ability to establish any causal relationship between lipids and diet quality or between lipids and incident CHD, albeit that the prospective study design alleviates some concerns about reverse causality.
In summary, our study identified multiple individual lipid species as potential markers of habitual diet quality assessed by AHEI among American Indian individuals and highlighted the associations of diet-related lipid species with risk of CHD. Future studies are needed to confirm our findings in other ethnic groups with different population settings.
Acknowledgments
We thank the Strong Heart Study participants, Indian Health Service facilities, and participating Tribal communities for their extraordinary cooperation and involvement, which has contributed to the success of the Strong Heart Study. The content expressed in this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Indian Health Service.
Author contributions
The authors’ responsibilities were as follows – JZ: conceived the study; XW, GM: conducted the statistical analyses; XW: drafted the manuscript; JZ, AF: supervised the study; YZ, OF: collected the liquid chromatography–mass spectrometry (LC-MS) data and performed initial quality control and data preprocessing for LC-MS data; and all authors contributed to the discussion and critically reviewed and approved the manuscript.
Conflicts of interest
No potential conflicts of interest relevant to this article were reported.
Funding
This study was supported by National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health (grant R01DK107532). The Strong Heart Study has been funded in whole or in part with federal funds from the National Heart, Lung, and Blood Institute, National Institutes of Health, and the Department of Health and Human Services under contract numbers 75N92019D00027, 75N92019D00028, 75N92019D00029, and 75N92019D00030. The study was previously supported by research grants R01HL109315, R01HL109301, R01HL109284, R01HL109282, and R01HL109319 and by cooperative agreements U01HL41642, U01HL41652, U01HL41654, U01HL65520, and U01HL65521 from the National Heart, Lung, and Blood Institute.
Data availability
The phenotype data used in this study can be requested through the Strong Heart Study (https://strongheart-study.org/). The lipidomic data can be obtained from the corresponding author on a reasonable request.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ajcnut.2023.12.024.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Pan A., Lin X., Hemler E., Hu F.B. Diet and cardiovascular disease: advances and challenges in population-based studies. Cell. Metab. 2018;27(3):489–496. doi: 10.1016/j.cmet.2018.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gao M., Jebb S.A., Aveyard P., Ambrosini G.L., Perez-Cornago A., Carter J., et al. Associations between dietary patterns and the incidence of total and fatal cardiovascular disease and all-cause mortality in 116,806 individuals from the UK Biobank: a prospective cohort study. BMC Med. 2021;19(1):83. doi: 10.1186/s12916-021-01958-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dibben G., Faulkner J., Oldridge N., Rees K., Thompson D.R., Zwisler A.D., et al. Exercise-based cardiac rehabilitation for coronary heart disease, Cochrane Database Syst. Rev. 2021;11(11):CD001800. doi: 10.1002/14651858.CD001800.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chiuve S.E., Fung T.T., Rimm E.B., Hu F.B., McCullough M.L., Wang M., et al. Alternative dietary indices both strongly predict risk of chronic disease. J. Nutr. 2012;142(6):1009–1018. doi: 10.3945/jn.111.157222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shan Z., Li Y., Baden M.Y., Bhupathiraju S.N., Wang D.D., Sun Q., et al. Association between healthy eating patterns and risk of cardiovascular disease. JAMA Intern. Med. 2020;180(8):1090–1100. doi: 10.1001/jamainternmed.2020.2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ibsen D.B., Christiansen A.H., Olsen A., Tjønneland A., Overvad K., Wolk A., et al. Adherence to the EAT-Lancet diet and risk of stroke and stroke subtypes: a cohort study. Stroke. 2022;53(1):154–163. doi: 10.1161/STROKEAHA.121.036738. [DOI] [PubMed] [Google Scholar]
- 7.Casas R., Castro-Barquero S., Estruch R., Sacanella E. Nutrition and cardiovascular health. Int. J. Mol. Sci. 2018;19(12):3988. doi: 10.3390/ijms19123988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wei F., Lamichhane S., Oresic M., Hyotylainen T. Lipidomes in health and disease: analytical strategies and considerations. TrAc Trends Anal. Chem. 2019;120 doi: 10.1016/j.trac.2019.115664. [DOI] [Google Scholar]
- 9.Lankinen M., Schwab U., Kolehmainen M., Paananen J., Nygren H., Seppänen-Laakso T., et al. A healthy nordic diet alters the plasma lipidomic profile in adults with features of metabolic syndrome in a multicenter randomized dietary intervention. J. Nutr. 2015;146(4):662–672. doi: 10.3945/jn.115.220459. [DOI] [PubMed] [Google Scholar]
- 10.Zhang T., Naudin S., Hong H.G., Albanes D., Männistö S., Weinstein S.J., et al. Dietary quality and circulating lipidomic profiles in 2 cohorts of middle-aged and older male Finnish smokers and American populations. J. Nutr. 2023;153:2389–2400. doi: 10.1016/j.tjnut.2023.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O'Gorman A., Morris C., Ryan M., O’Grada C.M., Roche H.M., Gibney E.R., et al. Habitual dietary intake impacts on the lipidomic profile. J. Chromatogr. B. Analyt. Technol. Biomed. Life. Sci. 2014;966:140–146. doi: 10.1016/j.jchromb.2014.01.032. [DOI] [PubMed] [Google Scholar]
- 12.Toledo E., Wang D.D., Ruiz-Canela M., Clish C.B., Razquin C., Zheng Y., et al. Plasma lipidomic profiles and cardiovascular events in a randomized intervention trial with the Mediterranean diet. Am. J. Clin. Nutr. 2017;106(4):973–983. doi: 10.3945/ajcn.116.151159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McCullough M.L., Chantaprasopsuk S., Islami F., Rees-Punia E., Um C.Y., Wang Y., et al. Association of socioeconomic and geographic factors with diet quality in US adults. JAMA Netw. Open. 2022;5(6) doi: 10.1001/jamanetworkopen.2022.16406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee E.T., Welty T.K., Fabsitz R., Cowan L.D., Le N.A., Oopik A.J., et al. The Strong Heart Study. A study of cardiovascular disease in American Indians: design and methods. Am. J. Epidemiol. 1990;132(6):1141–1155. doi: 10.1093/oxfordjournals.aje.a115757. [DOI] [PubMed] [Google Scholar]
- 15.North K.E., Howard B.V., Welty T.K., Best L.G., Lee E.T., Yeh J.L., et al. Genetic and environmental contributions to cardiovascular disease risk in American Indians: the strong heart family study. Am. J. Epidemiol. 2003;157(4):303–314. doi: 10.1093/aje/kwf208. [DOI] [PubMed] [Google Scholar]
- 16.Fretts A.M., Howard B.V., McKnight B., Duncan G.E., Beresford S.A., Mete M., et al. Associations of processed meat and unprocessed red meat intake with incident diabetes: the Strong Heart Family Study. Am. J. Clin. Nutr. 2012;95(3):752–758. doi: 10.3945/ajcn.111.029942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee E.T., Cowan L.D., Welty T.K., Sievers M., Howard W.J., Oopik A., et al. All-cause mortality and cardiovascular disease mortality in three American Indian populations, aged 45-74 years, 1984-1988. The Strong Heart Study. Am. J. Epidemiol. 1998;147(11):995–1008. doi: 10.1093/oxfordjournals.aje.a009406. [DOI] [PubMed] [Google Scholar]
- 18.SHS Strong Heart Study Operations Manual. Phase IV. Volume II: Morbidity and Mortality Surveillance Procedures. University of Oklahoma Health Sciences Center. 2001 https://strongheartstudy.org/portals/1288/Assets/documents/manuals/Phase%20IV%20Operations%20Manual.pdf?ver=2017-11-15-134610-080 [Internet] [accessed 2023-11-01]. Available from: [Google Scholar]
- 19.Miao G., Zhang Y., Huo Z., Zeng W., Zhu J., Umans J.G., et al. Longitudinal plasma lipidome and risk of type 2 diabetes in a large sample of American Indians with normal fasting glucose: the Strong Heart Family Study. Diabetes Care. 2021;44(12):2664–2672. doi: 10.2337/dc21-0451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Storey J.D. A direct approach to false discovery rates. J. R. Stat. Soc. B . 2002;64(3):479–498. [Google Scholar]
- 21.Storey John D., Tibshirani R. Statistical significance for genomewide studies. Proc. Natl. Acad. Sci. 2003;100(16):9440–9445. doi: 10.1073/pnas.1530509100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee E.T., Howard B.V., Wang W., Welty T.K., Galloway J.M., Best L.G., et al. Prediction of coronary heart disease in a population with high prevalence of diabetes and albuminuria: the Strong Heart Study. Circulation. 2006;113(25):2897–2905. doi: 10.1161/CIRCULATIONAHA.105.593178. [DOI] [PubMed] [Google Scholar]
- 23.Meierhofer D. Acylcarnitine profiling by low-resolution LC-MS. PLOS ONE. 2019;14(8) doi: 10.1371/journal.pone.0221342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O'Sullivan A., Gibney M.J., Brennan L. Dietary intake patterns are reflected in metabolomic profiles: potential role in dietary assessment studies. Am. J. Clin. Nutr. 2011;93(2):314–321. doi: 10.3945/ajcn.110.000950. [DOI] [PubMed] [Google Scholar]
- 25.Wedekind R., Kiss A., Keski-Rahkonen P., Viallon V., Rothwell J.A., Cross A.J., et al. A metabolomic study of red and processed meat intake and acylcarnitine concentrations in human urine and blood. Am. J. Clin. Nutr. 2020;112(2):381–388. doi: 10.1093/ajcn/nqaa140. [DOI] [PubMed] [Google Scholar]
- 26.Tong T.Y.N., Koulman A., Griffin J.L., Wareham N.J., Forouhi N.G., Imamura F. A combination of metabolites predicts adherence to the Mediterranean diet pattern and its associations with insulin sensitivity and lipid homeostasis in the general population: the Fenland Study, United Kingdom. J. Nutr. 2020;150(3):568–578. doi: 10.1093/jn/nxz263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bagheri M., Willett W., Townsend M.K., Kraft P., Ivey K.L., Rimm E.B., et al. A lipid-related metabolomic pattern of diet quality. Am. J. Clin. Nutr. 2020;112(6):1613–1630. doi: 10.1093/ajcn/nqaa242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li J., Guasch-Ferré M., Chung W., Ruiz-Canela M., Toledo E., Corella D., et al. The Mediterranean diet, plasma metabolome, and cardiovascular disease risk. Eur. Heart J. 2020;41(28) doi: 10.1093/eurheartj/ehaa209. 2645–2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Walker M.E., Song R.J., Xu X., Gerszten R.E., Ngo D., Clish C.B., et al. Proteomic and metabolomic correlates of healthy dietary patterns: the Framingham Heart Study. Nutrients. 2020;12(5):1476. doi: 10.3390/nu12051476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chandler P.D., Balasubramanian R., Paynter N., Giulianini F., Fung T., Tinker L.F., et al. Metabolic signatures associated with Western and Prudent dietary patterns in women. Am. J. Clin. Nutr. 2020;112(2):268–283. doi: 10.1093/ajcn/nqaa131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rosqvist F., Bjermo H., Kullberg J., Johansson L., Michaëlsson K., Ahlström H., et al. Fatty acid composition in serum cholesterol esters and phospholipids is linked to visceral and subcutaneous adipose tissue content in elderly individuals: a cross-sectional study. Lipids Health Dis. 2017;16(1):68. doi: 10.1186/s12944-017-0445-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang X., Sjögren P., Cederholm T., Ärnlöv J., Lindholm B., Risérus U., et al. Serum and adipose tissue fatty acid composition as biomarkers of habitual dietary fat intake in elderly men with chronic kidney disease. Nephrol. Dial. Transplant. 2014;29(1):128–136. doi: 10.1093/ndt/gfs478. [DOI] [PubMed] [Google Scholar]
- 33.Miao G., Fiehn O., Malloy K.M., Zhang Y., Lee E.T., Howard B.V., et al. Longitudinal lipidomic signatures of all-cause and CVD mortality in American Indians: findings from the Strong Heart Study. Geroscience. 2023;45:2669–2687. doi: 10.1007/s11357-023-00793-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schlitt A., Blankenberg S., Yan D., von Gizycki H., Buerke M., Werdan K., et al. Further evaluation of plasma sphingomyelin levels as a risk factor for coronary artery disease. Nutr. Metab (Lond). 2006;3:5. doi: 10.1186/1743-7075-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jiang X.C., Paultre F., Pearson T.A., Reed R.G., Francis C.K., Lin M., et al. Plasma sphingomyelin level as a risk factor for coronary artery disease. Arterioscler. Thromb. Vasc. Biol. 2000;20(12):2614–2618. doi: 10.1161/01.atv.20.12.2614. [DOI] [PubMed] [Google Scholar]
- 36.Nelson J.C., Jiang X.C., Tabas I., Tall A., Shea S. Plasma sphingomyelin and subclinical atherosclerosis: findings from the multi-ethnic study of atherosclerosis. Am. J. Epidemiol. 2006;163(10):903–912. doi: 10.1093/aje/kwj140. [DOI] [PubMed] [Google Scholar]
- 37.Jensen P.N., Fretts A.M., Hoofnagle A.N., McKnight B., Howard B.V., Umans J.G., et al. Circulating ceramides and sphingomyelins and the risk of incident cardiovascular disease among people with diabetes: the Strong Heart study. Cardiovasc. Diabetol. 2022;21(1):167. doi: 10.1186/s12933-022-01596-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.García-Jaramillo M., Lytle K.A., Spooner M.H., Jump D.B. A lipidomic analysis of docosahexaenoic acid (22:6, ω3) mediated attenuation of Western diet induced nonalcoholic steatohepatitis in male Ldlr-/-mice. Metabolites. 2019;9(11):252. doi: 10.3390/metabo9110252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Iannone V., Lok J., Babu A.F., Gómez-Gallego C., Willman R.M., Koistinen V.M., et al. Associations of altered hepatic gene expression in American lifestyle-induced obesity syndrome diet-fed mice with metabolic changes during NAFLD development and progression. J. Nutr. Biochem. 2023;115 doi: 10.1016/j.jnutbio.2023.109307. [DOI] [PubMed] [Google Scholar]
- 40.Wang H.J., Zakhari S., Jung M.K. Alcohol, inflammation, and gut-liver-brain interactions in tissue damage and disease development. World J. Gastroenterol. 2010;16(11):1304–1313. doi: 10.3748/wjg.v16.i11.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Simopoulos A.P. Omega-3 fatty acids in inflammation and autoimmune diseases. J. Am. Coll. Nutr. 2002;21(6):495–505. doi: 10.1080/07315724.2002.10719248. [DOI] [PubMed] [Google Scholar]
- 42.Majdan M., Bobrowska-Korczak B. Active compounds in fruits and inflammation in the body. Nutrients. 2022;14(12):2496. doi: 10.3390/nu14122496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim H., Anderson C.A., Hu E.A., Zheng Z., Appel L.J., He J., et al. Plasma metabolomic signatures of healthy dietary patterns in the chronic renal insufficiency cohort (CRIC) study. J. Nutr. 2021;151(10):2894–2907. doi: 10.1093/jn/nxab203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhu Q., Wu Y., Mai J., Guo G., Meng J., Fang X., et al. Comprehensive metabolic profiling of inflammation indicated key roles of glycerophospholipid and arginine metabolism in coronary artery disease. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.829425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen H., Wang Z., Qin M., Zhang B., Lin L., Ma Q., et al. Comprehensive metabolomics identified the prominent role of glycerophospholipid metabolism in coronary artery disease progression. Front. Mol. Biosci. 2021;8 doi: 10.3389/fmolb.2021.632950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hu Y., Hu F.B., Manson J.E. Marine omega-3 supplementation and cardiovascular disease: an updated meta-analysis of 13 randomized controlled trials involving 127 477 participants. J. Am. Heart Assoc. 2019;8(19) doi: 10.1161/JAHA.119.013543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ander B.P., Dupasquier C.M., Prociuk M.A., Pierce G.N. Polyunsaturated fatty acids and their effects on cardiovascular disease. Exp. Clin. Cardiol. 2003;8(4):164–172. [PMC free article] [PubMed] [Google Scholar]
- 48.Neff Warner L.R., Littman A.J., O'Leary M., Sarche M., Nelson L.A., Gray J.S., et al. Diet quality and depression in a cohort of American Indians: the Strong Heart Family Study. Am. Indian Alsk. Native Ment. Health Res. 2022;29(3):90–121. doi: 10.5820/aian.2903.2022.90. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The phenotype data used in this study can be requested through the Strong Heart Study (https://strongheart-study.org/). The lipidomic data can be obtained from the corresponding author on a reasonable request.