Abstract
In the present study, we report the finding of high concentrations of D-Asp (D-aspartate) in the retina of the cephalopods Sepia officinalis, Loligo vulgaris and Octopus vulgaris. D-Asp increases in concentration in the retina and optic lobes as the animal develops. In neonatal S. officinalis, the concentration of D-Asp in the retina is 1.8±0.2 μmol/g of tissue, and in the optic lobes it is 5.5±0.4 μmol/g of tissue. In adult animals, D-Asp is found at a concentration of 3.5±0.4 μmol/g in retina and 16.2±1.5 μmol/g in optic lobes (1.9-fold increased in the retina, and 2.9-fold increased in the optic lobes). In the retina and optic lobes of S. officinalis, the concentration of D-Asp, L-Asp (L-aspartate) and L-Glu (L-glutamate) is significantly influenced by the light/dark environment. In adult animals left in the dark, these three amino acids fall significantly in concentration in both retina (approx. 25% less) and optic lobes (approx. 20% less) compared with the control animals (animals left in a diurnal/nocturnal physiological cycle). The reduction in concentration is in all cases statistically significant (P=0.01–0.05). Experiments conducted in S. officinalis by using D-[2,3-3H]Asp have shown that D-Asp is synthesized in the optic lobes and is then transported actively into the retina. D-aspartate racemase, an enzyme which converts L-Asp into D-Asp, is also present in these tissues, and it is significantly decreased in concentration in animals left for 5 days in the dark compared with control animals. Our hypothesis is that the dicarboxylic amino acids, D-Asp, L-Asp and L-Glu, play important roles in vision.
Keywords: D-aspartate racemase, cephalopod, dicarboxylic amino acid, mollusc, vision
Abbreviations: D-AAO, D-amino acid oxidase; D-AspO, D-aspartate oxidase; GH, growth hormone; LH, luteinizing hormone; NAC, N-acetylcysteine; NMDA, N-methyl-D-aspartate; ODS, octadecylsilyl; OPA, o-phthalaldehyde; POD, horseradish peroxidase; TCA, trichloroacetic acid
INTRODUCTION
D-Asp (D-aspartate) is an endogenous amino acid found in the nervous and endocrine system of various animals. It was first found in the brain, stellate ganglia and axoplasm of the giant axon of the molluscs Octopus vulgaris (common octopus), Sepia officinalis (common cuttlefish) and Loligo vulgaris (common squid) [1,2]. Subsequently, D-Asp has been found in the nervous and endocrine tissues of many other animals, including the invertebrates Aplysia fasciata (sea hare) [3], Ciona intestinalis (sea squirt) [4], Penaeus japonicus (Kuruma prawn) and Jasus lalandii (rock lobster) [5] and the vertebrates Rana esculenta (green frog) [6], Podarcis sicula (Italian wall lizard) [7], Merlucius merlucius (European hake) and Solea solea (common sole) [8], Gallus gallus (chicken) [9], Rattus sp. (rat) [10–16] and Homo sapiens (human), in brain [17–19] and in cerebrospinal fluid [20,21]. As far as the physiological functions of D-Asp in the animal kingdom are concerned, important biological roles for this D-amino acid are now postulated for many invertebrates and, particularly, in higher vertebrates. In fact, it has been observed that in the brain of chicken [9], rat [10–12] and human [18], very high levels of D-Asp occur transiently during the last stage of the embryonic life or in the early post-natal life, suggesting that D-Asp is involved in the development of the nervous system in these animal species.
In addition to the role that D-Asp has in the nervous system, other studies have demonstrated that D-Asp also has an important role in the endocrine system. In adult rat, it has been demonstrated that D-Asp has neuronal and neuroendocrine roles [22–24]. D-Asp has been found at very high concentrations in adrenal, testis, pituitary, thymus and ovary [12]. Later, D-Asp was also found in very high concentrations in the pineal gland of rat (approx. 1.0 μmol/g of tissue), with a peak of concentration during the night (2830 pmol/pineal gland) [13,16]. The peak of D-Asp was concomitant with the higher concentration of melatonin in this gland (5.7 pmol/pineal gland), thus suggesting an involvement of D-Asp in the synthesis and release of melatonin [25]. High concentrations of D-Asp in the pineal gland were observed by Lee et al. [26], who also found D-Asp in the pituitary gland and retina [27]. In addition, it has been demonstrated that the pituitary gland has a high capacity to accumulate D-Asp, and that this amino acid has the capacity to induce the release and synthesis of testosterone either through the release of LH (luteinizing hormone) or by acting directly on the testes to release testosterone [22], as well as being implicated in testosterone synthesis in rat Leydig cells [23] and in spermatogenesis [24]. The implication of D-Asp in the maturation of sexual glands and in the synthesis and release of steroid hormones has been demonstrated to occur in the frog Rana esculenta [6] and in the lizard Podarcis sicula [7].
Parallel to the biochemical study, immunohistochemical studies using an anti-D-Asp antibody have also demonstrated the presence of D-Asp in a variety of cultured mammalian cells, e.g. cultured rat pinealocytes [25], pheochromocytoma PC12 cells [28], rat GH3 pituitary tumour cells [29] and pheochromocytoma MPT1 cells, a subclone of PC12 cells [30], as well as in animal tissues, e.g. various regions of rat brain [31,32], rat adrenal gland [33], pineal glands [26], the central nervous system of rat embryo E12 [15,16] cells, spermatids of rat testis [24,34], and rat pituitary and retina [27].
It has been demonstrated that NMDA (N-methyl-D-aspartate), a molecule well known for its neuroexcitatory activity and for its action on the L-Glu (L-glutamate) receptors belonging to the NMDA class, is an endogenous compound that is present in rat nervous system and endocrine glands [35,36]. This compound is biosynthesized in vivo from its precursor, D-Asp, with a SAM (S-adenosylmethionine)-dependent methyltransferase enzyme being responsible for its synthesis [35,36]. Both D-Asp and NMDA are mostly concentrated in the rat adenohypophysis, hypothalamus, brain and testis [35]. It has been demonstrated that NMDA induces the release of GnRH (gonadotropin-releasing hormone) from the hypothalamus, which in turn induces the synthesis and release at the adenohypophysis of LH, GH (growth hormone) [35] and prolactin [36] in rat. This phenomenon, which was based on the mutual action of D-Asp and NMDA in the synthesis and release of nervous and endocrine hormones, has also been demonstrated to occur in the tunicate Ciona intestinalis [4]. Finally, in addition to the release of LH, GH and prolactin, as mentioned above, more recently, D-Asp has also been demonstrated to be involved in the release of α-melanocyte-stimulating hormone, GABA (γ-aminobutyric acid) and dopamine [37]. D-Asp is present in all magnocellular neurons of the rat hypothalamus, and participates in oxytocin production during lactation, as well as increased oxytocin gene expression and decreased concentration of circulating oxytocin [38]. A last interesting observation is that D-Asp has also been found as a nuclear component of cells in the mammalian hypothalamo-neurohypophyseal system, and it is supposed that D-Asp interacts directly with DNA or nuclear proteins to activate/inactivate genes in order to control transcription [39]. A specific mRNA and related protein, steroidogenic acute regulatory protein, has also been demonstrated [40].
A racemase that specifically synthesizes D-Asp from L-Asp, D-aspartate racemase, has been found in rat brain [16] and in the mollusc blood shell Scapharca broughtonii [41], indicating that D-Asp is synthesized in vivo. In addition, a D-AspO (D-aspartate oxidase; EC 1.4.3.1), the enzyme which specifically oxidizes D-Asp into oxaloacetate has been found in various animals, and has been purified from O. vulgaris [1] and from beef kidney [42].
In another set of studies, using an enzymic method based on the use of D-AAO (D-amino acid oxidase; EC 1.4.3.3) and chromatographic methods based on the enantiomeric separation of amino acids with chiral HPLC stationary phases, D-amino acids, other than D-Asp, have been found in invertebrates and mammals, but at very low concentrations compared with D-Asp, except for D-Ala (D-alanine) and D-Ser (D-serine), which were found at concentrations approximately the same as that of D-Asp. In mammals, D-Ala has been reported to be present in human brain [17], in human cerebrospinal fluid [20] and in rat pituitary gland [14]. Other interesting studies have been performed on D-Ser. In rodents and humans, several groups have demonstrated that free D-Ser occurs predominantly in the brain, and persists at high levels (200–300 nmol/g) in embryonic and post-natal life [18,43]. However, while D-Asp was found at higher levels in the endocrine glands, D-Ser was found at high levels in the brain regions (cerebrum, hippocampus and hypothalamus) [18]. In addition, interesting studies have been conducted in rat that demonstrated that D-Ser selectively potentiates NMDA-receptor-mediated neurotransmission at the glycine site [44].
All the above results thus indicate that D-Asp has an important role in the neuroendocrine system. However, no in-depth studies have been conducted on the presence and role of D-Asp in the visual nervous system. In the present study, we have measured the concentration of D-Asp in the retina of the marine molluscs: S. officinalis, L. vulgaris and O. vulgaris, and have found that these tissues possess a consistent amount of D-Asp, and that there exists a relationship between the amount of D-Asp in the retina and D-aspartate racemase (the enzyme which synthesizes D-Asp), and their exposure to light. Experiments were conducted on adult S. officinalis and on embryos or early post-natal animals. Our hypothesis is that D-Asp could have an important role in vision.
MATERIALS AND METHODS
Materials
D-AAO was purchased in purified form from Roche Applied Science (Monza, Italy) or from Sigma Chemical Company (St. Louis, MO, U.S.A.). Standard amino acids, D-Asp, NMDA, D-Glu (D-glutamate), and other D- and L-amino acids, were from Sigma. The Sep-Pak® cartridges or columns of ODS (octadecylsilyl)-C18 resin, and the ultrafree centrifugal filter membranes with a cut-off of 30 kDa were from Waters-Millipore Corporation (Milford, MA, U.S.A.). Sephacryl S-200 resin was from Amersham Biosciences (Piscataway, NJ, U.S.A.). The cation-exchange resin AG 50W-X8 (100–200 mesh) and the anion-exchange resin AG 1W-X8 (100–200 mesh) were from Bio-Rad (Hercules, CA, U.S.A.). Acetonitrile and other organic solvents for HPLC were from Merck (Darmstadt, Germany) or Carlo Erba Reagenti (Milan, Italy). D-[2,3-3H]Asp (10–30 Ci/mmol; 1 Ci/ml) was purchased from Amersham Biosciences. All other chemicals used in this work were analytical-grade reagents.
Preparation and purification of D-AspO from O. vulgaris and from beef kidney
D-AspO is an enzyme able to oxidize D-Asp, NMDA and D-Glu, and is used as a tool for the determination of the above amino acids. Since D-AspO from the hepatopancreas of O. vulgaris oxidizes D-Asp and D-Glu, and, to a lesser extent, NMDA, whereas D-AspO from beef kidney oxidizes D-Asp and NMDA, and, to a lesser extent, D-Glu [46,47], enzymes from both sources were used. D-AspO from O. vulgaris was prepared according the described procedure [1], whereas the D-AspO from beef kidney was obtained by overexpression in Escherichia coli as described in [45], modified as follows: a single-stranded cDNA was synthesized from bovine kidney polyadenylated RNA, using reverse transcriptase and oligo(dT) as a primer cDNA, and was amplified by PCR using the single-stranded cDNA as a template and, as primers, BOVI (5′-ATGGATACAGTACGGATTGC-3′), complementary to the 5′ coding region, and BOVII (5′-TTGCTATCATTTCGGGTCAC-3′), complementary to the 3′ coding region of the cDNA of bovine kidney D-AspO. The resulting fragment was inserted into the expression vector pKK223-3, and the sequence was controlled by the deoxynucleotide termination procedure and inserted in E. coli JM105. Cells harbouring pKK223-3 D-AspO were grown in 1 litre of LB (Luria–Bertani) medium, containing 100 μg/ml ampicillin, at 37 °C. IPTG (isopropyl β-D-thiogalactoside) was added to a final concentration of 1 mM, and cells were grown for an additional 4–8 h. The bacterial suspension was centrifuged for 30 min at 3000 g, and the pellet was sonicated in 10 ml of 0.1 M citrate/phosphate buffer (McIlvaine buffer), pH 5.5, containing 30 mM sodium or potassium tartrate and 20 mM EDTA. The homogenate was heated at 55 °C for 5 min and centrifuged at 30000 g for 30 min. The supernatant was brought to 45% saturation with NH4SO4 and centrifuged as above. The precipitate was dissolved in 50 ml of 0.05 M Tris/HCl, pH 8.0, containing 10 mM sodium tartrate and 10 mM EDTA, and dialysed extensively against the same buffer. The dialysed sample was then added to a cation-exchange resin (DE-52 column, 2.5 cm×20 cm), equilibrated in 0.05 M Tris/HCl, pH 8.0, followed by 100 ml of the same buffer. The eluate was saturated to 45% with NH4SO4 and centrifuged as above. The pellet was dissolved in 5 ml of the same buffer and passed through a Sephacryl S-200 column (2.5 cm×80 cm) equilibrated in 0.1 M phosphate buffer, pH 7.0, containing 10 mM sodium or potassium tartrate. Fractions of 5 ml were collected, and those with the highest specific activity were pooled and concentrated to 2 ml by using an ultrafree filter membrane with cut-off of 30 kDa. The concentrated enzyme contained 5 mg/ml of protein and had an activity of 30 units/mg, where 1 unit is defined as the amount of the enzyme capable of oxidizing 1 μmol of D-Asp in 1 min at 37 °C.
Sample preparation for amino acid analysis
The retinas, optic lobes and brain from each adult animal were homogenized with 0.1 M TCA (trichloroacetic acid) at a ratio of 1:10, using an Ultra-Turrax T25 homogenizer, and were centrifuged for 10 min at 25000 g. The supernatants were passed through a column (1 cm×5 cm) of cation-exchange resin (AG 50WX8; 100–200 mesh) and washed with 12 ml of 0.001 M HCl. The amino acids were eluted with 8 ml of 4 M NH4OH, and the eluate was dried by evaporation in small Petri dishes under a hood over a warm plate at 40–50 °C. The residue was dissolved in 2 ml of distilled water and was purified further by passage through a Sep-Pak® cartridge containing 0.8 g of ODS-C18 (previously activated with methanol and washed with distilled water). After the sample was loaded on to the Sep-Pak® cartridge, it was washed with 2 ml of distilled water, and all the eluates were dried by evaporation as above, before dissolving the residues in water using a microlitre amount of water equivalent to the mg of tissue used.
Determination of D-Asp and L-amino acids by HPLC
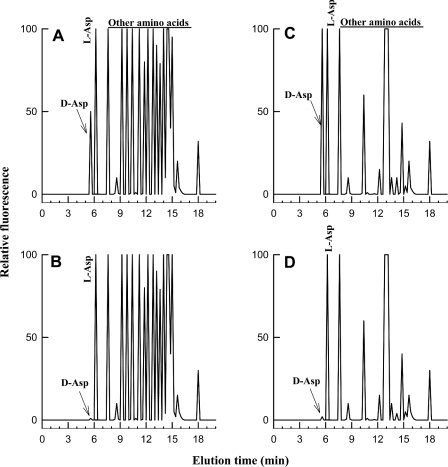
The method was based on the separation of D-Asp from other amino acids as described in [35,48] and modified as follows: 10 μl of purified sample was mixed with 100 μl of 0.5 M sodium pyrophosphate buffer, pH 9.5, and 20 μl of OPA (o-phthalaldehyde)/NAC (N-acetylcysteine) reagent (prepared by mixing 20 mg of OPA with 10 mg of NAC in 2 ml of 50% methanol). After 2 min, water was added to a final volume of 1000 μl, and 50 μl was injected onto a C18 Supelcosil column (0.45 cm×25 cm; Supelco, Belafonte, PA, U.S.A.) connected to a Beckman-Gold HPLC system. The column was eluted at 1.2 ml/min, with a gradient consisting of solution A (920 ml of water, 30 ml of 1 M citrate/phosphate buffer (McIlvaine buffer), pH 5.6, and 50 ml of acetonitrile) and solution B (90% acetonitrile in water). The programme gradient was 0–5% solution B for 10 min; 5–30% solution B for 30 min, 30–100% solution B for 10 min, staying at 100% solution B for 5 min and returning to 0% of solution B for 1 min. The fluorescence was read at an excitation wavelength of 330 nm and an emission wavelength of 450 nm. D-Asp was eluted with a peak at 5.6 min, followed by L-Asp 0.5 min later, and was well separated from other amino acids (Figure 1). To verify that the peak eluted at 5.6 min was really D-Asp, 10 μl of the sample was mixed with 20 μl of 0.5 M pyrophosphate buffer, pH 8.2, and 2 μl of purified D-AspO, and incubated for 20 min at 37 °C, then 100 μl of 0.5 M pyrophosphate buffer, pH 9.5, and 20 μl of OPA/NAC were added, before HPLC as before. The absence of the peak at elution time 5.6 min or its decrease confirmed the presence of D-Asp. A standard consisting of 10 μl of a mixture of amino acid standards (10 nmol of D-Asp and 20 nmol of each of the other L-amino acids) and all other components as used for the sample was carried out under the same conditions to give a standard curve. The areas of the peaks of amino acid standards were used to calculate the amount of D-Asp and other amino acids contained in the sample (Figure 1).
Figure 1. HPLC analyses of D-Asp and other amino acids in a standard amino acid mixture and in the retina of S. officinalis.
(A) Profile of a mixture of standard amino acids containing 12.5 nmol of D-Asp and 25 nmol of each amino acid injected into the HPLC column. (B) The same amino acid mixture as in (A), but after D-AspO treatment. (C) Free amino acids from 0.05 mg of retina chromatographed by HPLC under the same conditions as in (A). (D) The same sample as (C), but after D-AspO treatment. In (B) and (D), the peak corresponding to D-Asp is abolished by the action of D-AspO.
Colorimetric methods for the determination of the sum of D-Asp, D-Glu and NMDA, and the sum of other amino acids
Two colorimetric methods were used to determine D-amino acids. The first method was based on the determination of the α-oxoacids developed by the reaction between a D-amino acid and D-AspO or D-AAO. The second method was based on the determination of H2O2 developed by the same reaction as follows:
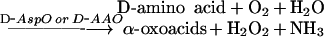 |
According to the specificity of the oxidases, when D-AspO was used, then D-Asp, D-Glu and NMDA were determined (see above), and when D-AAO was used, other D-amino acids [D-Pro, D-Met, D-Leu, D-Ile, D-Phe, D-Tyr, D-Ala, D-Trp, D-Ser, D-Thr, D-His, D-Arg, D-Val and D-Lys (using three-letter amino acid codes)] were oxidized [49].
Method 1: based on the determination of the α-oxoacids
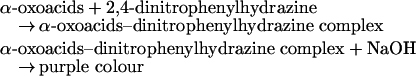 |
In order to determine the sum of D-Asp+D-Glu+NMDA, the following assay procedure was used. Into three Eppendorf tubes, 100 μl of the purified sample was added, along with 50 μl of 1 M Tris/HCl, pH 8.2. Then, to the first tube (sample), 2.5 μl of D-AspO from O. vulgaris and 2.5 μl of D-AspO from beef kidney (overexpressed) were added. To the second tube (sample+internal standard), 10 μl of D-Asp standard (1 μmol/ml) and the same amount of the two enzymes were added. To the third tube (blank sample), 10 μl of water was added. The three tubes were incubated for 30 min at 37 °C. Afterwards, 50 μl of 5 mM 2,4-dinitrophenylhydrazine (dissolved in 5 M HCl) was added and mixed, and tubes were left to incubate at room temperature (20–25 °C) for 10 min. Then, 700 μl of 1 M NaOH was added and mixed, and, after 5 min, the A445 was read against water.
To determine the sum of other D-amino acids, the same assay was used, but 2.5 μl of purified D-AAO was used instead of D-AspO, and D-Ala was used as standard instead of D-Asp.
Method 2: based on the determination of H2O2
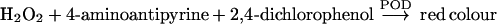 |
where POD is horseradish peroxidase.
In order to determine the sum of D-Asp+D-Glu+NMDA, the following modification of the method described in [50] was used. Into three Eppendorf tubes, 200 μl of the purified sample, 50 μl of a POD/H2O2 assay mixture [prepared by mixing 1 ml of 2 M Tris/HCl buffer, pH 8.2, 0.5 ml of 10 mM 4-aminoantipyrine, 0.5 ml of 8 mM 2,4-dichlorophenol and 20 μl of POD (2500 units/ml)] were mixed. Then, to the first tube (sample), 2.5 μl of D-AspO from O. vulgaris and 2.5 μl of D-AspO from beef kidney (overexpressed) were added. To the second tube (sample+internal standard), 10 μl of D-Asp standard (1 μmol/ml) and 2.5 μl of each D-AspO, as above, were added. To the third tube (blank sample), 10 μl of water was added. All tubes were incubated for 30 min at 37 °C. After that, 250 μl of water was added and mixed, and the A510 was read against water.
To determine the sum of other D-amino acids, the same assay was used, but 2.5 μl of purified D-AAO was used instead of D-AspO, and D-Ala was used as a standard instead of D-Asp. For both methods used, the concentration of the sum of D-Asp+D-Glu+NMDA was expressed as the total amount of corresponding D-Asp, and was calculated as follows:
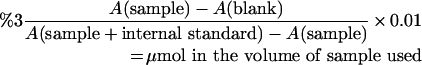 |
To determine the concentration of the sum of the other D-amino acids (D-Pro, D-Met, D-Leu, D-Ile, D-Phe, D-Tyr, D-Ala, D-Trp, D-Ser, D-Thr, D-His, D-Arg, D-Val and D-Lys), the same calculation was carried out, and the concentration of total D-amino acids were expressed as the total amount of corresponding D-Ala.
D-Asp and other amino acids in the retina and optic lobes of S. officinalis, and light and dark experiments
Since the highest concentration of D-Asp was observed in the retina of S. officinalis (Table 1), we conducted an experiment on this species to determine whether D-Asp in the retina and optic lobes was influenced by light. For this purpose, two groups of S. officinalis each consisting of eight animals were left to acclimatize for 3–4 days in circulating seawater (which was obtained via a direct connection with the seawater of the Bay of Naples). The first group was left in tanks maintained in an environment with 12 h of light and 12 h of darkness (to simulate the natural environment). The second group was left in tanks in a dark room. After 5 days in these conditions, the animals from both groups were killed, and the retinas and optic lobes were processed for the determination of D-amino acids.
Table 1. D-Asp acid in the retina of adult S. officinalis, L. vulgaris and O. vulgaris.
Values are the means±S.D., expressed as μmol/g of tissue obtained from eight adult animals using the HPLC and the colorimetric methods.
| D-Asp* | D-Glu+NMDA† | Other D-amino acids‡ | |||
|---|---|---|---|---|---|
| HPLC method | α-Oxoacid method | H2O2 method | α-Oxoacid method | H2O2 method | |
| S. officinalis (160–200 g body mass) | 2.60±0.30 | 0.35±0.05 | 0.33±0.04 | 0.48±0.06 | 0.44±0.05 |
| O. vulgaris (1200–1500 g body mass) | 2.30±0.25 | 0.30±0.06 | 0.28±0.05 | 0.35±0.06 | 0.40±0.09 |
| L. vulgaris (130–150 g body mass) | 1.60±0.20 | 0.25±0.05 | 0.26±0.04 | 0.35±0.03 | 0.32±0.04 |
* Specific determination of D-Asp using the HPLC method.
† D-Glu+NMDA was obtained by calculating the difference between the total concentration of D-Asp+D-Glu+NMDA, obtained using the colorimetric method, minus D-Asp, as obtained using the HPLC method.
‡ The sum of the D-amino acids, D-Pro, D-Met, D-Leu, D-Ile, D-Phe, D-Tyr, D-Ala, D-Trp, D-Ser, D-Thr, D-His, D-Arg, D-Val and D-Lys, obtained by using the colorimetric method based on the use of D-AAO.
D-Aspartate racemase and light/dark environment in the retina and optic lobes of S. officinalis
We next sought to determine whether endogenous D-Asp is synthesized in vivo from L-Asp by D-aspartate racemase and, if so, whether the enzymic activity is influenced by the light/dark conditions. Retinas and optic lobes obtained from eight adult S. officinalis were homogenized (1:5) in 0.02 M phosphate buffer, pH 7.5, containing 10 mM EDTA and a protease inhibitor cocktail (1:100; Sigma), and centrifuged at 30000 g for 30 min. Then, in Eppendorf tubes, 100 μl of the supernatant of the homogenate was mixed with 100 μl of 0.2 M L-Asp (sodium salt, neutral pH) and 20 μl of 0.5 M sodium tartrate to inhibit the D-AspO [42], and with 100 μl of 1 M citrate/phosphate buffer (McIlvaine buffer) at pH 5.0–7.5 or with 100 μl of 1 M Tris/HCl, at pH 8–9. Then the pH of each assay mixture was readjusted to the desired pH with 1–5 μl of 1 M NaOH or 1 M HCl, and incubated at 37 °C for 2 h. A blank sample was included at the same pH values as for the sample, but distilled water replaced L-Asp. After incubation, 50 μl of 1 M TCA was added to the sample, mixed and centrifuged at 12000 g for 20 min. The supernatant was purified on cation exchange as described above, and reduced to a volume of 500 μl in 0.2 M pyrophosphate buffer, pH 8.2, and the amount of D-Asp synthesized was determined using HPLC. The racemase activity was expressed in enzyme units/g of tissue, where 1 enzyme unit was defined as the μmol of D-Asp produced in 2 h of incubation under the above assay conditions. Other L-amino acids (L-Glu, L-Ala, L-Ser) were also tested under the same assay conditions as possible substrates for racemase activity. In order to determine whether racemase activity was influenced by the light, the same experiment was also conducted on the retina and optic lobes of S. officinalis left for 5 days in the dark.
D-Asp migration from optic lobes to retina of S. officinalis
This experiment was carried out on juvenile (20-day-old) S. officinalis. The eggs of S. officinalis, collected from the Bay of Naples by frogmen from the Zoological Station of Naples, were kept in circulating seawater at 18 °C. When the embryos were hatching, they were left in circulating seawater for 20 days, fed with plankton also collected from the Bay of Naples. At this stage, the animals weighed approx. 2–3 g. The optic lobes, retinas and central brain weighed approx. 10.4, 5.02 and 2.04 mg respectively. Five animals were chosen for the in vivo experiment. Each animal was immersed in seawater containing 5% ethyl alcohol until anaesthetized (within 4–5 min). A small portion of tissue between the eye and brain was opened (under a binocular microscope) to visualize the optic lobes, and then 1 μl of a solution of D-[2,3-3H]Asp (20±10 Ci/mmol; 1 Ci/ml) was injected into the optic lobes with a small needle. In total 2200000 d.p.m. (0.05 pmol of D-Asp) were injected. After injection, the animals were left in approx. 500 ml of normal oxygenated seawater for 12 h at 16–18 °C. During this time, the animals live normally without apparent suffering. After that, the animals were killed and the optic lobes, the retina adjacent to the treated optic lobe and the brain were taken from each animal that had been injected with D-Asp. Each tissue was homogenized in 100 μl of 0.01 M TCA and centrifuged at 10000 g for 20 min. Supernatant (20 μl) was mixed with 2 μl of 0.1 M NaOH (to neutralize the TCA), 10 μl of 0.1 M pyrophosphate buffer, pH 9.5, 20 μl distilled water and 5 μl of OPA-NAC. After 2 min, 50 μl of this mixture was injected on to the HPLC, and chromatographed as above to separate D-Asp from other amino acids. The peak corresponding to D-Asp was collected, and the radioactivity was measured in a scintillation counter.
Statistical analysis
Statistical analyses were performed using Statistical (StatSoft, 1997/98 Edition). Comparison of two groups was made using Student's t test. Three or more group comparisons were conducted using ANOVA with Duncans's post-hoc tests.
RESULTS
D-Asp in the retina of the cephalopods S. officinalis, L. vulgaris and O. vulgaris
In a previous study, Neidle and Dunlop [9] demonstrated that the retinas of chicken embryos and neonatal rats contain a consistent amount of D-Asp. In addition, these authors also observed that D-Asp increases in the chicken embryos at the thirteenth day of embryonic life, and in the rat at the seventh day after birth, suggesting that D-Asp had some then-unknown specific role [9]. Immunohistochemical studies conducted by Lee et al. [27] also have confirmed the presence of D-Asp in adult rat retinas. In the present study, using a specific method for the determination of D-Asp by HPLC (Figure 1) and two colorimetric methods based on the use of D-AspO and D-AAO, we found that the retinas of S. officinalis, O. vulgaris and L. vulgaris possess a consistent concentration of D-Asp (Table 1), and that this compound constitutes the only significant D-amino acid compared with the other D-amino acids present in this tissue. In fact, as shown in Table 1, the results obtained on the retinas from eight adult animals of each species of cephalopod indicate a mean value of D-Asp of 2.60±0.30 μmol/g of tissue in S. officinalis, and 2.30±0.25 and 1.6±0.20 μmol/g in O. vulgaris and L. vulgaris respectively. When the retina samples were analysed using the colorimetric methods based on the use of D-AspO from beef kidney and from O. vulgaris hepatopancreas, we found that the sum of D-Glu+NMDA was 0.30±0.06 and 0.25±0.05 in the retina of O. vulgaris and L. vulgaris respectively, using the colorimetric hydrazone method. Analogously, using the colorimetric method based on the determination of H2O2, we found that total D-Glu+NMDA was 0.33±0.04 μmol/g tissue in the retina of S. officinalis, and 0.28±0.05 and 0.26±0.04 in that of O. vulgaris and L. vulgaris respectively. Thus D-Glu+NMDA was only 12–15% of D-Asp (Table 1). Roughly the same results were found for total other amino acids, determined by the use of D-AAO. Also, in this case, the sum of total D-amino acids (D-Pro, D-Met, D-Leu, D-Ile, D-Phe, D-Tyr, D-Ala, D-Trp, D-Ser, D-Thr, D-His, D-Arg, D-Val and D-Lys), was much less when compared with the concentration of D-Asp (Table 1).
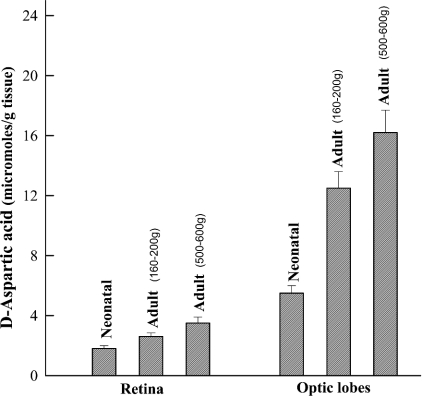
D-Asp in the retina and optic lobes of S. officinalis during development
We have compared the concentration of D-Asp in the retina and optic lobes of S. officinalis during the development of the animal. The results have demonstrated that in both retina and optic lobes, D-Asp increases with the increasing age of the animals (Figure 2). As they hatch, the animals already possess D-Asp in the retina at a concentration of 1.8±0.2 μmol/g of tissue, whereas in the optic lobes, D-Asp occurs at 5.5±0.4 μmol/g of tissue. When the animals reach a body mass of 160–200 g, which corresponds to an age of approx. 3–6 months (according to zoologists), the concentration in the retina reaches the value of 2.6±0.25 μmol/g of tissue, and in the optic lobes, it is 12.5±1.1 μmol/g of tissue. Finally, when the animals reach a body mass of 500–600 g, at 6–12 months of age, the concentration of D-Asp reaches 3.5±0.40 μmol/g in retina and 16.2±1.5 μmol/g in the optic lobes (Figure 2). Thus a progressive increment of D-Asp occurs in proportion to the age of the animal. The other common amino acids present in the retina and optic lobes also increase with age, but their increase is very much less evident than that observed for D-Asp (results not shown).
Figure 2. D-Asp in the retina and optic lobes of S. officinalis during development.
Neonatal animals were taken as soon as they hatched. Adult animals were at 160–200 g of body mass, corresponding to an age of approx. 3–6 months, and animals with body mass of 500–600 g constitute animals of approx. 6–12 months of age. Results are means±S.D. obtained from eight animals for each group.
D- and L-Asp and L-Glu in the retina and optic lobes: influence of light and dark
The presence D-Asp in the retina of the cephalopods in consistent amounts led us to hypothesize that D-Asp in the retina could have a role in vision. In order to verify this possibility, we conducted experiments to determine D-Asp and other amino acids in the retinas of a group of eight adult S. officinalis left for 5 days in an environment of 12 h of light and 12 h of darkness (controls), and of another group of eight S. officinalis left for 5 days in the dark. The results obtained from this experiment indicated that, in the retinas of animals which lived in the dark, the concentrations of D-Asp, and also of L-Asp and L-Glu, were decreased significantly compared with the control animals in the light/dark cycle. We found, in fact, that the mean values of these three amino acids obtained on S. officinalis that were left in a diurnal nocturnal cycle (controls) were of 2.60±0.25, 2.18±0.2 and 3.56±0.36 μmol/g of retina for D-Asp, L-Asp and L-Glu respectively, whereas the mean values of the same amino acids obtained in the retinas of the group of S. officinalis left in the dark were 2.05±0.24, 1.78±0.22 and 2.78±0.25 μmol/g of retina respectively (Table 2). Thus the decrease in concentration of these amino acids between the control animals and the animals left in the dark was 1.27-, 1.22- and 1.28-fold, and, in all cases, the difference was significant for P<0.01 (Table 2). All the other amino acids present in significant amounts in the retina (L-Ser, glycine, taurine and L-Ala) did not show any difference in concentration between the retinas of control animals and the retinas of animals that were left in the dark (Table 2).
Table 2. D-Asp and L-amino acids in the retina and optic lobes of S. officinalis in control animals and in animals left in the dark.
Values are the mean±S.D. expressed as μmol/g of tissue, obtained by HPLC analysis from eight adult animals of body mass 160–200 g. *P<0.01; **P<0.05.
| Retina from control animals | Retina from animals left in dark | Ratio control/dark | Optic lobes from control animals | Optic lobes from animals left in dark | Ratio control/dark | |
|---|---|---|---|---|---|---|
| D-Asp | 2.60±0.25 | 2.05±0.24 | 1.27* | 12.5±1.10 | 10.4±0.80 | 1.20* |
| L-Asp | 2.18±0.20 | 1.78±0.22 | 1.22* | 2.80±0.35 | 2.43±0.30 | 1.15** |
| L-Glu | 3.56±0.36 | 2.78±0.25 | 1.28* | 18.8±2.40 | 16.0±2.20 | 1.17** |
| L-Ser | 0.35±0.04 | 0.34±0.05 | 1.02 | 1.10±0.14 | 1.14±0.15 | 0.96 |
| Gly | 1.04±0.05 | 1.02±0.04 | 1.02 | 5.30±0.60 | 5.20±0.50 | 1.02 |
| Taurine | 13.0±1.40 | 12.6±1.35 | 1.03 | 42.3±3.30 | 41.5±3.10 | 1.02 |
| L-Ala | 0.85±0.15 | 0.80±0.16 | 1.02 | 4.50±0.44 | 4.48±0.50 | 1.0 |
Since the optic lobes of the cephalopods are connected to the retinas through blood vessels and numerous nerves [51], we also determined the concentration of free amino acids in the optic lobes in order to determine whether this tissue could also be implicated in the changes in amino acids as a function of the light/dark environment. The results obtained have indicated that D-Asp, L-Asp and L-Glu were decreased 1.2-, 1.15- and 1.17-fold respectively in the optic lobes of the animals that have lived in the dark, with statistical significance of P<0.01, 0.05 and 0.05 respectively (Table 2). In addition, it is of particular interest to observe that, in the optic lobes of S. officinalis, the concentration of D-Asp is very high (12.5±1.10 μmol/g of tissue) and was 4.46 times higher than that of L-Asp. Also L-Glu in this tissue was very high, but no significant amount of D-Glu was observed (Table 2).
D-Aspartate racemase concentration at the light and dark environment
The presence of D-Asp in the retina raised the question of where does the D-Asp come from? A racemase which converts L-Asp into D-Asp has been found in rat brain [16] and in the mollusc Scapharca broughtonii (blood shell) [41]. Therefore we thought that a similar enzyme could also be present in the nervous system of the cephalopods. Thus we determined the presence of D-aspartate racemase in the retinas and optic lobes of adult S. officinalis in control animals using L-Asp and other L-amino acids as substrates at pH 5–10. One interesting result obtained was that among the various L-amino acids used (L-Asp, L-Glu, L-Ala and L-Gly), only when L-Asp was used as substrate, was D-Asp synthesized (results not shown). A second interesting result was that in both retina and optic lobes, the optimum activity was at pH 8.5 (Figure 3). However, there was a large difference in concentration of D-aspartate racemase between retina and optic lobes. The optic lobes posses a concentration of this enzyme that is approx. 6-fold higher than in the retina. In fact, as shown in Figure 3, at pH 8.5, the retina exhibited racemase activity of 3.0±0.4 units/g of tissue compared with a value of 18±2.1 in optic lobes (Figure 3).
Figure 3. D-Aspartate racemase in the retina and optic lobes of S. officinalis at various pH values.
Bars 1–4 from the left are the values of racemase activity found in the retina of S. officinalis measured at pH 7.5, 8.0, 8.5 and 9.0 respectively, and using L-Asp as substrate to generate D-Asp. Bars 5–8 from the left are the values found in the optic lobes of the same animals at pH 7.5, 8.0, 8.5 and 9.0 respectively. The values are the enzyme units/g of tissue and are the means±S.D. obtained from eight adult animals.
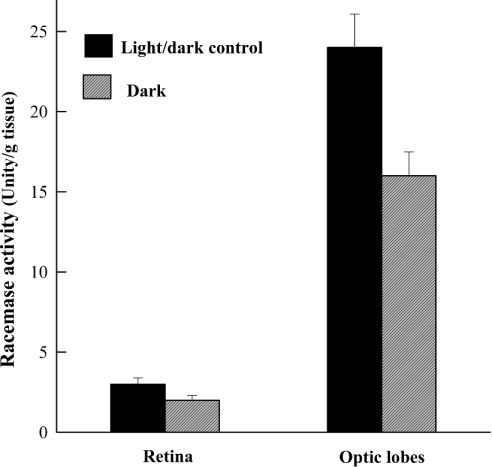
To determine whether a relationship existed between the amount of racemase activity and the light exposure, we determined the activity of this enzyme in the retinas and optic lobes from control animals, and from animals which were left for 5 days in the dark. The results obtained from this study (Figure 4) indicate that the concentration of this enzyme decreased significantly in both retina and optic lobes in animals following the dark treatment. In retina, the racemase activity decreased from 3.0±0.4 units/g of tissue in the control animal to 2.0±0.3 units/g of tissue in the animals left at dark (P<0.01). However, in the optic lobes, this enzyme was present at higher concentration than in retina, at 24.0±2.1 units/g tissue. Interestingly, a very significant decrease in the activity of racemase occurred in this tissue when the animals were left in the dark, from 24.0±2.1 units/g of tissue in the control animals to 16.0±1.5 units/g of tissue in the animals left in the dark (P<0.01) (Figure 4). These results thus support our hypothesis that there is a direct correlation between the concentration of D-Asp and racemase activity, suggesting that the synthesis of endogenous D-Asp is regulated by the aspartate racemase.
Figure 4. D-Aspartate racemase in the retina and optic lobes of S. officinalis at light/dark changes.
The two left-hand bars represent the values of racemase activity found in the retina of adult animals left for 5 days in an environment of 12 h of light and 12 h of dark (control), and in the retina of animals left for 5 days only in the dark. The two right-hand bars represent the racemase activity in the optic lobes of the same animals from which the retinas were taken. Results are means±S.D. obtained from eight animals. D-aspartate racemase in both retina and optic lobes significantly decreased in the animals in the dark (1.5 times compared with the control animals).
Transport of D-[2,3-3H]aspartate from optic lobes to retina of S. officinalis
The results reported in Table 2 indicate that, in S. officinalis, D-Asp is present in both retina and in optic lobes. However, in optic lobes, the concentration of D-Asp is almost 5 times higher than in retina. These results are in accord with the fact that D-aspartate racemase in the retina is at a very low concentration compared with that present in the optic lobes. Thus these results led us to hypothesize that D-Asp is synthesized in the optic lobes and is then transferred to the retina. To verify this hypothesis, we injected a known amount of radioactive D-Asp into the optic lobes of S. officinalis, and, after 12 h, we determined the concentration of radiolabelled D-Asp in the retina and in the optic lobes. The results obtained have indicated that transport of D-[2,3-3H]-Asp from optic lobes to retina occurs. In fact, as shown in Table 3, after 12 h of injection of radiolabelled D-Asp into the optic lobes, the radioactivity associated with D-Asp was found to be 220000±12000 d.p.m. in optic lobes and 150000±9500 d.p.m. in the retina. When the values were reported in terms of specific activity (d.p.m. per mg of tissue/nmol of D-Asp per mg of tissue), we found that, in optic lobes, the specific activity was 4807, whereas in the retina, it was 23714 (Table 3). This result indicates that D-Asp is synthesized in the optic lobes and is then transported to the retina.
Table 3. Transport of radioactive D-Asp from optic lobes to retina of S. officinalis.
D-[2,3-3H]aspartic acid (1 μl) containing 20±10 Ci/nmol (1 mCi/ml) was injected into one of the optic lobes of a juvenile S. officinalis of approx. 20 days of age (approx. 3 g of body mass). After 12 h, the optic lobes (into which the D-[2,3-3H]Asp was injected) and the retinas (connected to the optic lobes) were homogenized in 50 μl of 0.1 M TCA, centrifuged at 12000 g for 10 min, and the supernatant was chromatographed by HPLC. The peak corresponding to D-Asp was collected, and the radioactivity was determined. Results are means±S.D.
| Tissue mass (mg) | Endogenous D-Asp+D-[2,3-3H]Asp (nmol/mg of tissue) | Total d.p.m. per whole tissue | d.p.m./mg of tissue | Specific activity [(d.p.m./mg of tissue)/(nmol D-Asp/mg of tissue)] | |
|---|---|---|---|---|---|
| Optic lobe | 10.42±0.60 | 4.40±0.26 | 220000±12000 | 21153±2250 | 4807±750 |
| Retina | 5.02±0.42 | 1.26±0.21 | 150000±9500 | 29880±3250 | 23714±2234 |
DISCUSSION
Our finding of a consistent concentration of D-Asp in the retinas of cephalopods gives substantial support for a physiological role of D-Asp in the retina and the vision phenomenon. Our previous studies showed very high concentrations of D-Asp in the nervous system of cephalopods, including brain, optic lobes and stellate ganglia [1,2]. In the present study, we show that D-Asp is also present in the retinas of S. officinalis, O. vulgaris and L. vulgaris at consistent concentrations. The retina is a delicate and very important nervous membrane at the back of the eye, on to which the images of external objects are focused. In mammals, the retina consists of an outer pigmented layer and an inner nervous stratum, which, in turn, is composed of eight microscopic layers, named outwards from within as follows: (i) layer of optic nerve fibres, (ii) layer of ganglion cells, (iii) inner plexiform layer, (iv) inner nuclear layer, (v) outer plexiform layer, (vi) outer nuclear layer, (vii) layer of rods and cones, and (viii) pigment layer. Each of these cellular layers is involved in the capture of images and transfer to the central nervous system where they are elaborated. In S. officinalis, O. vulgaris and L. vulgaris, the lens is spherical, approx. 1 cm in diameter and projects considerably beyond the front surface of the eye. In contrast with that of mammals, the pupil of cephalopods changes shape according the light direction, therefore vision is more efficient, since light hits the retina perpendicularly to specialized areas, such that collimation is not needed, and each specialized area receives light through one of the pupil's vertical segments only [51]. In the retinas of rat and chicken, Neidle and Dunlop [9] found a significant transient amount of D-Asp before birth and in early post-natal stages, suggesting that D-Asp could have an important role in vision. The presence of D-Asp in neonatal rat retina also was documented by Lee et al. [27] through immunohistochemical studies. They found that rat retinas contain free D-Asp, and that, at 7 days of age, the immunopositivity was more intense than in 3-day-old rats.
In the present study, we demonstrated that in all three cephalopods studied, D-Asp represents the only amino acid in D-form at consistent concentration. The other D-amino acids found, including D-Glu and NMDA, were at significantly lesser amounts than D-Asp (Table 1).
Among the three cephalopods examined, S. officinalis contains the highest quantity of D-Asp (2.60±0.30 μmol/g of tissue) followed by O. vulgaris and L. vulgaris, with concentrations of 2.30±0.25 and 1.60±0.20 μmol/g of tissue respectively. The sum of D-Glu+NMDA was 0.25–0.35 μmol/g of tissue, and the sum of the D-amino acids, D-Pro, D-Met, D-Leu, D-Ile, D-Phe, D-Tyr, D-Ala, D-Trp, D-Ser, D-Thr, D-His, D-Arg, D-Val and D-Lys, was 0.35–0.48 μmol/g of tissue, thus approx. 5-fold less concentrated than that of D-Asp.
One of the interesting results obtained in the present work concerns the presence of D-Asp in the retina and optic lobes of S. officinalis during the development of the animal. D-Asp, in fact, is already present in these tissues at a consistent concentration when the animals hatch, and, during development of the animal, this amino acid increases significantly with the increasing age of the animals (Figure 4). The concentrations of the other amino acids present in these tissues also increase with age, but their increase is very much less evident than that observed for D-Asp (results not shown), suggesting that this amino acid is necessary at birth, and that is needed more than other amino acids during development. Another important result regards the occurrence of D-Asp, L-Asp and L-Glu in the retina and optic lobes of S. officinalis as a function of light. When S. officinalis were left in the dark for 5 days, the levels of these amino acids were significantly reduced in these tissues (Table 2), whereas the other amino acids present in same tissues (L-Ser, glycine, taurine and L-Ala) were not changed, meaning specifically that D-Asp, L-Asp and L-Glu have a role in vision. The same phenomena that occur in the retina also occur in the optic lobes. This probably takes place because the optic lobes of the cephalopods are connected to the retinas through blood vessels and nerves.
One question raised in the present work was where does D-Asp come from in the nervous system of the cephalopods, and in which tissue it is synthesized? In our previous work, we demonstrated that, in rat, D-Asp is synthesized from L-Asp through the action of an aspartate racemase, an enzyme which transforms L-Asp into D-Asp [16,41]. Therefore we sought to determine if this enzyme could also exist in cephalopods which have a consistent concentration of D-Asp. Experiments conducted on S. officinalis indeed demonstrated that an aspartate racemase is present in the retina and optic lobes, and that this enzyme has a maximum of activity at pH 8.5 (Figure 3). It was also found that if the animals were left in an environment without light, then the concentration of the aspartate racemase significantly decreased in the retina and optic lobes by 1.5 times (P<0.01) (Figure 4). Based on these results, we hypothesized that an aspartate racemase is physiologically responsible for the formation of D-Asp. However, we cannot exclude the possibility that other compounds generated by the L-Asp or L-Glu metabolism would also be precursors for D-aspartate synthesis through a series of intermediate reactions. It should also be noted that during the dark experiments, D-Asp, as well as racemase activity and L-Asp, are reduced. An answer to this matter could be that, although the racemase is reduced in darkness, there may still be enough enzymic activity remaining to convert a portion of L-Asp into D-Asp.
A further interesting question was where D-Asp in the retina of S. officinalis comes from. Is it synthesized in this tissue or it does come from some other tissues were it is synthesized? Experiments using radiolabelled D-Asp have demonstrated that D-Asp is synthesized in optic lobes and is then transferred to the retina (Table 3). In fact, after 12 h from the injection of radiolabelled D-Asp in the optic lobes, the radioactivity associated with D-Asp in the retina increased by approx. 1.5 times, as expressed as d.p.m./mg of tissue, and 4–5 times, as expressed as d.p.m. per mg of tissue/nmol of D-Asp per mg of tissue (Table 3). L-Asp and L-Glu, which are at high concentrations in the retina and optic lobes, are probably also synthesized in the optic lobes and then transferred to the retina.
In conclusion, the results obtained from the present study indicate that L-Asp, L-Glu and, in particular, D-Asp are molecules that participate in important physiological events in the retina. Concentrations of these three amino acids and, in particular, D-Asp decrease in the retina when the animal is left in the dark, indicating that they have an important role in vision. D-Asp, in particular, is the compound which merits more emphasis than the other two dicarboxylic amino acids due to the fact that D-amino acids are not as common as the L-forms and that they are metabolized only by D-AspO. In addition, among other D-amino acids also present in the retina and optic lobes, D-Asp is the amino acid comparatively more concentrated and that other D-amino acids present do not change in concentration during the dark environment. All the results obtained in the present study, however interesting, do not permit us to make any possible hypothesis concerning the physiological role of L-Asp, L-Glu and, in particular, of D-Asp in vision, and future experiments will be devoted to further our understanding of this phenomenon.
Acknowledgments
K. P., M. T. and G. F. gratefully acknowledge financial support from the following NIH (National Institutes of Health) grants MBRS/SCORE 5SO6GM45455, MIRT 5T37TW00033 and MBRS/RISE 1R25GM9244.
References
- 1.D'Aniello A., Giuditta A. Identification of D-aspartic acid in the brain of Octopus vulgaris. J. Neurochem. 1977;29:1053–1057. doi: 10.1111/j.1471-4159.1977.tb06508.x. [DOI] [PubMed] [Google Scholar]
- 2.D'Aniello A., Giuditta A. Presence of D-aspartate in squid axoplasm and in other regions of the cephalopod nervous system. J. Neurochem. 1978;31:1107–1108. doi: 10.1111/j.1471-4159.1978.tb00155.x. [DOI] [PubMed] [Google Scholar]
- 3.D'Aniello A., Nardi G., Vetere A., Ferguson G. P. Occurrence of free D-aspartic acid in the circumsoesophageal ganglia of Aplysia fasciata. Life Sci. 1992;52:733–736. doi: 10.1016/0024-3205(93)90235-u. [DOI] [PubMed] [Google Scholar]
- 4.D'Aniello A., Spinelli P., De Simone A., D'Aniello S., Branno M., Aniello F., Fisher G. H., Di Fiore M. M., Rastogi R. K. Occurrence and neuroendocrine role of D-aspartic acid and N-methyl-D-aspartic acid in Ciona intestinalis. FEBS Lett. 2003;552:193–198. doi: 10.1016/s0014-5793(03)00921-9. [DOI] [PubMed] [Google Scholar]
- 5.Okuma E., Fujita F., Amano H., Noda H., Abe H. Distribution of free D-amino acids in the tissues of crustaceans. Fish. Sci. 1995;61:157–160. [Google Scholar]
- 6.Di Fiore M. M., Assisi L., Botte V., D'Aniello A. D-Aspartic acid is implicated in the control of testosterone production by the vertebrate gonad: studies on the female green frog Rana esculenta. J. Endocrinol. 1998;156:199–207. doi: 10.1677/joe.0.1570199. [DOI] [PubMed] [Google Scholar]
- 7.Assisi L., Botte V., D'Aniello A., Di Fiore M. M. Enhancement of aromatase activity by D-aspartic acid in the ovary of lizard Podarcis sicula. Reproduction. 2001;121:803–808. doi: 10.1530/rep.0.1210803. [DOI] [PubMed] [Google Scholar]
- 8.D'Aniello A., Di Fiore M. M., Fisher G. Occurrence of D-aspartic acid in animal tissues and its role in the nervous and endocrine system. Trends Comp. Biochem. Physiol. 1998;4:1–21. [Google Scholar]
- 9.Neidle A., Dunlop D. S. Developmental changes of free D-aspartic acid in the chicken embryo and in the neonatal rat. Life Sci. 1990;46:1517–1522. doi: 10.1016/0024-3205(90)90424-p. [DOI] [PubMed] [Google Scholar]
- 10.Dunlop D. S., Neidle A., McHale D., Dunlop D. M., Lajtha A. The presence of free D-aspartic acid in rodents and man. Biochem. Biophys. Res. Commun. 1986;142:27–32. doi: 10.1016/s0006-291x(86)80329-1. [DOI] [PubMed] [Google Scholar]
- 11.D'Aniello A., D'Onofrio G., Pischetola M., Vetere A., Petrucelli L., Fisher G. H. Biological role of D-amino acid oxidase and D-aspartate oxidase: effects of D-amino acids. J. Biol. Chem. 1993;268:26941–26949. [PubMed] [Google Scholar]
- 12.Hashimoto A., Nishikawa T., Oka T., Hayashi T., Takahashi K. Widespread distribution of free D-aspartate in rat periphery. FEBS Lett. 1993;331:4–8. doi: 10.1016/0014-5793(93)80286-4. [DOI] [PubMed] [Google Scholar]
- 13.Imay K., Fukushima T., Hagiwara K., Santa T. Occurrence of D-aspartic acid in rat brain pineal gland. Biomed. Chromatogr. 1995;9:106–109. doi: 10.1002/bmc.1130090211. [DOI] [PubMed] [Google Scholar]
- 14.Hamase K., Homma H., Takigawa Y., Fukushima T., Santa T., Imai K. Regional distribution and postnatal changes of D-amino acids in rat brain. Biochim. Biophys. Acta. 1997;1334:214–222. doi: 10.1016/s0304-4165(96)00095-5. [DOI] [PubMed] [Google Scholar]
- 15.Schell M. J., Cooper O. B., Snyder S. H. D-Aspartate localizations imply neuronal and neuroendocrine roles. Proc. Natl. Acad. Sci. U.S.A. 1997;94:2013–2018. doi: 10.1073/pnas.94.5.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wolosker H., D'Aniello A., Snyder S. H. D-Aspartate disposition in neuronal and endocrine tissues: ontogeny, biosynthesis and release. Neuroscience. 2000;100:183–189. doi: 10.1016/s0306-4522(00)00321-3. [DOI] [PubMed] [Google Scholar]
- 17.Fisher G. H., D'Aniello A., Vetere A., Padula L., Cusano G., Man E. H. Free D-aspartate and D-alanine in normal and Alzheimer brain. Brain Res. Bull. 1991;26:983–985. doi: 10.1016/0361-9230(91)90266-m. [DOI] [PubMed] [Google Scholar]
- 18.Hashimoto A., Kumashiro S., Nishikawa T., Oka T., Takahashi K., Mito T., Takashima S., Doi N., Mizutani Y., Kaneco T., Ootomo E. Embryonic development and postnatal changes in free D-aspartate and D-serine in the human prefrontal cortex. J. Neurochem. 1993;61:348–351. doi: 10.1111/j.1471-4159.1993.tb03575.x. [DOI] [PubMed] [Google Scholar]
- 19.D'Aniello A., Lee J. M., Petrucelli L., Di Fiore M. M. Regional decreases of free D-aspartate levels in Alzheimer's disease. Neurosci. Lett. 1998;250:131–134. doi: 10.1016/s0304-3940(98)00451-0. [DOI] [PubMed] [Google Scholar]
- 20.Fisher G. H., Petrucelli L., Carden C., Emory C., Frey W. H., II, Amaducci L., Sorbi S., Sorrentino G., Borghi M., D'Aniello A. Free D-amino acids in human cerebrospinal fluid of Alzheimer disease, multiple sclerosis, and healthy control subjects. Mol. Chem. Neuropathol. 1994;23:115–123. doi: 10.1007/BF02815405. [DOI] [PubMed] [Google Scholar]
- 21.Fisher G. H., Lorenzo N., Abe H., Fujita E., Frey W. H., Emory C., Di Fiore M. M., D'Aniello A. Free D- and L-amino acids in ventricular cerebrospinal fluid from Alzheimer and normal subjects. Amino Acids. 1998;15:263–269. doi: 10.1007/BF01318865. [DOI] [PubMed] [Google Scholar]
- 22.D'Aniello A., Di Cosmo A., Di Cristo C., Annunziato L., Petrucelli L., Fisher G. H. Involvement of D-aspartic acid in the synthesis of testosterone in rat testes. Life Sci. 1996;59:97–104. doi: 10.1016/0024-3205(96)00266-4. [DOI] [PubMed] [Google Scholar]
- 23.Nagata Y., Homma H., Lee J. A., Imai K. D-Aspartate stimulation of testosterone synthesis in rat Leydig cells. FEBS Lett. 1999;444:160–164. doi: 10.1016/s0014-5793(99)00045-9. [DOI] [PubMed] [Google Scholar]
- 24.D'Aniello A., Di Fiore M. M., D'Aniello G., Colin F. E., Lewis G., Setchell B. P. Secretion of D-aspartic acid by the rat testis and its role in endocrinology of the testis and spermatogenesis. FEBS Lett. 1998;436:23–27. doi: 10.1016/s0014-5793(98)01087-4. [DOI] [PubMed] [Google Scholar]
- 25.Takigawa Y., Homma H., Lee J., Fukushima T., Santa T., Iwatsubo T., Imay K. D-Aspartate uptake into cultured rat pinealocytes and the concomitant effect on L-aspartate levels and melatonin secretion. Biochem. Biophys. Res. Commun. 1998;248:641–647. doi: 10.1006/bbrc.1998.8971. [DOI] [PubMed] [Google Scholar]
- 26.Lee J. A., Homma H., Sakai K., Fukushima T., Tashiro T., Iwatsubo T., Yoshikawa M., Imai K. Immunohistochemical localization of D-aspartate in the rat pineal gland. Biochem. Biophys. Res. Commun. 1997;231:505–508. doi: 10.1006/bbrc.1996.5902. [DOI] [PubMed] [Google Scholar]
- 27.Lee J., Homma H., Tashiro T., Iwatsubo T., Imai K. D-Aspartate localization in the rat pituitary gland and retina. Brain Res. 1999;838:193–199. doi: 10.1016/s0006-8993(99)01718-7. [DOI] [PubMed] [Google Scholar]
- 28.Moriyama Y., Yamada H., Hayashi M., Oda T., Yamaguchi A. Identification of D-aspartate in rat pheochromocytoma PC12 cells. Neurosci. Lett. 1998;248:57–60. doi: 10.1016/s0304-3940(98)00308-5. [DOI] [PubMed] [Google Scholar]
- 29.Long Z., Lee J., Okamoto T., Nimura N., Imay K., Homma H. D-Aspartate in a prolactin-secreting cloning strain of rat pituitary tumor cells (GH3) Biochem. Biophys. Res. Commun. 2000;276:1143–1147. doi: 10.1006/bbrc.2000.3573. [DOI] [PubMed] [Google Scholar]
- 30.Long Z., Sekine M., Adaki M., Furuchi T., Imay K., Nimura N., Homma H. Cell density inversely regulates D- and L-aspartate levels in rat pheochromocytoma MPT1 cells. Arch. Biochem. Biophys. 2002;404:92–97. doi: 10.1016/s0003-9861(02)00241-2. [DOI] [PubMed] [Google Scholar]
- 31.Sakai K., Homma H., Lee J. A., Fukushima T., Santa T., Tashiro K., Iwatsubo T., Imai K. Emergence of D-aspartic acid in the differentiating neurons of the rat central nervous system. Brain Res. 1998;351:65–71. doi: 10.1016/s0006-8993(98)00599-x. [DOI] [PubMed] [Google Scholar]
- 32.Long Z., Homma H., Lee J., Fukushima T., Santa T., Tashiro K., Iwatsubo T., Yamada R., Imai K. Biosynthesis of D-aspartate in mammalian cells. FEBS Lett. 1998;434:231–235. doi: 10.1016/s0014-5793(98)00986-7. [DOI] [PubMed] [Google Scholar]
- 33.Sakai K., Homma H., Lee J., Fukushima T., Santa T., Tashiro K., Iwatsubo T., Imai K. D-Aspartic acid localization during postnatal development of rat adrenal gland. Biochem. Biophys. Res. Commun. 1997;235:433–436. doi: 10.1006/bbrc.1997.6783. [DOI] [PubMed] [Google Scholar]
- 34.Sakai K., Homma H., Lee J., Fukushima T., Santa T., Tashiro K., Iwatsubo T., Imai K. Localization of D-aspartic acid in elongate spermatids in rat testis. Arch. Biochem. Biophys. 1998;351:96–105. doi: 10.1006/abbi.1997.0539. [DOI] [PubMed] [Google Scholar]
- 35.D'Aniello A., Di Fiore M. M., Fisher G. H., Milone A., Seleni A., D'Aniello S., Perna A., Ingrosso D. Occurrence of D-aspartic acid and N-methyl-D-aspartic acid in rat neuroendocrine tissues and their role in the modulation of luteinizing hormone and growth hormone release. FASEB J. 2000;14:699–714. doi: 10.1096/fasebj.14.5.699. [DOI] [PubMed] [Google Scholar]
- 36.D'Aniello G., Tolino A., D'Aniello A., Fisher G. H., Di Fiore M. M. The role of the aspartic acid and N-methyl-D-aspartic acid in the regulation of prolactin release. Endocrinology. 2000;141:3862–3870. doi: 10.1210/endo.141.10.7706. [DOI] [PubMed] [Google Scholar]
- 37.Pampillo M., Scimonelli T., Bottino M. C., Duvilanski B. H., Rettori V., Seilicovich A., Lasaga M. The effect of D-aspartate on luteinizing hormone-releasing hormone, α-melanocyte-stimulating hormone, GABA and dopamine release. NeuroReport. 2002;13:2341–2344. doi: 10.1097/00001756-200212030-00034. [DOI] [PubMed] [Google Scholar]
- 38.Wang H., Wolosker H., Pevsner J., Snyder S. H., Selkoe D. J. Regulation of rat magnocellular neurosecretory system by D-aspartate: evidence for biological role(s) of a naturally occurring free D-amino acid in mammals. J. Endocrinol. 2000;167:247–252. doi: 10.1677/joe.0.1670247. [DOI] [PubMed] [Google Scholar]
- 39.Wang H., Wolosker H., Morris J. F., Pevsner J., Snyder S. H., Selkoe D. J. Naturally occurring free D-aspartate is a nuclear component of cells in the mammalian hypothalamo-neurohypophyseal system. Neuroscience. 2002;109:1–4. doi: 10.1016/s0306-4522(01)00545-0. [DOI] [PubMed] [Google Scholar]
- 40.Nagata Y., Homma H., Matsumoto M., Imai K. Stimulation of steroidogenic acute regulatory protein (StAR) gene expression by D-aspartate in rat Leydig cells. FEBS Lett. 1999;454:317–320. doi: 10.1016/s0014-5793(99)00840-6. [DOI] [PubMed] [Google Scholar]
- 41.Watanabe T., Shibata K., Kera Y., Yamada R. Occurrence of free D-aspartate and aspartate racemase in the blood shell Scapharca broughtonii. Amino Acids. 1998;14:353–360. doi: 10.1007/BF01318854. [DOI] [PubMed] [Google Scholar]
- 42.Negri A., Massey V., Williams C. H., Jr D-Aspartate oxidase from beef kidney: purification and properties. J. Biol. Chem. 1987;262:10026–10034. [PubMed] [Google Scholar]
- 43.Hashimoto A., Oka T., Nishikawa T. Anatomical distribution and postnatal changes in endogenous free D-aspartate and D-serine in rat brain and periphery. Eur. J. Neurosci. 1995;7:1657–1663. doi: 10.1111/j.1460-9568.1995.tb00687.x. [DOI] [PubMed] [Google Scholar]
- 44.Shell M. J., Molliver M. E., Snyder S. H. D-Serine as synaptic modulator: localization to astrocytes and glutamate-stimulated release. Proc. Natl. Acad. Sci. U.S.A. 1995;92:3948–3952. doi: 10.1073/pnas.92.9.3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Negri A., Tedeschi G., Ceciliani F., Ronchi S. Purification of beef kidney D-aspartate oxidase overexpressed in Escherichia coli and characterization of its redox potentials and oxidative activity towards agonists and antagonists of excitatory amino acid receptors. Biochim. Biophys. Acta. 1999;1431:212–222. doi: 10.1016/s0167-4838(99)00027-8. [DOI] [PubMed] [Google Scholar]
- 46.D'Aniello A., Vetere A., Petrucelli L. Further study on the specificity of D-amino acid oxidase and of D-aspartate oxidase and time course for complete oxidation of D-amino acid. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 1993;105B:731–734. doi: 10.1016/0305-0491(93)90113-j. [DOI] [PubMed] [Google Scholar]
- 47.Tedeschi G., Negri A., Ceciliani F., Ronchi S., Vetere A., D'Aniello G., D'Aniello A. Properties of the flavoenzyme D-aspartate oxidase from Octopus vulgaris. Biochim. Biophys. Acta. 1994;1207:217–222. doi: 10.1016/0167-4838(94)00071-9. [DOI] [PubMed] [Google Scholar]
- 48.Aswad D. W. Determination of D- and L-aspartate in amino acid mixtures by high performance liquid chromatography after derivatization with a chiral adduct of o-phthaldialdehyde. Anal. Biochem. 1984;118:405–407. doi: 10.1016/0003-2697(84)90106-4. [DOI] [PubMed] [Google Scholar]
- 49.Dixon M., Kleppe K. D-Amino acid oxidase. II. Specificity, competitive inhibition and reaction sequence. Biochim. Biophys. Acta. 1965;96:357–365. [Google Scholar]
- 50.Marks V. An improved glucose-oxidase method for determining blood, C.S.F. and urine glucose levels. Clin. Chim. Acta. 1959;4:395–397. doi: 10.1016/0009-8981(59)90110-x. [DOI] [PubMed] [Google Scholar]
- 51.Young J. Z. London: Oxford University Press; 1971. The anatomy of the nervous system of Octopus vulgaris. [Google Scholar]