Abstract
Joint kinematic instability, arising from congenital or acquired musculoskeletal pathoanatomy or from imbalances in anabolism and catabolism induced by pathophysiological factors, leads to deterioration of the composition, structure and function of cartilage and, ultimately, progression to osteoarthritis (OA). Alongside articular cartilage degeneration, synovial fluid lubricity decreases in OA owing to a reduction in the concentration and molecular weight of hyaluronic acid and surface-active mucinous glycoproteins that form a lubricating film over the articulating joint surfaces. Minimizing friction between articulating joint surfaces by lubrication is fundamental for decreasing hyaline cartilage wear and for maintaining the function of synovial joints. Augmentation with highly viscous supplements (that is, viscosupplementation) offers one approach to re-establishing the rheological and tribological properties of synovial fluid in OA. However, this approach has varied clinical outcomes owing to limited intra-articular residence time and ineffective mechanisms of chondroprotection. This Review discusses normal hyaline cartilage function and lubrication and examines the advantages and disadvantages of various strategies for restoring normal joint lubrication. These strategies include contemporary viscosupplements that contain antioxidants, anti-inflammatory drugs or platelet-rich plasma and new synthetic synovial fluid additives and cartilage matrix enhancers. Advanced biomimetic tribosupplements offer promise for mitigating cartilage wear, restoring joint function and, ultimately, improving patient care.
Introduction
Osteoarthritis (OA) is a debilitating musculoskeletal disease that affects hundreds of millions of individuals worldwide and the incidence of OA is increasing with the aging population1,2. Despite the high prevalence of OA and decades of research aimed at improving clinical outcomes, strikingly few treatment options are available. Canonical treatment options become increasingly more invasive with disease severity and include lifestyle adjustments and physical therapy, NSAID use, intraarticular glucocorticoid injections and total joint arthroplasty3–6. Beginning in the 1990s7, viscosupplementation emerged as a novel strategy for treating medium-severity OA. This approach involves administration of an exogenous hyaluronic acid solution, often formulated as sodium hyaluronate (the sodium salt of hyaluronic acid), to the affected joint. The rationale for viscosupplementation stems from the observation that the concentration and molecular weight of hyaluronic acid in the synovial fluid decreases in OA, and results in a reduction in synovial fluid lubricity8. Thus, introducing additional amounts of high-molecular-weight sodium hyaluronate into the synovial space is hypothesized to restore lubricity and protect the cartilage from further wear. However, conflicting clinical reports call into question the efficacy of viscosupplementation.
To improve upon the strategy underpinning viscosupplementation, several lubricating materials are undergoing preclinical evaluation as OA treatments. For this approach, termed tribosupplementation, the materials target specific aspects of cartilage lubrication and, in some cases, provide additional functionality to the treatment strategy. Several tribosupplements have been constructed as linear polymers to mimic the structure of hyaluronic acid; however, growing interest is emerging in hydrogel or crosslinked polymer networks, as well as micro-particle- and nano-particle-based formulations, to enhance lubrication, drug delivery and joint residency times.
With an emphasis on improving tribosupplement retention and lubrication, in this Review, we discuss the structure and function of cartilage, in both healthy and OA conditions, we evaluate the chondroprotective efficacy of clinically approved viscosupplements, and we comparatively assess the effectiveness of preclinical tribosupplements categorized as fluid additives or matrix enhancers (Fig. 1). As the composition and structure of a material affect its lubricant function, we categorize these materials as linear, hydrogel or particle materials. We conclude by summarizing current strategies and discussing future directions in this field, with an emphasis on improving tribosupplement performance.
Fig. 1 |. Types of viscosupplementation and tribosupplementation.
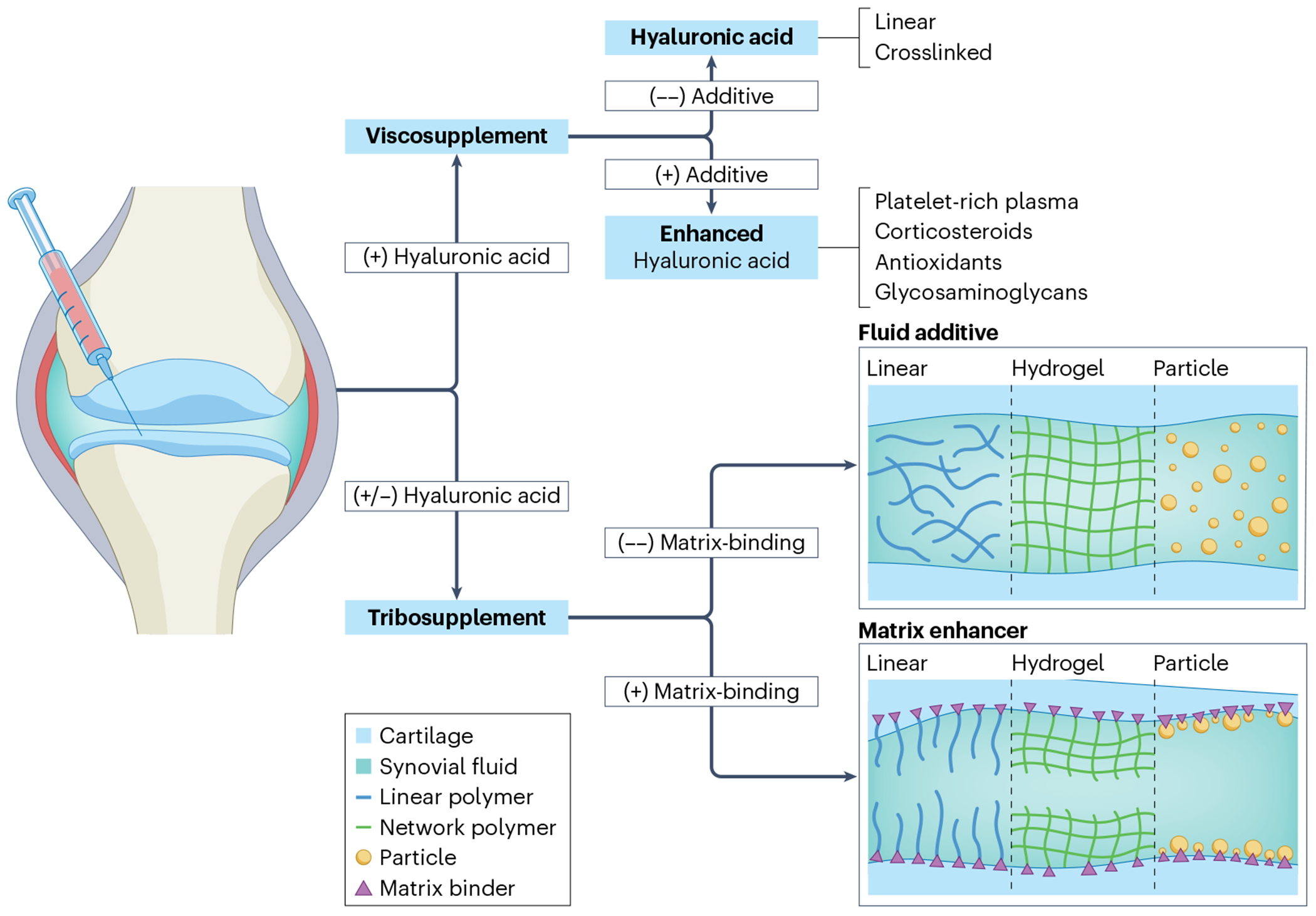
This figure depicts the various categories of clinical, hyaluronic acid-based viscosupplements and tribosupplements that are discussed in this Review. The term viscosupplement describes an exogenous hyaluronic acid-based solution. Viscosupplements can be further subdivided on the basis of structure (linear versus cross-linked) and composition (administered alone versus in combination with additional components that enhance the drugs’ therapeutic effects). Tribosupplements are alternative non-hyaluronic acid-based lubricants that can be further divided by structure (linear polymer, hydrogel or particle) and matrix-binding capacity (that is, whether the tribosupplement remains suspended in the synovial fluid or contains a matrix binder that localizes it to the cartilage surface).
Hyaline cartilage structure and function
Hyaline cartilage possesses exquisite load-bearing and friction-lowering properties, owning to its precise composition and structure, such that this tissue withstands compressive loads and shear forces over the course of a lifetime. In this section, we provide a summary of the synovial joint in its healthy state and describe the changes that occur during OA that lead to the impaired function.
Healthy cartilage
Lubrication of the joint to minimize friction between the articulating joint surfaces and decrease hyaline cartilage wear is fundamental to normal synovial joint function in health and disease. Hyaline cartilage (also known as articular cartilage) (Fig. 2) is a hydrated, avascular, aneural, alymphatic connective tissue that provides a smooth, nearly frictionless surface on the joint while supporting the applied compressive loads during diarthrodial joint motion. The extracellular matrix (ECM) of cartilage contains mainly water and anionic proteoglycans, comprising glycosaminoglycans (primarily chondroitin-6-sulfate and keratan sulfate) bound to a core protein, that are constrained by an organized fibrous type II collagen network. This network bestows the tissue with dual rheological properties and tribological properties that are essential for diarthrodial joint performance9. Chondrocytes within the cartilage vary in shape, number, size and activity, depending on the anatomical zone and mechanochemical microenvironment in which they reside. The superficial zone of cartilage begins at the articular surface and comprises 10–20% of the cartilage volume. The interposed transition zone (making up 40–60% of the cartilage volume) connects the surface layer to the deep zone, which anchors the tissue to the subchondral bone plate (consisting of 30% of the cartilage volume)10,11. In the superficial zone, the water content is highest (~80% water); collagen (type II and IX) is abundant and the fibres are arranged parallel to the articular surface along the predominant direction of joint sliding10,11. Surface-active hyaluronic acid, proteoglycan 4 (PRG4, also known as lubricin and superficial zone protein) and phosphocholine lipids, bound to the lamina splendens, synergistically work together to protect the tissue from interfacial shear forces, achieving coefficients of friction as low as ~10−3. The abundant chondrocytes adjacent to the sliding surface have a flattened morphology. In the transition zone, proteoglycans are present that are intertwined with arching collagen fibres to increase the fixed negative charge density and to constrain water within the matrix to help support compressive loads. Within this zone, chondrocytes are sparse and spherical. In the deep zone, radially oriented collagen fibres perpendicular to the subchondral plate anchor the tissue to the bone; this zone has the lowest water content (65%) and highest fixed negative charge density (20–30% of tissue water content)10–12. Chondrocytes are abundant in the deep zone, are arranged in columns that run parallel to the collagen fibres and become hypertrophic as they approach the tide mark and zone of calcified cartilage.
Fig. 2 |. The composition, morphology and biomechanics of cartilage and synovial fluid in the healthy and osteoarthritic states.
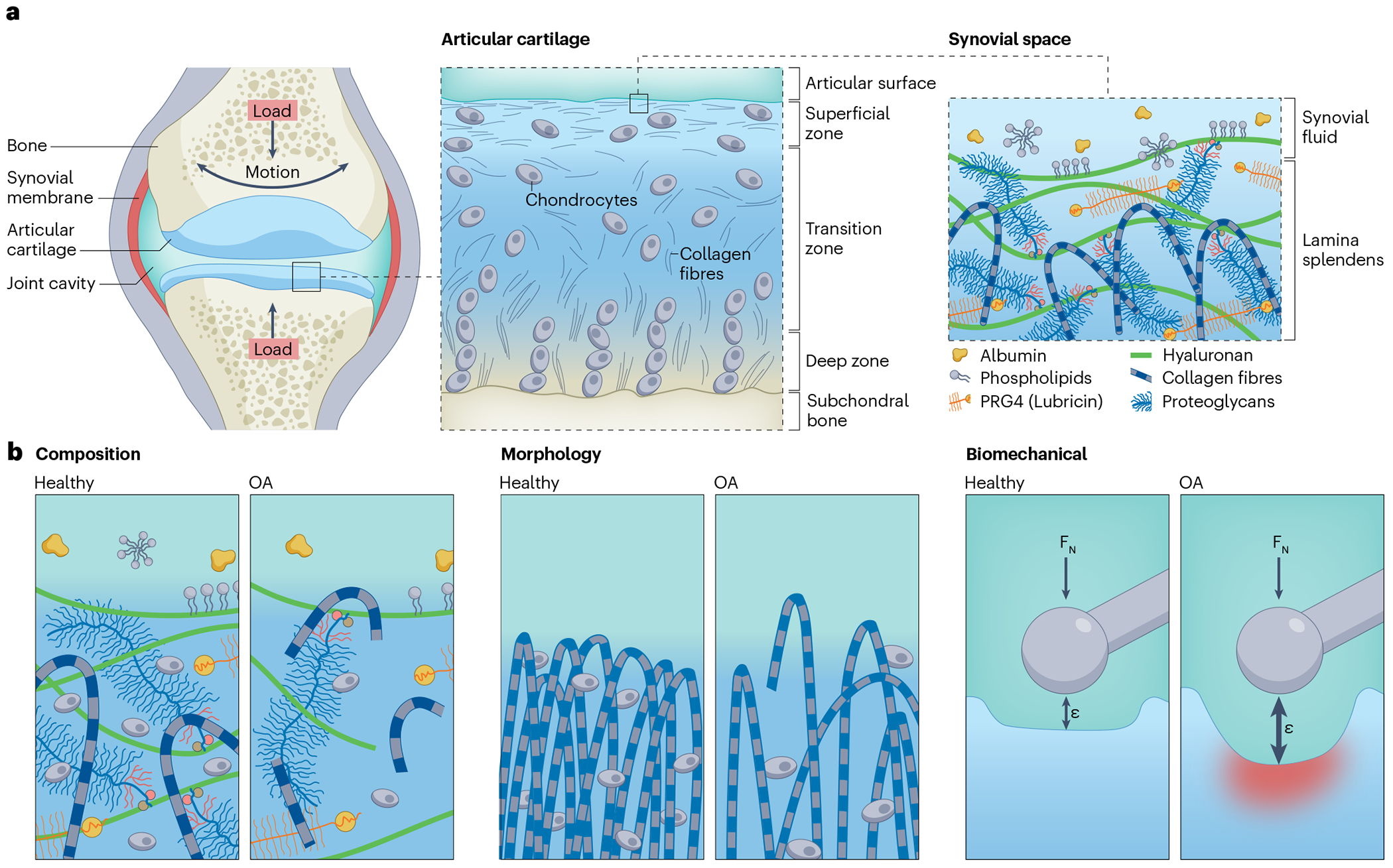
a, Hyaline cartilage consists of three layers: the superficial zone, transition zone and deep zone, each with a different morphology, composition and structure. Chondrocytes are embedded within the extracellular matrix (ECM) of cartilage and synthesize and maintain the ECM constituents. The superficial zone interfaces with the synovial fluid, which contains hyaluronic acid and proteoglycan 4 (PRG4). Hyaluronic acid is an anionic, non-sulphated, high-molecular-weight biopolymer that endows synovial fluid with its viscosity. PRG4, a surface-active glycoprotein, attaches to the lamina splendens, binds to hyaluronic acid present in the synovial fluid to localize it to the articular surface and thereby provides a discontinuous, sacrificial layer that enhances superficial zone pressurization and boundary lubrication. b, During osteoarthritis (OA), the composition, morphology and biomechanics of cartilage and synovial fluid change. Compositionally, osteoarthritic cartilage contains decreased amounts of hyaluronic acid, PRG4, and type II collagen compared with healthy tissue because of wear and an aberrant balance between anabolism and catabolism. Similarly, in the synovial fluid, the concentration and molecular weight of sodium hyaluronate decreases and the concentration of PRG4 decreases during OA. OA also results in morphological changes to cartilage as the structure of the collagen matrix transitions from the characteristic arching fibrils, producing a matrix that is perpendicular to the subchondral bone in the middle and deep zones and parallel to the articular surface, to a disorganized distribution of fibrils. Ultimately, the compositional and morphological changes in OA reduce cartilage stiffness, such that the same applied force (FN) produces a larger strain (ε) in osteoarthritic cartilage than in healthy cartilage.
In hyaline cartilage, the type II collagen fibrillar network provides structure and tensile strength, whereas the abundant hydrophilic, negatively charged, sulphated glycosaminoglycans retain interstitial water, conferring compressive stiffness and elastohydrodynamic lubrication. Pressurization of entrapped interstitial fluid supports more than 90% of applied load (known as interstitial fluid load support)13–15. With joint loading, compression deforms the type II collagen network, decreasing pore volumes and relatively increasing the local fixed negative charge density, which jointly retard interstitial fluid transport. Subject to this strain-dependent decrease in tissue permeability, the increased viscous drag of interstitial fluid flowing through the porous type II collagen network accounts for the tissue’s non-linear, viscoelastic behaviour16. Besides affording load support, the interstitial fluid also contributes to joint lubrication. Compressive deformation induces fluid efflux from the cartilage, creating an interposed fluid film between the articulating joint surfaces that decreases the coefficient of friction, protecting the tissue from mechanical shear wear. The coefficient of friction inversely linearly correlates with the interstitial fluid load support, achieving its lowest values when the interstitial fluid load support is greatest13,17. During an extended period of compressive deformation, unbound interstitial fluid gradually dissipates. Subsequently, the coefficient of friction increases, resulting in mechanically suboptimal conditions. Over time, any aberration in this process affects the structural components of the joint, such as the superficial type II collagen fibrillar network, emphasizing the importance of maintaining a healthy lubrication environment for preventing structural degradation18.
Synovial fluid, a dialysate of blood plasma, is the naturally occurring lubricant entrapped between articulating joint surfaces that, via elastohydrodynamic lubrication, imparts essential functions such as shock absorption, load support and nearly frictionless sliding motion19–21. These functions rely on the synergistic action of its constituents. Besides serum proteins, such as albumin and g-globulin, synovial fluid contains hyaluronic acid, surface-active phospholipids, and PRG4 that are produced by fibroblast synovial lining cells and superficial zone chondrocytes. Mechanically, synovial fluid exhibits non-Newtonian fluid characteristics (that is, non-linear viscosity). During high strain rate activities such as running and jumping, entanglement and hydrophobic interaction of PRG4 with hyaluronic acid molecules cause the synovial fluid to behave like an elastic-solid, enhancing its ability to support the applied loads on the joint22–24. Boundary lubrication within the synovial joints is a result, at least in part, of adsorption of PRG4 to the articular surface. The adsorbed PRG4 interacts with and retains hyaluronic acid at the articular surface, which in turn complexes with surface-active phospholipids to form a hydration film that ultimately lowers the coefficient of friction via a process known as ‘hydration lubrication’25,26.
Osteoarthritis
OA manifests clinically with pain and decreased joint function as the hyaline cartilage progressively degrades, and can be defined as either primary (idiopathic) or secondary (resulting from injury or another disease). Although the aetiology of OA is multi-factorial (Fig. 2), this disease typically results from mechanical injury as a consequence of trauma, joint instability, ligamentous deficiency, skeletal malalignment, obesity or anatomical deformity. Mechanical overloading induces surface wear and superficial zone delamination27 that propagate to structural tissue failure (manifesting as fissuring or fibrillation28), tissue swelling29, compositional loss of glycosaminoglycan30 and derangement of the type II collagen network31. Mechanical injury also incites an inflammatory response that increases the production of cytokines and catabolic enzymes by macrophages, neutrophils, lymphocytes, chondrocytes and synoviocytes, which further break down the tissue. Specifically, elevated cytokines (such as IL-1β and TNF) in the synovial fluid in OA induce enzyme-mediated cartilage degeneration via matrix metalloproteinases (MMPs)32,33. Depletion of cartilage glycosaminoglycan and the subsequent degradation of the type II collagen fibrillar network increases hydraulic permeability, reduces interstitial fluid load support and decreases cartilage stiffness, compromising the overall biomechanical integrity of the cartilage14. Concomitant with these pathological processes in cartilage, synovial fluid lubricity also decreases in OA owing to a decrease in the concentration and molecular weight of hyaluronic acid and a decrease in the concentration of PRG4. These changes decrease the rheological and tribological properties of the synovial fluid23,34–38, independently contributing to the propagation of OA pathophysiology39,40.
Amelioration of OA requires a comprehensive approach, including correcting the mechanical factors that contribute to acute or chronic chondrocyte injury and tissue wear, as well as dampening the inflammatory cascade and neutralizing catabolic enzymes that degrade cartilage. Furthermore, this approach involves reconstituting damaged cartilage material properties by augmenting tissue ECM constituents. Currently, no device or pharmacological treatment mitigates cartilage wear and imparts chondroprotection after the induction of OA.
Cartilage lubrication
The prevention of cartilage wear requires an understanding of lubrication to rationally design tribosupplements and lubricating biomacromolecules. The time-dependent behaviour of cartilage is a critical aspect of its biomechanical response. Cartilage exhibits viscoelastic properties that influence its response to mechanical loading over time. This dynamic behaviour is particularly crucial in the context of evaluating the effectiveness of viscosupplements and tribosupplements, as it affects various modes of lubrication. In general, the mode of lubrication and the coefficient of friction between articulating surfaces depend on the thickness and viscosity of the interposed fluid layer between the opposing surfaces, as well as the velocity and magnitude of the contact force. Two fundamental modes govern lubrication: fluid-film lubrication and boundary lubrication. Fluid-film lubrication involves the formation of an optimized fluid-film layer that reduces friction during articulation. By contrast, boundary lubrication occurs when surface-active molecules form a protective layer between the contacting surfaces. Understanding these primary modes of lubrication is pivotal for designing viscosupplements.
Classically, the mode of lubrication for non-deformable articulating surfaces (for example, metal surfaces) is delineated by the Stribeck curve. This curve depicts the coefficient of friction as a function of the Hersey number (a dimensionless number that is derived by multiplying the sliding velocity by the lubricant viscosity, and dividing by load per unit length)41. Boundary lubrication occurs at low Hersey numbers (that is, at high loads and low speeds, and with low-viscosity lubricants); as the articulating surfaces are in contact, separated only by a thin interposed lubricant layer, the coefficient of friction is high and relatively constant. This type of setting is where boundary lubricants such as PRG4 are most effective. Fluid-film lubrication occurs at high Hersey numbers (that is, at low loads and high speeds, and with high-viscosity lubricants); the coefficient of friction is lowered as the interposed lubricating fluid drives the articulating surfaces apart. Mixed-mode lubrication, a combination of these two modes, occurs at intermediate Hersey numbers; a fluid-film begins to form during this mode of lubrication, reducing direct contact between asperities on the articulating surfaces. The coefficient of friction is a function of both the surface chemistry and the lubricant viscosity, and viscous lubricants, such as sodium hyaluronate supplements, are most effective during mixed-mode lubrication42,43. Once the hydrated tissue reaches equilibrium under load44, cartilage lubrication becomes increasingly reliant on the properties of the lubricant, as the interstitial fluid load support diminishes to zero13–15,17,43. Modified versions of the Stribeck curve exist for semi-permeable, deformable surfaces that incorporate additional intermediary lubrication modes such as elastohydrodynamic, weeping, squeeze-film, rehydration and boosted modes to account for the influence of cartilage compliance on articulation45–49. Elastohydrodynamic lubrication is unique to cartilage: loading induces fluid efflux from the tissue, creating an interposed fluid-film entrained between the articulating joint surfaces. The coefficient of friction depends on the properties of the lubricant, as well as the geometry, surface characteristics (such as roughness and surface chemistry) and material properties (such as interstitial fluid load support) of the contacting tissues50.
Designing viscosupplements is a formidable task when confronted with the myriad challenges presented by osteoarthritic degeneration. The formulation must navigate not only the challenges posed by elevated cytokines and reduced synovial fluid lubricity but also tackle the complexities of compromised surface chemistry, altered cartilage structure (such as fibrillation, reduced fixed charge density and lesions) and shifted biomechanics (such as deteriorated compressive and tensile properties and heightened permeability) inherent in OA.
Clinical viscosupplements
Synovial fluid lubricity declines in OA owing to a decrease in the concentration and molecular weight of hyaluronic acid and a decrease in the concentration of PRG4, diminishing its rheological and tribological properties23,34–38. Thus, viscosupplementation of the depleted synovial fluid is a treatment for OA and is based on the theory that supplementing the synovial fluid with exogenous, gel-like hyaluronic acid derivatives via intra-articular injection will enhance synovial fluid viscosity, lubricity and function by rejuvenating the non-Newtonian fluid properties of synovial fluid51. Besides this physicochemical mechanism, pharmacologically, hyaluronic acid has various antioxidative, anti-nitrosative, anti-inflammatory, reparative and analgesic effects that occur via multiple biological mechanisms52. Studies indicate that hyaluronic acid inhibits various pathological processes, including IL-1β-induced MMP activity in synovial fluid, the mRNA expression of various pro-inflammatory molecules (including cyclooxygenase 2 and prostaglandin E2 (PGE2)) in synovial fibroblasts, and the expression of a disintegrin and metalloproteinase with thrombospondin motifs 4 (ADAMTS4, also known as aggrecanase 1) in chondrocytes. Hence, hyaluronic acid reduces synovitis and decreases levels of cytokines in the synovial fluid. These cytokines are known to provoke inflammatory cascades and stimulate the production of MMPs that degrade cartilage ECM in OA53,54. The analgesic effects of hyaluronic acid in synovial joints might arise from several mechanisms. First, hyaluronic acid might inhibit the synthesis of pain-inducing substances such as PGE2 and bradykinin. Furthermore, hyaluronic acid directly interacts with hyaluronic acid receptors on nociceptive pain nerve endings55.
Hyaluronic acid viscosupplementation
Commercially available sodium hyaluronate viscosupplements vary with respect to their molecular weight, degree of crosslinking, compositional origin and recommended frequency of intra-articular injections56–68 (Fig. 3). The majority of these viscosupplements are FDA approved for the treatment of OA, with the exception of Suplasyn (Zuellig Pharma), which is clinically approved in Canada for OA treatment. Systematic reviews and meta-analyses evaluating the clinical efficacy of viscosupplementation are inconclusive and contradictory, leading the American Academy of Orthopedic Surgeons and the American College of Rheumatology not to recommend the use of viscosupplements for treating hand, knee or hip OA69,70. However, given the limited array of treatment options available for OA, sodium hyaluronate viscosupplements are widely used in the clinic and constitute a multibillion dollar business.
Fig. 3 |. Clinical results for several clinically approved viscosupplements.
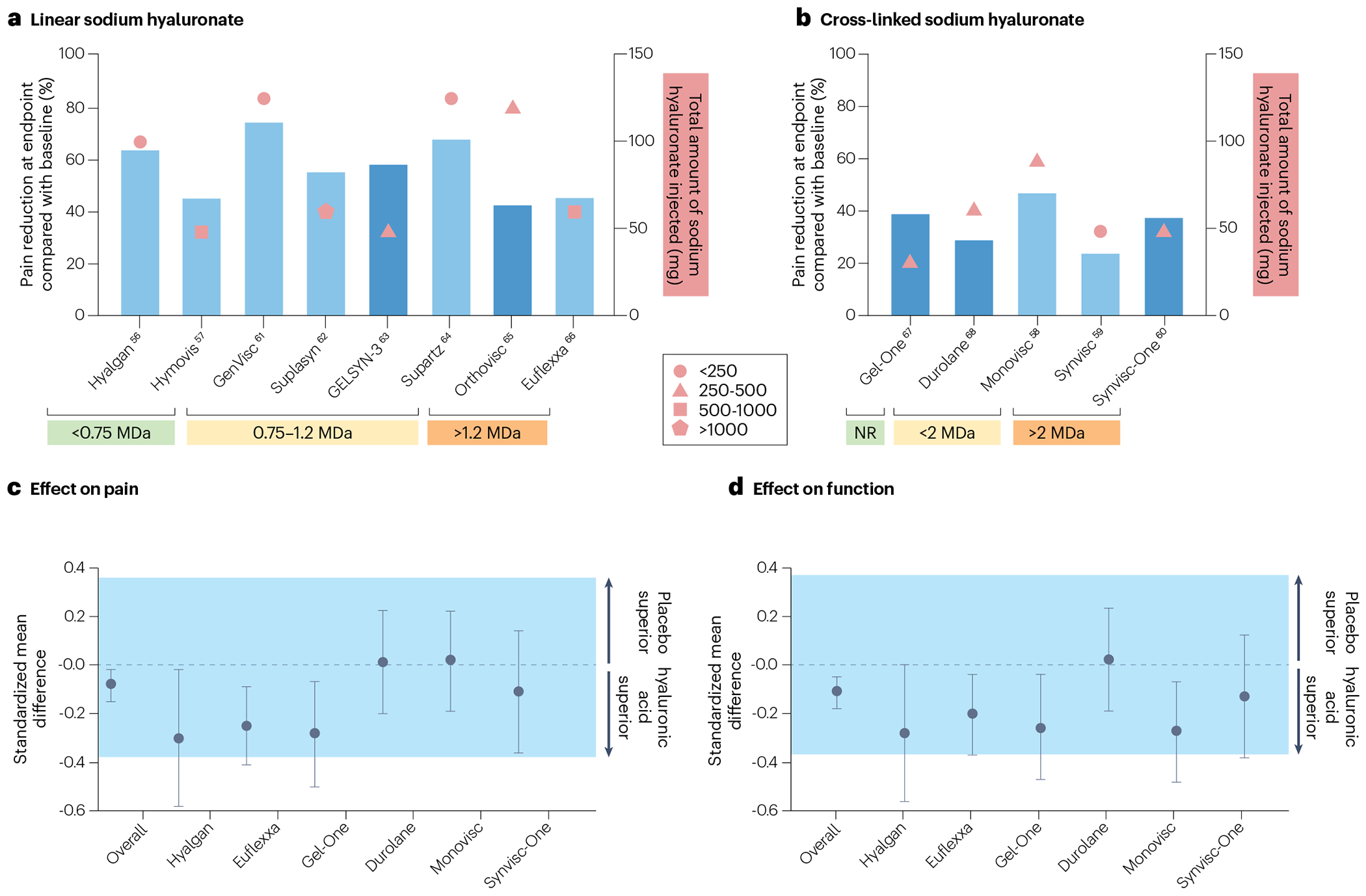
a,b, Results from clinical trials of linear viscosupplements (a) and crosslinked viscosupplements (b). On the x-axis, the viscosupplements are grouped by molecular weight of sodium hyaluronate. The left y-axis, corresponding to the bars, represents the relative decrease in average pain at the study end point compared with the average pain reported at baseline. The dark blue bars represent Western Ontario and McMaster Universities Osteoarthritis pain scores whereas the light blue bars represent visual analogue scale pain. The right y-axis, corresponding to the symbols, represents the total amount of sodium hyaluronate injected. The shape reflects the size of the clinical trial (key). c,d, The clinical outcomes for pain (c) and function (d) for those trials that included a placebo control, and a summary estimate of 24 placebo-controlled trials for pain and 19 placebo-controlled trials for function (Overall), are compared using standardized mean differences82. A standardized mean difference of less than 0 indicates that the viscosupplementation is favoured over placebo. The shaded region demonstrates the values for which clinical outcomes between viscosupplementation and placebo are equivalent, on the basis of a minimal clinically significant difference of 0.37. Data are represented as mean with 95% confidence intervals. All of the viscosupplements are FDA approved, except for Suplasyn, which is approved for clinical use in Canda. NR, not reported.
Several prospective randomized placebo control studies report that intra-articular injections of sodium hyaluronate or crosslinked sodium hyaluronate decrease pain, improve joint function59,66,71,72 and are reparative to the cartilage matrix, as evidenced by an increase in cartilage proteoglycan content (measured by T1Rho MRI) at 6 weeks and 3 months after treatment73. Patients with symptomatic primary knee OA treated with an injection of Hylan G-F20 (marketed as Synvisc (three injections) or Synvisc-One (single injection), Sanofi; a high-molecular weight derivative of sodium hyaluronate composed of crosslinked hylan polymers) showed improvements in Western Ontario and McMaster Universities Osteoarthritis (WOMAC) pain scores, compared with those patients treated with a saline placebo, 26 weeks after the injection60. However, this treatment had no effect on gait velocity. Nevertheless, the patients treated with five weekly intra-articular injections of sodium hyaluronate (Hyalgan, Sanofi) had improved knee function and longer walking times for up to 12 months following the treatment74.
Given the influence of the molecular weight of sodium hyaluronate on synovial fluid lubricity, understanding the effect of the molecular weight of sodium hyaluronate on viscosupplement efficacy is important. In an investigation of 1,187 patients with late-stage OA, treatment with the high-molecular-weight viscosupplement Hylan G-F20 delayed total knee arthroplasty by an average of 2 years75. Although a systematic review reported no clinically significant reduction in pain following injections of crosslinked sodium hyaluronate for OA, a sub-analysis of those studies suggested that higher molecular weight preparations resulted in notable reductions in WOMAC pain scores76. A comparison of intermediate molecular weight (800–1,500 kDa) and low molecular weight (500–730 kDa) sodium hyaluronate preparations77 suggests that intermediate-molecular-weight hyaluronic acid supplements provide better pain relief. However, another evaluation comparing high-molecular-weight (6–7 MDa) with intermediate-molecular-weight (0.5–1.5 MDa) sodium hyaluronate viscosupplements showed improvements in WOMAC pain scores for both groups, independent of molecular weight78.
Several prospective randomized placebo-controlled trials contradict the positive findings of these studies and indicate that sodium hyaluronate and crosslinked sodium hyaluronate injections have limited efficacy for treating mild to moderate knee OA, irrespective of the number of intra-articular injections, structure or molecular weight of the hyaluronic acid supplement78–82. For example, one trial found that treatment with high-molecular-weight sodium hyaluronate (Artzal, Seikagaku Corp; also sold as Supartz and Artz in different countries) or Hylan G-F20 (Synvisc-One) was no better than placebo81. Blinded, randomized, controlled trials comparing intra-articular injection of high-molecular-weight sodium hyaluronate or Hylan G-F20 with saline revealed no differences in WOMAC pain scores or cartilage preservation as measured by MRI79,83. Also, in another trial, increasing the number of intra-articular injections did not affect the efficacy of the treatment: intra-articular injections of 1.5 MDa sodium hyaluronate (Orthovisc) for 3, 4 or 5 sequential weeks failed to relieve knee pain or recover function at 3, 6, 9 and 12 months following treatment80. A meta-analysis of various large placebo-controlled trials found only a marginal superiority of viscosupplementation over placebo in outcomes of both pain reduction and function improvement that was not clinically significant82 (Fig. 3c,d). However, given the limited number of trials included in this analysis (24 and 19 trials for the pain and function assessments, respectively), additional placebo-controlled trials that focus on the effect of viscosupplementation on patient outcomes, including improvements in pain, function and quality of life, are needed before obtaining a definitive answer on the efficacy of viscosupplementation.
The limited ability of hyaluronic acid viscosupplements to consistently mitigate pain and/or modify OA cartilage pathology is multi-factorial. These obstacles include the short intra-articular residence time of sodium hyaluronate owing to enzymatic degradation, and the inability to correct the provocative pathoanatomy and/or abnormal joint kinetics and joint kinematics that induced OA84,85.
Enhanced hyaluronic acid viscosupplementation
The inconsistent clinical benefits observed with sodium hyaluronate and crosslinked sodium hyaluronate intra-articular injections has led to supplementation with additional substances that target specific aspects of OA pathophysiology, as discussed in this section86.
Platelet-rich plasma.
Platelet-rich plasma (PRP), an autologous derivative of whole blood, is purported to reduce pain, slow OA progression and recover joint function by harnessing the inherent properties of platelets, including their anti-inflammatory properties and high concentration of growth factors that promote chondrocyte proliferation and chondrogenesis87–93. PRP added to sodium hyaluronate viscosupplements is intended to combine the biological effects of PRP with the mechanical effects of sodium hyaluronate, and several trials have compared intra-articular injections of PRP alone, sodium hyaluronate alone, PRP and sodium hyaluronate, and saline placebo92–99. Compared with saline treatment, WOMAC scores improved with PRP treatment, but when comparing PRP treatment or combined PRP and sodium hyaluronate treatment with sodium hyaluronate treatment alone, PRP showed no added benefit in reducing pain or improving joint function92–99. This result suggests that the addition of PRP to sodium hyaluronate treatment does not enhance the therapeutic effect of the viscosupplement.
Glucocorticoids.
Glucocorticoids suppress the inflammatory response and reduce pain in OA-affected joints, and, given their safety profile and long history of use as an intra-articular injection, are a prime candidate to combine with hyaluronic acid viscosupplementation100,101. In patients with symptomatic knee OA, intra-articular administration of Cingal (Anika Therapeutics; a combination of sodium hyaluronate (Monovisc, Anika Therapeutics) and triamcinolone hexacetonide (a glucocorticoid)) reduced the average WOMAC pain scores at 1 and 3 weeks compared with sodium hyaluronate (Monovisc) alone. However, this effect was not seen 6 and 26 weeks after the injection, suggesting that the beneficial effects of the corticosteroid on reducing inflammation were short lived and did not improve the long-term efficacy of the sodium hyaluronate102. Although Cingal is currently the only commercially available formulation that combines sodium hyaluronate and a glucocorticoid, several other preclinical formulations are under investigation, with variations in terms of structure and amount of sodium hyaluronate, concentration of glucocorticoids and glucocorticoid used that might have additional clinical benefits86.
Antioxidants.
Antioxidants are reactive oxygen species (ROS) scavengers that minimize ROS-associated degradation and clearance of intra-articular sodium hyaluronate103. Thus, to increase joint resident time, several sodium hyaluronate viscosupplements contain various antioxidants. Treatment with Ostenil Plus (TRB Chemedica; 20 mg/ml sodium hyaluronate and 5 mg/ml of mannitol) improved WOMAC pain, stiffness and total scores in both patients with knee OA and patients with hip OA relative to baseline and untreated individuals104–106. In a 6-month study, Ostenil Plus performed similarly to sodium hyaluronate (Monovisc) and Hylan G-F20 (Synvisc) in reducing WOMAC pain scores, and performed slightly better than sodium hyaluronate (Monovisc) at maintaining low WOMAC stiffness scores, suggesting that the addition of mannitol prolongs linear sodium hyaluronate joint residency time, producing a similar effect to crosslinked sodium hyaluronate, Hylan G-F20 (refs. 107,108). Similarly, HAppyVisc (Labrha; 15.5 mg/ml sodium hyaluronate and 35 mg/ml mannitol) reduced WOMAC pain scores over 6 months, as did sodium hyaluronate without an antioxidant (Euflexxa, Ferring Pharmaceuticals)109. Single injections of HAppyCross (Labrha; 16 mg/ml Hylan and 35 mg/ml mannitol) administered to patients with early-stage knee or hip OA mitigated pain scores at follow-up compared with baseline110,111. However, by comparison, HAppyCross was less effective in patients with late-stage hip OA110,111. Intra-articular administration of Synolis VA (Aptissen; 20 mg/ml sodium hyaluronate and 40 mg/ml sorbitol) decreased joint pain for both patients with knee OA and patients with hip OA at follow-up compared with baseline, but Synolis VA was no better than Hylan G-F20 (Synvisc-One) at reducing WOMAC pain scores 168 days after treatment112. Overall, the combination of sodium hyaluronate with antioxidants provides a therapeutic effect that is similar to that of sodium hyaluronate alone, suggesting that the ROS-scavenging function might not extend the joint-residency time of the material as intended.
Glycosaminoglycans.
Glycosaminoglycans have dual physicochemical and biological characteristics: physically, these molecules organize water molecules to create a viscoelastic, lubricious liquid in aqueous solution and biologically, these molecules reduce the expression of cytokines and matrix-degrading proteins and also stimulate chondrocyte expression of additional proteoglycans113. Glycosaminoglycans have been added to sodium hyaluronate viscosupplements to exploit this dual functionality. In a clinical trial, serial injections of Arthrum HCS (LCA Pharmaceutical; 20 mg/ml sodium hyaluronate and 20 mg/ml chondroitin sulfate) at 1, 3 and 6 months notably reduced the WOMAC pain scores of patients with OA compared with the initial pain scores at study initiation114. A meta-analysis examining the benefit of glycosaminoglycan supplementation (that is, comparing Arthrum HCS versus sodium hyaluronate (Arthrum H, LCA Pharmaceutical)) found that the addition of glycosaminoglycans decreased the initial WOMAC pain score 1 month after the last serial injection, but had no effect at 3 and 6 months115. These results suggest that the addition of glycosaminoglycans provides only short-term benefits to the formulation, possibly owing to rapid clearance of the glycosaminoglycans from the joint space; methods to extend their joint residence time might be beneficial, but further investigation is necessary.
Tribosupplements
In the following section, we use the more general term for cartilage lubricants, tribosupplements, to differentiate them from the previously discussed sodium hyaluronate-based viscosupplements. Tribosupplements employ physicochemical mechanisms to re-establish synovial fluid viscosity, lubricity and chondroprotection by rejuvenating the non-Newtonian synovial fluid properties25,116,117. Important biochemical and architectural motifs critical to endogenous synovial fluid lubricant function serve as inspiration for the design of tribosupplements118–120. These emerging tribosupplements provide biomechanical support, lubrication and/or storage capabilities121,122, and can be split into two categories: free-floating fluid additives or cartilage-bound matrix enhancers that target cartilage via specific reactive domains123.
Fluid additives
Fluid-additive tribosupplements can be split into additional categories: linear polymers, hydrogels and particles (Table 1). These synthetic tribosupplements augment OA synovial fluid viscoelasticity to enhance fluid load support and minimize direct cartilage surface contact during mixed-mode lubrication22,119,122,124–127. Hydrogel and particle constructs offer a versatile system for integrating additional functionalities, beyond lubricating effects. These enhancements can include impeding enzymatic degradation, enabling localized delivery of disease-modifying OA drugs, and/or incorporating stimuli-responsive systems that react to environmental cues128.
Table 1 |.
Tribosupplement fluid additives
| Name | Description | Therapeutic highlights | Refs. | |
|---|---|---|---|---|
| In vitro results and rheological and tribological properties | Effects in vivo and in animal models of OA | |||
| Linear tribosupplement fluid additives | ||||
| HA/PA and HA/PM | Hyaluronic acid backbone grafted with poly(acryloylamino-2-methyl-1-propanesulfone) or poly(2-methacryloyloxyethyl phosphorylcholine) | In a cartilage-on-steel configuration, the coefficient of friction of the combination of HA/PA and HA/PM was less than the coefficient of friction of synovial fluid with both healthy and degraded cartilage | In an ACLT rat model of OA, treatment decreased the OARSI scores compared with saline treatment and the scores were similar to that of healthy animals | 129 |
| Poly(7-oxanorbornene-2-carboxylate) | Analogue of sodium hyaluronate | The tribosupplement has similar rheological and tribological properties to that of healthy synovial fluid and is resistant to hyaluronidase-mediated degradation. In ex vivo cartilage-on-cartilage tribology tests, the polymer produced a coefficient of friction lower than that of saline | Following injection into healthy rabbits, 75% of the residual polymer remained in the joints at day 11. In a rat meniscal tear model of OA, treatment decreased cartilage degeneration compared with saline treatment | 130 |
| rhPRG4 | Recombinant form of human PRG4 | In a cartilage-on-glass tribology test, this tribosupplement produced a coefficient of friction similar to that of synovial fluid and less than that of saline Lubrication enhanced when there is free rhPRG4 or sodium hyaluronate in the bulk solution |
In an ACLT rat model of OA, treatment reduced OARSI score, enhanced PRG4 expression and production, and decreased urinary CTX-II levels compared with saline treatment. In a porcine DMM model of OA, treatment reduced the macroscopic OA score, increased PRG4 production, reduced urinary CTX-II levels and reduced synovial fluid and serum IL-1β levels | 133–136 |
| SynLubricin | Codon-scrambled recombinant form of human PRG4 | Reduced the coefficient of friction of articulating PRG4-depleted cartilage to a similar extent to natural PRG4 and to a greater extent than saline | N/A | 137 |
| LUB:1 | Truncated recombinant human PRG4 | Efficiently bound to the cartilage surface and reduced the coefficient of friction in a cartilage-on-glass configuration when compared with saline treatment | Reduced microscopic OA score in a DMM rat model compared with saline treatment | 138 |
| pMPC | Linear or crosslinked polymer of 2-methacryloyloxyethyl phosphorylcholine | Linear and network pMPC achieved a lower coefficient of friction than saline when lubricating cartilage-on-cartilage articulation. Network pMPC reduced the compressive strain more than linear pMPC and saline. Network pMPC provided better lubrication and cushioning for the articular cartilage upon repeated exposure than linear pMPC | Intra-articular injection of network pMPC into the joint of a healthy horse was safe and well-tolerated | 140 |
| Hydrogel tribosupplement fluid additives | ||||
| HA-VS/SH-2-PEG | Divinyl sulfone-modified hyaluronic acid crosslinked to thiol-terminated poly(ethylene glycol) | HA-VS/SH-2-PEG alone or dispersed in sodium hyaluronate solution reduced the coefficient of friction compared with saline in a steel-on-steel tribological configuration. In vitro, 20% or 100% of the encapsulated triamcinolone acetonide was released over 30 days in the presence or absence of hyaluronidase, respectively | Treatment reduces macroscopic and microscopic signs of disease in an ACL transection rabbit model of knee OA | 141 |
| HA-GG | Gellan gum hydrogel chemically modified with sodium hyaluronate | HA-GG was not susceptible to degradation by hyaluronidase and was cytocompatible with chondrocytes and synoviocytes in vitro. HA-GG decreased the post-wear surface roughness of titanium articulating on titanium compared with sodium hyaluronate | N/A | 142 |
| N-chitosan/ADH/HA-ALD hydrogel | Hydrogel formed by in situ crosslinking of N-carboxyethyl chitosan, adipic acid dihydrazide and aldehyde-modified sodium hyaluronate | This tribosupplement has dynamic self-healing rheological properties as assessed in a recovery test involving successive increases and decreases in applied strain | In a rat model of monoiodoacetate-induced OA, treatment increased cartilage glycosaminoglycan content, and decreased cytokine expression, OARSI score and signs of pain compared with sodium hyaluronate treatment and untreated controls at 2 and 12 weeks | 143 |
| Particle tribosupplement fluid additives | ||||
| poly(DMA-co-MPC) | A block polymer composed of N-(3,4-dihydroxy-phenethyl) methacrylamide and 2-methacryloxyethyl phosphorylcholine, which spontaneously forms spherical aggregates | The coefficient of friction decreases with increasing polymer concentration and increasing MPC content in tribological tests of polystyrene on silica. ROS scavenging increases with increasing polymer concentration and DMA content. The tribosupplement reduces the catabolic and pro-inflammatory response of TNF-treated osteoblastic cells in vitro | N/A | 148 |
| pMPC-liposomes | Poly(2-methacryloyloxyethyl phosphorylcholine) decorated liposomes | The coefficient of friction between two mica surfaces was reduced with pMPC-liposome treatment relative to PEG-liposome treatment | Following intra-articular administration in healthy mice, pMPC-liposomes had a greater joint residency time than both PEG-liposomes and sodium hyaluronate | 149,150 |
| HA@MPC | Sodium hyaluronate methacrylate anhydride particles, decorated with poly(2-methacryloyloxyethyl phosphorylcholine) | In tribology tests using a universal materials tester in a pin-on-disc configuration, the HA@MPC particles lowered the coefficient of friction more than sodium hyaluronate. Increased crosslinking slowed the release of a loaded drug (diclofenac sodium) | Treatment with HA@MPC particles encapsulating the drug diclofenac sodium resulted in increased cartilage glycosaminoglycan content and expression of collagen II and aggrecan, and decreased OARSI scores, compared with treatment with saline, sodium hyaluronate or unloaded HA@MPC in rats with DMM-induced OA | 152 |
| PMs@NF | Nanofat decorated PLGA porous microspheres | PMs@NF decreased the coefficient of friction between polyethylene and Ti6Al4V surfaces in a tribology test. In vitro, treatment with PMs@NF increased the expression of collagen II and aggrecan and decreased the expression of MMP1, IL-1β and TAC1 in TNF-exposed chondrocytes compared with no treatment | In rats with DMM-induced OA, PMs@NF treatment increased the joint space and the amount of type II collagen and aggrecan in the cartilage and decreased the osteophyte volume and OARSI scores compared with saline treatment | 153 |
| MGS@DMA-SBMA | Gelatin microspheres coated with poly(dopamine methacrylamide-to-sulfobetaine methacrylate) | MGS@DMA-SBMA reduced the coefficient of friction between PTFE and Ti6Al4V surfaces compared with saline. In vitro, treatment with diclofenac sodium-loaded MGS@DMA-SMBA increased the expression of type II collagen and aggrecan, and decreased the expression of MMP13, ADAMTS5 and TAC1 expression in TNF-exposed chondrocytes compared with no treatment | In rats with DMM-induced OA, treatment with diclofenac sodium-loaded MGS@ DMA-SMBA increased the joint space and the amount of type II collagen and aggrecan in the cartilage, and decreased the osteophyte volume and OARSI scores compared with untreated animals | 157 |
| Lipo@HMs | Liposomes embedded within hydrogel microspheres | Worn Lipo@HMs reduced the coefficient of friction between polyethylene and stainless-steel surfaces compared with liposome-free hydrogel microspheres and saline | In a rat OA model induced by ACLT and medial meniscectomy, treatment with rapamycin-loaded Lipo@HMs (RAPA@Lipo@HM) reduced osteophyte formation and Mankin score, and preserved the expression of type II collagen and aggrecan, compared with sham treatment or treatment with PBS, liposome-free hydrogel microspheres or unloaded Lipo@HMs | 158 |
| Poly(acrylic acid) microgels | Microgels made from poly(acrylic acid) | Poly(acrylic acid) microgels resuspended in saline achieved similar viscosities to saline and much lower viscosities than bovine synovial fluid or sodium hyaluronate (Hymovis, Fidia Farmaceutici). In a cartilage-on-glass tribology test, poly(acrylic acid) microgels reduced the coefficient of friction between the surfaces compared with saline, to a degree that was comparable with bovine synovial fluid and sodium hyaluronate (Hymovis, Fidia Farmaceutici) | N/A | 159 |
ACLT, anterior cruciate Ligament transection; ADAMTS5, A disintegrin and metalloproteinase with thrombospondin motifs 5; DMM, destabilization of the medial meniscus; HA@MPC, hyaluronic acid methacrylate anhydride particles Loaded with diclofenac sodium and decorated with pMPC; HA/PA, a hyaluronic acid backbone grafted with poly(acryloylamino-2-methyl-1-propanesulfone); HA/PM, a hyaluronic acid backbone grafted poly(2-methacryloyloxyethyl phosphorylcholine); HA-VS/SH-2-PEG, HA-divinyl sulfone/thiol-2-poly (ethylene glycol); Lipo@HMs, hyaluronic acid methacrylate anhydride-based hydrogel microspheres with Lubricious surface Liposomes; MGS@DMA-SBMA, poly(dopamine methacrylamide-to-sulfobetaine methacrylate)-coated microfluidic gelatin methacrylate spheres; MMP1, matrix metalloproteinase-1; N/A, not applicable; OA, osteoarthritis; N-chitosan/ADH/HA-ALD, N-carboxyethyl chitosan, adipic acid dihydrazide and aldehyde-modified hyaluronic acid; OARSI, Osteoarthritis Research Society International; pMPC, polymeric 2-methacryloyloxyethyl phosphorylcholine; PMS@NF, poly(lactic-co-glycolic acid) porous microspheres coated with nanofat; poly(DMA-co-MPC), poly(N-(3,4-dihydroxyphenethyl) methacrylamide-co-2-methacryloxyethyl phosphorylcholine); PRG4, proteoglycan 4; rhPRG4, recombinant human lubricin; ROS, reactive oxygen species; TAC1, tachykinin precursor 1.
Linear polymers.
Linear fluid-additive tribosupplements consist of linear and/or bottle-brush polymers that remain suspended within the synovial fluid following delivery to the synovial space. These constructs are inspired by the naturally occurring lubricating molecules found within synovial fluid: hyaluronic acid and PRG4 (which are linear and bottle-brush polymers, respectively). For example, researchers have constructed semi-synthetic brush-like polymers composed of a hyaluronic acid backbone grafted with poly(acryloylamino-2-methyl-1-propanesulfone) (HA/PA) or poly(2-methacryloyloxyethyl phosphorylcholine) (HA/PM) (Fig. 4), which emulate articular cartilage’s brush-like biopolymers that consist of a hyaluronan backbone and hydrophilic side chains129. In a 3D in vitro model of degraded human cartilage, combined treatment with HA/PA and HA/PM performed better than treatment with either polymer alone; the polymers generated a lubricating surface layer that lowered the coefficient of friction, reduced cartilage fibrosis and degradation and enhanced chondrogenesis129. After intra-articular injection in healthy rats, HA/PA and HA/PM remained in the joint for up to 8 weeks129. Intra-articular injection of the combined HA/PA and HA/PM solution in a rat anterior cruciate ligament transection (ACLT) model of OA mitigated disease and regenerated cartilage better than saline, HA/PA or HA/PM treatment alone, as evidenced by decreased Osteoarthritis Research Society International (OARSI) scores, as well as enhanced type II collagen and aggrecan staining, such that the OARSI scores were comparable with those of healthy cartilage129.
Fig. 4 |. Structures of various linear tribosupplements.
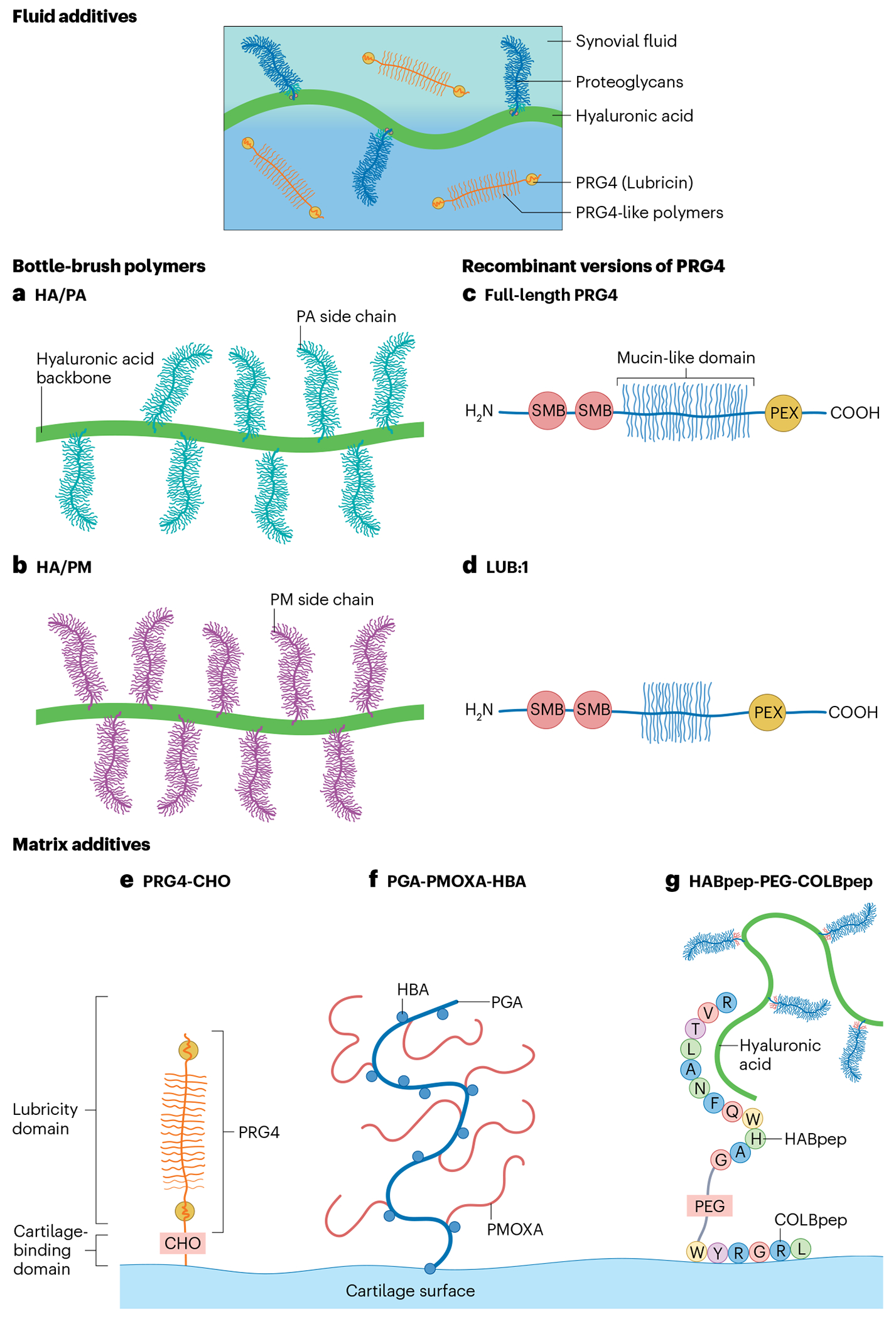
a–d, Various linear polymers are under investigation as tribosupplements, including fluid additives and matrix enhancers. These include fluid additives that mimic the bottlebrush polymers present in synovial fluid, such as HA/PA (a) and HA/PM (b) and linear polymers that mimic proteoglycan 4 (PRG4, also known as lubricin), such as full-length PRG4 (c) and LUB:1 (d). HA/PA and HA/PM consist of synthetic polymers poly(acryloylamino-2-methyl-1-propanesulfone) (PA), and poly(2-methacryloyloxyethyl phosphorylcholine) (PM) covalently bound to an hyaluronic acid (HA) backbone, respectively. Full-length PRG4 is derived from natural sources or produced via recombinant protein synthesis. The yield of recombinant PRG4 is maximized by truncating the mucin-like domain to form LUB:1. e–g, Matrix additives are also under investigation that contain a cartilage-binding domain, as well as a lubricity domain, including PRG4-CHO (e), PGA-PMOXA-HBA (f) and HABPep-PEG-COLBPep (g). PRG4-CHO contains succinimidyl-4-formylbenzamide, which enables PRG4 to covalently attach to the articular surface and improve lubricity. For PGA-PMOXA-HBA, the HBA domains covalently bond to cartilage and the PMOXA blocks create a lubricious film between articulating surfaces. HABPep-PEG-COLBPep consists of PEG flanked by a collagen-binding peptide (COLBpep) and a HA-binding peptide (HABpep). The COLBpep allows the construct to specifically bind collagen at the cartilage surface, whereas the HABpep interacts with HA suspended in synovial fluid to promote lubrication.
A hyaluronidase-resistant, high-molecular-weight (>2 MDa), anionic, poly(7-oxanorbornene-2-carboxylate) sodium hyaluronate analogue has also been developed130–132. Aqueous solutions of the polymer, at various concentrations, had shear-thinning and thixotropic characteristics similar to those of healthy synovial fluid. Ex vivo bovine and human cartilage-on-cartilage friction tests demonstrated that these polymers reduced the average kinetic coefficient of friction relative to healthy synovial fluid and saline. Intra-articular administration of these polymers in a rat meniscal tear model of OA decreased cartilage degeneration and had modest chondroprotective effects compared with saline injection130.
PRG4 is a surface-active, mucin-like glycoprotein, that functions as a lubricant in the eyes, throat and synovial joints84,85. Various investigators have assessed the ability of full-length recombinant human PRG4 (rhPRG4 and SynLubricin) and recombinant truncated PRG4 (LUB:1) to mimic the functional performance of native PRG4 (Fig. 4c,d). Assessed as a boundary lubricant, rhPRG4 reduced the coefficient of friction within an articulating cartilage-on-glass configuration in a dose-dependent manner and was most effective at reducing the coefficient of friction when bound at the cartilage surface alongside being present in the bulk solution133. This recombinant polymer produced a similar coefficient of friction to native synovial fluid, and reduced the coefficient of friction to a greater degree when in a solution with sodium hyaluronate134. In vivo, intra-articular administration of rhPRG4 in a rat ACLT model of OA reduced the OARSI scores, enhanced PRG4 expression and production, and decreased urinary levels of C-terminal crosslinked telopeptide of type II collagen (CTX-II) compared with no treatment or saline treatment135. Similarly, in a porcine model of OA induced by destabilization of the medial meniscus (DMM), rhPRG4 treatment reduced the macroscopic OA score, increased PRG4 production in the synovial fluid, decreased urinary CTX-II levels and reduced IL-1β levels in the synovial fluid and serum compared with saline treatment, but did not improve the microscopic OA scores136.
SynLubricin, a codon scrambled recombinant form of PRG4 designed to improve the manufacturing efficiency of PRG4 while maintaining its function as a boundary lubricant, reduced the coefficient of friction of articulating cartilage to a greater degree than saline and to a similar extent to native synovial fluid137. Similarly, a truncated version of rhPRG4, LUB:1, improved the manufacturing efficiency of the material compared with non-truncated rhPRG4 while maintaining its activity as a boundary lubricant; LUB:1 administration decreased cartilage wear in rats with DMM-induced OA, as measured by microscopic scoring of the affected cartilage, compared with saline treatment138. In humans, rhPRG4 is tolerated as an eye-drop treatment, as evidenced by the completion of a clinical trial in patients with moderate dry eye disease, further supporting its good safety profile139.
Using polymeric 2-methacryloyloxyethyl phosphorylcholine (pMPC) as a bioinspired model system, investigators have examined the relationship between the architecture of a polymer and its tribosupplement performance140. MPC-based polymers have highly hydrated phosphocholine head-groups that can function as efficient lubricant elements, where the positively and negatively charged chemical groups form a hydrated lubricating layer. When applied to the surface of an ex vivo articulating cartilage-on-cartilage configuration, both linear (non-crosslinked) and network (crosslinked) pMPC reduced the coefficient of friction and compressive strain compared with saline treatment. Upon repeated administration of pMPC, with an intermediate phase of saline treatment, network pMPC outperformed linear pMPC in its ability to reduce the coefficient of friction and cushion the cartilage surfaces, demonstrating that pMPC with a crosslinked architecture functions as a superior tribosupplement to pMPC with a linear architecture, and remains effective with multiple applications. Intra-articular injection of network pMPC into healthy equine carpi is safe and does not affect total synovial fluid white blood cell count, joint effusion or histological assessment compared with saline treatment 10 weeks post-injection, suggesting that pMPCs have a good safety profile140.
Building upon clinically established viscosupplementation, the available evidence suggests that linear fluid-additive tribosupplements composed of semisynthetic polymers, synthetic polymers and recombinant proteins can reduce the coefficient of friction of articulating surfaces, in a similar manner to sodium hyaluronate or PRG4, and protect cartilage in several animal models of OA. Although these materials are the next logical step in the pursuit of a clinical alternative to viscosupplementation, the exploration of additional functionalities to fine tune the performance of viscosupplementation is an exciting new area of research. In the following two sections we discuss the design and use of more complex fluid-additive tribosupplements.
Hydrogels.
Hydrogels are crosslinked swollen polymer networks. Following administration to the synovial space, these fluid additives remain in the synovial fluid. One such hydrogel-based system consists of divinyl sulfone-modified sodium hyaluronate crosslinked to thiol-terminated poly(ethylene glycol) (HA-VS/SH-2-PEG); various properties of this system can be controlled by altering the mass ratios of sodium hyaluronate, including its viscoelasticity, resistance to hyaluronidase degradation, and capacity for releasing triamcinolone acetonide (a synthetic corticosteroid)141. Increasing the molecular weight of PEG in this formulation boosted its viscosity and shear-thinning profile141. HA-VS/SH-2-PEG hydrogels reduced the coefficient of friction relative to unmodified sodium hyaluronate in steel-on-steel and pin-on-disc tribology tests141. In an in vivo study using an ACL transection rabbit model of knee OA, intra-articular injection of HA-VS/SH-2-PEG mitigated both macroscopic and microscopic manifestations of OA, resulting in lower Pelletier and histological Mankin scores at the lateral femoral condyles and tibial plateaux relative to saline treatment or treatment with a sodium hyaluronate141.
Gellan gum is a naturally occurring, anionic, linear polysaccharide that, when ionically crosslinked, is mechanically stable and resistant to enzymatic degradation. Hydrogels consisting of sodium hyaluronate chemically modified gellan gum (HA-GG), formed via esterification of free hydroxyl and carboxyl groups, have characteristics of each constituent: sodium hyaluronate imparts lubricity and viscoelasticity whereas gellan gum imparts elasticity and enzymatic stability142. HA-GG gels are easily injected intra-articularly and possess tuneable viscoelasticity, resistance to hyaluronidase and chondrocyte and synoviocyte cytocompatibility142. In tribological wear tests between titanium interfaces, HA-GG gels decreased the post-wear surface roughness relative to linear sodium hyaluronate (Artz, Seikagaku)142; however, HA-GG gels have not been evaluated using ex vivo tissue constructs or in vivo animal models.
Chitosan-based hydrogels are also under investigation as viscosupplements. Chitosan possesses cationic amines that bind to the anionic articular cartilage surface and contains hydroxyl groups that facilitate functionalization. A hydrogel formed by in situ crosslinking of N-carboxyethyl chitosan, adipic acid dihydrazide and aldehyde-modified sodium hyaluronate has self-healing properties, the ability to reform intermolecular bonds and restore its material properties following mechanical perturbation; this latter property is particularly desirable as it enables the formulation to pass through a small-gauge needle and remain efficacious in the synovial space143. In vivo, utilizing a rat model of monoiodoacetate-induced OA, intra-articular administration of this hydrogel decreased the production of TNF and IL-1β, increased the expression of proteoglycans, relieved pain and improved weight-bearing capacity at 2 and 12 weeks post-injection compared with saline or sodium hyaluronate treatment143.
Similar to cross-linked viscosupplements such as Hylan G-F20, hydrogel fluid-additive tribosupplements exhibit prolonged joint residence times and reduced susceptibility to enzymatic degradation. The ability of a hydrogel to both encapsulate and release a drug means that the tribosupplement can also have pharmacological activity, enabling it to address OA pathologies beyond poor lubrication.
Particles.
Polymer-based and/or lipid-based particle fluid additives remain within the synovial fluid following administration to the synovial space and are attractive tribosupplements because of their lubricity in aqueous solution and, similar to their hydrogel counterparts, their capacity to carry and deliver a drug cargo to the joint space. In fact, poly(lactic-co-glycolic) acid (PLGA) microspheres loaded with the corticosteroid triamcinolone acetonide, as an extended-release formulation, was approved by the FDA in 2017 as a treatment for OA knee pain. Although not designed as a cartilage lubricant, these microspheres are a potential therapeutic option and studies to extend the indication to hip OA are underway144–146.
Particle-based additives offer ease of preparation, controllability of size and physicochemical properties, biocompatibility, drug-delivering capabilities and optimization of tribology performance122,124,147. One example is the block polymer poly(DMA-co-MPC), which is composed of N-(3,4-dihydroxyphenethyl) methacrylamide (DMA) and MPC and spontaneously forms spherical aggregates, driven by the hydrophobicity of DMA and hydrophilicity of MPC148. This block polymer was cytocompatible at high concentrations, imparted antioxidant activity by eliminating ROS radicals and decreased friction between silica and polystyrene surfaces compared with water148.
Liposomes composed of hydrogenated soy phosphatidylcholine (HSPC) lipids are one type of lipid-based particle under investigation as tribosupplements. As liposomes tend to aggregate with time, the researchers incorporated distearoylphosphatidylethanolamine (DSPE) conjugated to either pMPC or PEG chains into the liposome formulations (referred to as pMPC-liposomes and PEG-liposomes, respectively) to improve their stability149,150. The pMPC-liposomes and PEG-liposomes remained stable for up to 3 months at 25 °C and pH 5.8–6.2, unlike bare liposomes that only remained stable for up to 7 days149. The coefficient of friction provided by the boundary layers of the pMPC-liposomes was comparable with that of bare liposomes, and much lower than that of PEG-liposomes, as determined by surface force balance measurements between smooth mica surfaces149. In vivo, after intra-articular administration in healthy mice, the joint retention half-life of PEG-liposomes was independent of particle diameter, but decreased with decreasing PEG molecular weight150. By contrast, the half-life of the pMPC-liposomes correlated positively with the liposome diameter, with larger (~170 nm) liposomes having a half-life fourfold longer than that of small-diameter liposomes (~80 nm). Additionally, the larger diameter pMPC-liposomes had a half-life fourfold longer than that of size-matched PEG-liposomes150, suggesting that ionic interactions between pMPC and the charged synovial fluid constituents might also be important for joint retention.
The drug-delivery capabilities of particle additives are an important consideration. NSAIDs are a mainstay of OA treatment but can cause deleterious off-target systemic adverse effects151. To achieve localized delivery of the NSAID diclofenac sodium, alongside enhanced joint lubrication, researchers have investigated electrosprayed hyaluronic acid methacrylate anhydride (HAMA) particles loaded with diclofenac sodium and decorated with pMPC (HA@MPC)152. In tribological testing (using a universal materials tester in a pin-on-disc configuration), the MPC-coated HAMA particles decreased the coefficient of friction of the interacting surfaces relative to saline and unmodified particles152. The release rate of diclofenac sodium from HA@MPC particles incubating in saline, observed over 6 days, correlated inversely with the concentration of HAMA present in the particles; crosslinking the particles reduced their surface pore size and extended the drug release period152. Importantly, in rats with DMM-induced OA, intra-articular administration of drug-loaded HA@MPC particles reduced the OARSI scores compared with treatment with unloaded or unmodified HAMA particles152.
Spherical micro-particles that function as ‘biological ball bearings’ are also under investigation and reduce friction at sliding interfaces via a rolling mechanism. To generate such ‘biological ball bearings’, researchers have coated PLGA microspheres with lubricious and bioactive nanofat153. Nanofat is a specialized form of fat tissue that has been emulsified into very small particles and contains lubricating lipids, bioactive growth factors and stem cells. Although all of the physiological mechanisms of nanofat have not been elucidated, it contains bioactive molecules (such as cytokines) that have anti-inflammatory and analgesic properties154. The combination of nanofat with PLGA porous microspheres forms a 3D porous network called PMs@NF153. In tribological testing (involving sliding a polyethylene ball onto a Ti6Al4V disc), the PMs@NF decreased the coefficient of friction over a range of concentrations, sliding speeds and compressive loads. In in vitro cultures of TNF-treated chondrocytes, PMs@NF was cytocompatible, upregulated the expression of various anabolic mediators (including collagen and aggrecan) and downregulated the expression of various pro-inflammatory mediators (including MMP1, IL-1β and tachykinin precursor 1 (TAC1))153. Injecting PMs@NF into the knee of rats with DMM-induced OA mitigated disease progression, as evidenced by a reduction in osteophyte formation, improvement in gait and reduction in OARSI score when compared with saline-injected controls153.
3,4-dihydroxy-L-phenylalanine is a naturally occurring amino acid that is secreted by mussels to help them adhere to surfaces155. Dopamine, a derivative of dihydroxy phenylalanine, has similar adhesive properties156. Inspired by the ball-bearing devices mentioned previously and mechanisms of mussel adhesion, researchers have developed porous gelatin microspheres (MGSs) coated with a poly(dopamine methacrylamide-to-sulfobetaine methacrylate) block-co-polymer to form MGS@DMA-SBMA157. The adhesive properties of dopamine are used to bind the polymer to the MGS surface. On the particle surface, the negatively charged head groups of the poly-sulfobetaine methacrylate create a hydration layer for lubrication. In a polyethylene ball-on-Ti6Al4V disc-based tribological test, MGS@DMA-SBMA lowered the coefficient of friction compared with uncoated MGS or saline157. MGS@DMA-SBMA loaded with the NSAID diclofenac sodium (MGS (diclofenac sodium)@DMA-SBMA) was chondroprotective in vitro and in vivo157. In in vitro assays of chondrocytes exposed to TNF, treatment with MGS@DMA-SBMA increased the mRNA expression of collagen and aggrecan, and decreased the mRNA expression of catabolic enzymes compared with saline treatment157. Furthermore, intra-articular administration of MGS (diclofenac sodium)@DMA-SBMA decreased joint space narrowing, osteophyte volume and histopathological OARSI scores in rats with DMM-induced OA157.
Lipids form hydration layers on the cartilage surface, and when the surface-attached lipid layers are broken, the hydration layers are renewed by lipids present in the synovial fluid. Inspired by this biological process, researchers have developed HAMA-based hydrogel microspheres with lubricious surface liposomes (Lipo@HM) endowed with the ability to form self-renewing hydration layers158. In a polyethylene sphere-on-stainless steel disc model of articulation, Lipo@HMs reduced the coefficient of friction of the interacting surfaces as the surface of the hydrogel microspheres were worn to expose increasing amounts of lubricating liposomes. The coefficient of friction reached a plateau once a critical number of liposomes were exposed, and finally increased as the Lipo@HMs were squeezed out of the contact area158. In the same model of articulation, pre-worn Lipo@HMs reduced the coefficient of friction compared with liposome-free hydrogel microspheres and saline (by 25% and 50%, respectively)158. For these Lipo@HMs, biolubrication arises via two mechanisms: ball-bearing-like mechanics decrease the interfacial sliding friction via a rolling mechanism, and reformation of hydration layers enables continuous lubrication. Rapamycin has also been incorporated into these liposomes (RAPA@Lipo@HMs) to maintain cellular homeostasis via promoting autophagy. In an in vivo ACLT and medial meniscectomy rat model of OA, RAPA@Lipo@HMs alleviated joint wear and reduced cartilage degeneration compared with sham treatment or treatment with PBS, liposome-free hydrogel microspheres or unloaded Lipo@HMs, as demonstrated by a decrease in osteophyte volume, reduction in Mankin score and preservation of type II collagen and aggrecan expression158. As biolubricants mainly function by reducing mechanical wear, the in vivo results of treating OA with RAPA@Lipo@HMs suggest that mitigating OA requires supplemental biological interventions that upregulate the expression of anabolic genes and downregulate the expression of catabolic, pro-inflammatory genes.
Attempts to design novel tribosupplements often involve producing a material with similar rheological properties (including viscosities) to those of commercially available viscosupplements, on the assumption that such properties are necessary for effective friction reduction. Recent studies examining poly(acrylic acid) microgels (PAA microgels) as tribosupplements challenge this paradigm. PAA microgels prepared at diameters ranging from 5 μm to 28 μm and resuspended in saline achieved much lower viscosities than both bovine synovial fluid and commercially available sodium hyaluronate (Hymovis, Fidia Farmaceutici), yet were able to maintain comparable coefficients of friction to these materials in a cartilage-on-glass tribological test159. These results challenge the field to reconsider the relationship between rheological and tribological performance, and to focus the design of materials on reducing friction as opposed to increasing viscosity.
Particles are ubiquitous within the drug-delivery field; however, their engineerability is expanding their use into additional biomedical applications, such as their use as tribosupplements. In this context, such particles might decrease the coefficient of friction between articulating surfaces, while retaining their role as a drug-delivery vehicle, thereby addressing multiple aspects of OA pathophysiology, which is crucial to halting and reversing OA progression.
Matrix enhancers
Matrix-binding tribosupplements rejuvenate the abraded chondroprotective superficial zone of cartilage by providing a layer that enhances boundary mode lubrication, similar to PRG4 (refs. 21,116,119,160). Classified as linear polymers, hydrogels or particles, these matrix enhancers comprise two domains: one that binds to the cartilage surface, either covalently or non-covalently, and one that imparts lubricity (Table 2).
Table 2 |.
Tribosupplement matrix enhancers
| Name | Description | Therapeutic highlights | Refs. | |
|---|---|---|---|---|
| In vitro results and rheological and tribological properties | Effects in vivo and in animal models of OA | |||
| Linear tribosupplement matrix enhancers | ||||
| PRG4-CHO | Aldehyde-modified PRG4 | When applied to PRG4-depleted cartilage explants, treatment with PRG4-CHO increased the concentration of PRG4 at the cartilage surface compared with treatment with unmodified PRG4. In tribology testing of PRG4-depleted cartilage explants, PRG4-CHO reduced the coefficient of friction of articulating cartilage surfaces compared with saline, achieving a level of lubrication equivalent to that of unmodified PRG4 | N/A | 161,162 |
| PGA-PMOXA-HBA polymers | Poly(2-methyl-2-oxazoline) and hydroxybenzaldehyde grafted onto a poly(glycolic acid) backbone | PGA-PMOXA-HBA reduced the coefficient of friction of degraded cartilage to that of healthy cartilage in ball-on-disc tribology tests. PGA-PMOXA-HBA with cyclic PMOXA brushes achieved lower coefficients of friction at lower grafting densities than PGA-PMOXA-HBA with linear PMOXA brushes. PGA-PMOXA-HBA did not affect the cell viability of treated cartilage explants. Increasing the molecular weight of linear-PMOXA, the ratio of PMOXA to PGA or the copolymer concentration improved the formation of a biopassive layer that decreased the adsorption of serum proteins on aminolized silicon oxide substrates and cartilage surfaces | N/A | 163–165 |
| HABPep-PEG-ColBPep | Construct made from hyaluronic acid-binding peptide, PEG, and collagen II binding peptide | Tribological tests of healthy and osteoarthritic cartilage found that HABPep-PEG-ColBPep treatment along with the addition of exogenous sodium hyaluronate reduced the coefficient of friction compared with saline treatment, independent of disease stage. HABPep-PEG-ColBPep increased the hyaluronic acid concentration at the surface of healthy and PRG4-depleted cartilage by 2.2-fold and 1.3-fold, respectively | In healthy rats, HABPep-PEG-ColBPep increased the joint retention time of hyaluronic acid | 166 |
| pDMAEA-co-PEG | Block copolymer consisting of poly(2-dimethylamino ethyl acrylate) and poly(ethylene glycol) | Treatment reduced the coefficient of friction of articulating cartilage against a glass surface compared with saline, and the individual blocks administered as homo-polymers, reducing the coefficient of friction in a dose-dependent manner | N/A | 167 |
| PAA-g-PEG | A bottlebrush polymer made of poly(ethylene glycol) grafted onto a poly(acrylic acid) backbone | Ex vivo, PAA-g-PEG binds to the surface of cartilage within 60 min. Treatment of articulating cartilage with PAA-g-PEG reduced the coefficient of friction compared with non-lubricated and LUB:1-lubricated surfaces. Treatment of explanted cartilage did not lower the coefficient of friction compared with saline treatment | In a rat ACLT model, PAA-g-PEG treatment improved the OARSI scoring of tibial cartilage degeneration. Treatment did not exacerbate synovitis | 123,168 |
| Hydrogel tribosupplement matrix enhancers | ||||
| pECM biomaterial ink | An ink mixture of thiol-modified sodium hyaluronate and ECM particles | pECM ink was extrudable and polymerized within 30 min after printing. Layering of thiol-modified sodium hyaluronate-only ink atop a bulk layer of pECM ink reduced the surface roughness, coefficient of friction, stiffness and adhesion force of the resulting construct. Chondrocytes seeded on top of ECM ink constructs can distribute throughout the scaffold and remain viable. The customized constructs can be printed for implantation into cartilage defects | N/A | 169 |
| Particle tribosupplement matrix enhancers | ||||
| PEG-4MAL microgel | Poly(ethylene glycol)-maleimide microgels containing PLGA nanoparticles | PEG-4MAL microgels containing PLGA nanoparticles encapsulating a fluorescent dye (rhodamine B) continuously released the dye over 16 days in vitro | Decorating the PLGA nanoparticles with peptides that target synoviocytes or collagen II extended the period of joint retention in both healthy rats and rats with DMM-induced OA. These microgels accumulated at the synovial membrane and did not induce cartilage degradation; however, treatment with the microgels did not improve the disease state of the joints compared with untreated controls | 170 |
ECM, extracellular matrix; HABPep-PEG-ColBPep, HA binding peptide-poly(ethylene glycol)-type II collagen-binding peptide; N/A, not applicable; OA, osteoarthritis; PAA-g-PEG, poly(acrylic acid)-g-poly(ethylene glycol); pDMAEA-co-PEG, poly(2-dimethylamino ethyl acrylate)-co-poly(ethylene glycol); PEG-4MAL, 4-arm poly(ethylene glycol) maleimide; PGA-PMOXA-HBA, poly(glycolic acid)-poly(2-methyl-2-oxazoline)-hydroxybenzaldehyde; PLGA, poly(lactic-co-glycolic acid); PRG4, proteoglycan 4; PRG4-CHO, aldehyde-modified PRG4.
Linear polymers.
Linear matrix enhancers are linear and/or bottle-brush polymers that contain a cartilage reactive domain. This domain ensures that, following introduction into the synovial space, the enhancer binds or localizes to the cartilage surface. This interaction brings the tribosupplement to the damaged cartilage, the tissue that would benefit most from improved lubricity during OA.
To achieve improved superficial zone localization and recruitment of unbound PRG4, researchers have generated an aldehyde-modified version of PRG4 (PRG4-CHO) that binds to the cartilage surface161 (Fig. 4e). Inclusion of this aldehyde modification did not seem to affect the protein structure or lubricating function of PRG4 and led to a twofold increase in PRG4 binding compared with unmodified PRG4 on both native cartilage and PRG4-depleted explants161,162. Tribology testing on PRG4-depleted cartilage explants following incubation in saline, healthy synovial fluid, unmodified PRG4, PRG4-CHO or PRG4-SHAM (unmodified PRG4 exposed to the same buffer conditions as PRG4-CHO) revealed that the static coefficient of friction (resistance to start-up motion) was highest for saline and lowest for synovial fluid162. However, this coefficient varied with duration (ranging from 1.2 to 1,200 s) of cartilage interstitial fluid depressurization, which simulates the amount of time between standing and the first sliding motion experienced by the cartilage surface162. At shorter prolonged standing times, the static coefficient of friction for PRG4, PRG4-SHAM, and PRG4-CHO was equivalent and lower than that of saline; however, at longer prolonged standing times, the static coefficient of friction for PRG4-CHO and PRG4-SHAM was equivalent to that of saline. Overall, both PRG4-CHO and PRG4-SHAM preparations had equivalent effects to PRG4, effectively reducing the kinetic coefficient of friction (resistance to a steady sliding motion) to a level lower than that of saline, but not to the same extent as synovial fluid162. Thus, aldehyde modification enhances adsorption of PRG4 to the cartilage surface without affecting lubricity.
Inspired by the lubricious and adsorptive domains of PRG4, researchers have created PGA-PMOXA-HBA polymers, composed of alternating brushes of poly(2-methyl-2-oxazoline) (PMOXA) and hydroxybenzaldehyde (HBA) that are grafted onto a poly(glycolic acid) (PGA) backbone163–165 (Fig. 4f). Increasing the density and the molar mass of the PMOXA side chains grafted onto the PGA backbone affects the material properties of the resulting film, by producing a thicker film with increased water content and an improved bio-passive layer that impedes adsorption of serum proteins163–165. Application of fluorescein-labelled PGA-PMOXA-HBA copolymers resulted in a greater fluorescent intensity on enzymatically digested cartilage (used to simulate the surface of osteoarthritic cartilage) than on healthy cartilage, suggesting that PGA-PMOXA-HBA preferentially binds to damaged cartilage over healthy cartilage163–165. Notably, in ball-on-disc tribology tests, PGA-PMOXA-HBA treatment restored the coefficient of friction of degraded cartilage to that of healthy cartilage164.
To promote sodium hyaluronate localization at the articular surface, researchers have developed the tribosupplement HABPep-PEG-ColBPep, composed of a hyaluronic acid-binding peptide (HABpep), PEG and type II collagen binding peptide (ColBpep)166 (Fig. 4g). The increased local concentration of sodium hyaluronate at the articulating surface produces a hydrated fluid film that reduces the coefficient of friction. In healthy rats, intra-articular injection of HABPep-PEG-ColBPep increased the in vivo retention time of rhodamine-labelled sodium hyaluronate by 12-fold versus untreated166. In tribological testing of healthy and OA human cartilage, treating the tissue with HABPep-PEG-ColBPep and sodium hyaluronate, followed by washing away the unbound sodium hyaluronate and conducting the friction measurement in a bath of saline, reduced the coefficient of friction to a similar extent to cartilage samples not treated with HABPep-PEG-ColBPep and tested in a bath of a sodium hyaluronate solution. These results suggests that the material tightly binds to sodium hyaluronate, and functions as a boundary lubricant even in the absence of solubilized sodium hyaluronate166. Fluorescence imaging of the cartilage after friction testing confirmed that HABPep-PEG-ColBPep concentrates the limited hyaluronic acid present in OA synovial fluid to the cartilage surface166.
The diblock polymer poly(2-dimethylamino ethyl acrylate)-co-poly(ethylene glycol) (pDMAEA-co-PEG) is another matrix enhancer tribosupplement under investigation. In this formulation, pDMAEA functions as the cartilage binding block and PEG serves as the lubrication block167. Application of pDMAEA-co-PEG to an articulating cartilage surface reduced the coefficient of friction compared with treatment with saline or the individual blocks of the homopolymers, suggesting that localization of the polymer to the articular surface improves the lubricating ability of the material167. Another PRG4-mimetic matrix enhancer, prepared by grafting PEG onto a PAA core (PAA-g-PEG), leverages the terminal thiol of the bottle-brush polymer to bind the material to the cartilage surface through interactions with collagen fibres123. Fluorescently labelled PAA-g-PEG bound to the surface of cartilage within 60 min, and the construct reduced the coefficient of friction of articulating cartilage compared with PRG4-stripped samples lubricated with saline and LUB:1-lubricated samples; however, the coefficient of friction was not reduced to the level provided by native PRG4 (ref. 123). In an ACLT rat model of OA, treatment with PAA-g-PEG did not improve the OARSI scoring of tibial cartilage degeneration. Furthermore, on explanted cartilage, treatment did not reduce the coefficient of friction compared with saline treatment, suggesting that treatment with PAA-g-PEG did not protect the cartilage from surface roughening; however, the treatment was well tolerated as there was no evidence of worsened synovitis168.
Overall, linear matrix enhancers, inspired by PRG4, build upon their linear fluid-additive counterparts by localizing the tribosupplement to the cartilage surface, thus ensuring that the material lubricates the tissue most in need of lubrication. When localized to these interfaces, linear matrix enhancers reduce the coefficient of friction of the ex vivo articulating surface; however, additional preclinical testing in large animal models of OA are needed to assess their safety and performance.
Hydrogels.
Hydrogel matrix enhancers are a newer iteration of tribosupplement in which the network polymers contain a cartilage reactive group that secures the tribosupplement to the cartilage surface and localize it to the articulating surface. Extrusion-based 3D printing is an adaptive technology that enables the creation of layered constructs of a specific size and shape, using a range of printable materials. However, this approach requires the formulation of biomaterial inks that meet ‘printability’ requirements (including optimal viscosity and the ability to exhibit shear-thinning behaviour). Additionally, these inks must have the desirable physicochemical and mechanical properties, while also meeting biological prerequisites for the intended target tissue (such as demonstrating bioactivity and biocompatibility). To produce a mechanically robust, layered scaffold that replicates the zonal structure of cartilage and restores the surface lubrication of hyaline cartilage, one study described an innovative biomaterial ink derived from ECM particles (pECM biomaterial ink)169. This ink contains sodium hyaluronate that has been chemically modified with thiol groups such that it crosslinks directly to the decellularized cartilage ECM particles via disulfide bonds. This process resulted in the formation of a 3D network of ECM particles169. Properties of this material, such as the construct mechanics, nutrient diffusion and cell viability, could be tuned by changing the composition and concentration of the ECM in-fill. Co-printing two inks (the pECM biomaterial ink and an ink containing thiol-modified sodium hyaluronate only) generated a layered scaffold, consisting of a dense layer made with pECM and a lubricious layer on top made with the thiol-modified sodium hyaluronate only, such that the overall scaffold had bulk compressive and surface mechanical properties (including coefficient of friction, adhesion and roughness) that approached those of native cartilage169. The addition of macropores increased the viability of cells introduced into the scaffold169. In ex vivo studies, filling cartilage defects with 3D-printed scaffolds manufactured from pECM biomaterial ink decreased the coefficient of friction and surface roughness, and increased the compressive modulus and adhesive force, compared with untreated controls169. Such 3D-printable bioinks are an exciting technology for resurfacing osteoarthritic cartilage. Future in vivo studies are needed to further evaluate their performance.
Particles.
Particle matrix enhancers are another emerging group of tribosupplements. Similar to other matrix enhancers, these particles contain a cartilage reactive group for binding to the cartilage surface. For example, using microfluidic fabrication technology, researchers have synthesized nanocomposite 4-arm-poly(ethylene glycol)-maleimide (PEG-4MAL) microgels that contain PLGA nanoparticles (200–400 nm NP) decorated with cartilage-targeting peptides (HAP-1 or WYR). HAP-1 binds and targets fibroblast-like synoviocytes, which are the main cell type of the synovium and produce high levels of inflammatory cytokines during OA, whereas WYR binds to the α1 chain of type II collagen in cartilage170. In vitro experiments found that the PEG-4MAL microgels containing the synoviocyte-targeting peptide or cartilage-targeting peptide specifically bound to synoviocytes (human and rabbit) or bovine articular cartilage, respectively170. Microgels containing fluorescent rhodamine B-loaded PLGA NPs continuously released the fluorophore over 16 days, suggesting that drugs loaded into the particles would follow a similar release profile170. After intra-articular injection in rats with DMM-induced OA, the PEG-4MAL microgels remained in the joint for at least 3 weeks without inducing further joint degeneration as measured by EPIC-μCT and histology; however, the PEG-4MAL microgels did not improve the disease state of the treated joints compared with untreated controls170. Given these encouraging results, the drug-delivery and lubrication capabilities of these types of material should be investigated further.
Conclusions
In OA, a combination of factors contribute to altered lubrication of the synovial joint. Today, no currently approved devices or pharmacological treatments impart chondroprotection after the induction of OA. Various linear and crosslinked sodium hyaluronate viscosupplements are now available that have analgesic effects that are equivalent to administered NSAIDS, but do not provide chondroprotection. The short joint residence time of these viscosupplements has led to the clinical development of enhanced viscosupplementation, including co-delivery of sodium hyaluronate or crosslinked sodium hyaluronate with drugs that target unaddressed pathologies86.
The natural lubricating capabilities of hyaluronic acid, PRG4 and surface-active phospholipids have inspired the design, synthesis and evaluation of tribosupplements. As biolubricants mainly function by reducing wear, when used in isolation these substances have limited chondroprotective and/or chondroregenerative effects. Mitigating OA requires supplemental pharmacological interventions that upregulate anabolic mediators and downregulate catabolic, pro-inflammatory mediators. Multifunctional nanoparticles, micelles and/or hydrogels that perform both as a tribosupplement and delivery system for disease-modifying small molecules or protein therapeutic molecules represent a key advance and future direction worthy of study. Increasing the joint residency time and localizing bioactive molecules to the sites of tissue damage should also improve their therapeutic efficiency.
Future work must define the stages of OA when tribosupplements will be most effective, including establishing objective success criteria and minimally clinically significant thresholds. OA affects all synovial joint structures, including the synovium, subchondral bone, meniscus, patellar tendon, quadriceps muscle and infrapatellar fat pad. One important avenue to explore is which tissues are the pain generators that are amenable to pain mitigation through biomechanical interventions. The interplay between rejuvenating the composition and material properties of synovial tissue and fluid, and the alleviation of pain while restoring joint function warrants mechanistic validation through independent animal experiments. In these studies, inflammatory molecules (such as IL-1, IL-2 and TNF) and known pain neuropeptides (such as substance P) should be measured in the affected tissues to better understand the nociceptive and neuropathic aspects of OA-related pain and how improving synovial fluid lubrication and cartilage tissue properties might mitigate this pain.
The majority of viscosupplement and tribosupplement in vivo work uses rodent models of mechanically induced OA in young animals. However, the growth plates never close, and bone regeneration can occur in these animals, unlike in humans. Rodent joints are extremely small compared with joints in larger animals and humans, and the cartilage has a different composition and structure to humans (including the presence of a thick layer of calcified cartilage). Furthermore, quadruped models might not replicate the pathomechanics (such as the kinematics) of humans. These limitations, combined with a lack of studies in older animals, as well as outcome differences between males and females, hamper translation, and is a call to the community to consider age and sex differences in future small and large animal studies171.
Amelioration of OA requires a comprehensive approach that addresses the multitude of factors involved. Continued research by interdisciplinary teams composed of clinicians, veterinarians, biomedical engineers, chemists and biologists will afford new materials and treatment approaches specifically designed to synergistically interact within the synovial space and recapitulate the molecular and macromolecular interactions present in a healthy joint.
Key points.
In osteoarthritis, compositional changes to the synovial fluid reduce the lubricating ability of the joint and can lead to propagation of cartilage wear.
Clinically approved viscosupplements are aimed at restoring synovial fluid lubricity and reducing the inflammatory response, but their efficacy remains nebulous.
Enhanced viscosupplements combine sodium hyaluronate with additional materials (such as glucocorticoids and antioxidants) that target specific aspects of osteoarthritis, but the benefits of these additions seem minimal.
Tribosupplementation, the delivery of non-hyaluronan-based lubricants to the joint, shows some promise but is largely in the preclinical stages of development; this approach includes fluid additives and matrix enhancers.
Fluid additives are cartilage lubricants (with a linear, hydrogel or particle structure) that remain suspended in the synovial fluid following intraarticular injection and comprise linear, hydrogel and particle structures.
Matrix enhancers are cartilage lubricants (with a linear, hydrogel or particle structure) that, in addition to containing a lubricious domain, contain a domain that binds to the cartilage surface.
Acknowledgements
C.D.D.M. acknowledges support from the National Science Foundation Graduate Research Fellowship Program (DGE-1840990). T.B.L. acknowledges support from the National Institutes of Health (NIH; F31 AR075386). J.M. acknowledges support from the Academy of Finland (348410, 357787), Instrumentarium Science Foundation (190021) and the Orion Research Foundation sr. B.D.S. acknowledges support from the Harvard Catalyst Foundation. A.J., D.T.F., T.P.S., M.B., M.B.A. and M.W.G. acknowledge support from Boston University and the Wallace H. Coulter Foundation.
Glossary
- Boundary lubrication
The mode of lubrication in which the two sliding surfaces are only separated by a thin film of lubricant, such that imperfections along the surfaces can come into contact with each other.
- Fluid-film lubrication
The mode of lubrication in which two sliding surfaces are completely separated by a film of lubricant.
- Hydration lubrication
The phenomenon of water molecules clustering around charged groups to form hydration layers that maintain extremely low coefficients of friction.
- Interstitial fluid load support
The proportion of an applied load that is supported by pressurized fluid entrapped within the cartilage matrix.
- Non-Newtonian fluid
A fluid that has a variable viscosity depending on the stress applied to it.
- Rheological properties
The deformation properties of a material, often described in terms of viscosity, storage modulus and loss modulus.
- Shear-thinning
A rheological behaviour in which the viscosity of a fluid decreases with increasing shear strain.
- Tribological properties
The frictional properties of a material during rubbing; lubricants are intended to affect tribological properties by decreasing friction between two rubbing surfaces.
- Tribosupplementation
Delivery of a material, other than an exogenous hyaluronic acid solution, to a synovial joint, with the intention of improving cartilage lubrication as a treatment for osteoarthritis.
- Viscosupplementation
Delivery of an exogenous hyaluronic acid solution, often formulated as sodium hyaluronate, to an osteoarthritic joint, to restore the lubricity of the synovial fluid.
Footnotes
Competing interests
A patent was filed and is owned by Boston University on poly(7-oxanorbornene-2-carboxylate), a tribosupplement formulation described in the Review, and the patent is available for licensing (US8378064B2). M.W.G. is an inventor listed on the patent. No IP has been licensed to the author. All other authors declare no competing interests.
Peer review information Nature Reviews Rheumatology thanks the anonymous reviewers for their contribution to the peer review of this work.
References
- 1.Long H. et al. Prevalence trends of site-specific osteoarthritis from 1990 to 2019: findings from the Global Burden of Disease Study 2019. Arthritis Rheumatol. 74, 1172–1183 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hootman JM, Helmick CG, Barbour KE, Theis KA & Boring MA Updated projected prevalence of self-reported doctor-diagnosed arthritis and arthritis-attributable activity limitation among US adults, 2015–2040. Arthritis Rheumatol. 68, 1582–1587 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arden NK et al. Non-surgical management of knee osteoarthritis: comparison of ESCEO and OARSI 2019 guidelines. Nat. Rev. Rheumatol 17, 59–66 (2021). [DOI] [PubMed] [Google Scholar]
- 4.Bijlsma JWJ, Berenbaum F & Lafeber FPJG Osteoarthritis: an update with relevance for clinical practice. Lancet 377, 2115–2126 (2011). [DOI] [PubMed] [Google Scholar]
- 5.Sinusas K. Osteoarthritis: diagnosis and treatment. Am. Fam. Physician 85, 49–56 (2012). [PubMed] [Google Scholar]
- 6.Maudens P, Jordan O & Allémann E Recent advances in intra-articular drug delivery systems for osteoarthritis therapy. Drug. Discov. Today 23, 1761–1775 (2018). [DOI] [PubMed] [Google Scholar]
- 7.Balazs EA & Denlinger JL Viscosupplementation: a new concept in the treatment of osteoarthritis. J. Rheumatol. Suppl 39, 3–9 (1993). [PubMed] [Google Scholar]
- 8.Legré-Boyer V. Viscosupplementation: techniques, indications, results. Orthop. Traumatol. Surg. Res 101, S101–S108 (2015). [DOI] [PubMed] [Google Scholar]
- 9.Venn M & Maroudas A Chemical composition and swelling of normal and osteoarthrotic femoral head cartilage. I. Chemical composition. Ann. Rheum. Dis 36, 121–129 (1977). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sophia Fox AJ, Bedi A & Rodeo SA The basic science of articular cartilage: structure, composition, and function. Sports Health 1, 461–468 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carballo CB, Nakagawa Y, Sekiya I & Rodeo SA Basic science of articular cartilage. Clin. Sports Med 36, 413–425 (2017). [DOI] [PubMed] [Google Scholar]
- 12.Chen SS, Falcovitz YH, Schneiderman R, Maroudas A & Sah RL Depth-dependent compressive properties of normal aged human femoral head articular cartilage: relationship to fixed charge density. Osteoarthritis Cartilage 9, 561–569 (2001). [DOI] [PubMed] [Google Scholar]
- 13.Ateshian GA The role of interstitial fluid pressurization in articular cartilage lubrication. J. Biomech 42, 1163–1176 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mäkelä JTA, Han SK, Herzog W & Korhonen RK Very early osteoarthritis changes sensitively fluid flow properties of articular cartilage. J. Biomech 48, 3369–3376 (2015). [DOI] [PubMed] [Google Scholar]
- 15.Park S, Krishnan R, Nicoll SB & Ateshian GA Cartilage interstitial fluid load support in unconfined compression. J. Biomech 36, 1785–1796 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mansour JM & Mow VC The permeability of articular cartilage under compressive strain and at high pressures. J. Bone Joint Surg. Am 58, 509–516 (1976). [PubMed] [Google Scholar]
- 17.Krishnan R, Kopacz M & Ateshian GA Experimental verification of the role of interstitial fluid pressurization in cartilage lubrication. J. Orthop. Res 22, 565–570 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McCutchen CW The frictional properties of animal joints. Wear 5, 1–17 (1962). [Google Scholar]
- 19.Radin EL, Paul IL, Swann DA & Schottstaedt ES Lubrication of synovial membrane. Ann. Rheum. Dis 30, 322 (1971). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ateshian GA & Wang H Rolling resistance of articular cartilage due to interstitial fluid flow. Proc. Inst. Mech. Eng. H 211, 419–424 (2016). [DOI] [PubMed] [Google Scholar]
- 21.Walker PS, Dowson D, Longfield MD & Wright V Lubrication of human joints. Ann. Rheum. Dis 28, 194 (1969). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ludwig TE, Hunter MM & Schmidt TA Cartilage boundary lubrication synergism is mediated by hyaluronan concentration and PRG4 concentration and structure. BMC Musculoskelet. Disord 16, 386 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bonnevie ED, Galesso D, Secchieri C, Cohen I & Bonassar LJ Elastoviscous transitions of articular cartilage reveal a mechanism of synergy between lubricin and hyaluronic acid. PLoS One 10, e0143415 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Greene GW et al. Adaptive mechanically controlled lubrication mechanism found in articular joints. Proc. Natl Acad. Sci. USA 108, 5255–5259 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seror J, Zhu L, Goldberg R, Day AJ & Klein J Supramolecular synergy in the boundary lubrication of synovial joints. Nat. Commun. 2015 61(6), 1–7 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jahn S & Klein J Hydration lubrication: the macromolecular domain. Macromolecules 48, 5059–5075 (2015). [Google Scholar]
- 27.Oungoulian SR et al. Articular cartilage wear characterization with a particle sizing and counting analyzer. J. Biomech. Eng 135, 024501 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Workman J, Thambyah A & Broom N The influence of early degenerative changes on the vulnerability of articular cartilage to impact-induced injury. Clin. Biomech 43, 40–49 (2017). [DOI] [PubMed] [Google Scholar]
- 29.Steinmeyer J, Knue S, Raiss RX & Pelzer I Effects of intermittently applied cyclic loading on proteoglycan metabolism and swelling behaviour of articular cartilage explants. Osteoarthritis Cartilage 7, 155–164 (1999). [DOI] [PubMed] [Google Scholar]
- 30.Hossain MJ et al. Anisotropic properties of articular cartilage in an accelerated in vitro wear test. J. Mech. Behav. Biomed. Mater 109, 103834 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Santos S, Emery N, Neu CP & Pierce DM Propagation of microcracks in collagen networks of cartilage under mechanical loads. Osteoarthritis Cartilage 27, 1392–1402 (2019). [DOI] [PubMed] [Google Scholar]
- 32.McNulty AL, Rothfusz NE, Leddy HA & Guilak F Synovial fluid concentrations and relative potency of interleukin-1 alpha and beta in cartilage and meniscus degradation. J. Orthop. Res 31, 1039–1045 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Berg WB, van den, Loo FA, van de, Zwarts WA & Otterness IG Effects of murine recombinant interleukin 1 on intact homologous articular cartilage: a quantitative and autoradiographic study. Ann. Rheum. Dis 47, 855 LP–855863 (1988). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fam H, Bryant JT & Kontopoulou M Rheological properties of synovial fluids. Biorheology 44, 59–74 (2007). [PubMed] [Google Scholar]
- 35.Elsaid KA, Jay GD, Warman ML, Rhee DK & Chichester CO Association of articular cartilage degradation and loss of boundary-lubricating ability of synovial fluid following injury and inflammatory arthritis. Arthritis Rheum. 52, 1746–1755 (2005). [DOI] [PubMed] [Google Scholar]
- 36.Elsaid KA et al. Decreased lubricin concentrations and markers of joint inflammation in the synovial fluid of patients with anterior cruciate ligament injury. Arthritis Rheum. 58, 1707–1715 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ludwig TE, McAllister JR, Lun V, Wiley JP & Schmidt TA Diminished cartilage-lubricating ability of human osteoarthritic synovial fluid deficient in proteoglycan 4: restoration through proteoglycan 4 supplementation. Arthritis Rheum. 64, 3963–3971 (2012). [DOI] [PubMed] [Google Scholar]
- 38.Watkins AR & Reesink HL Lubricin in experimental and naturally occurring osteoarthritis: a systematic review. Osteoarthritis Cartilage 28, 1303–1315 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bonnevie ED & Bonassar LJ A century of cartilage tribology research is informing lubrication therapies. J. Biomech. Eng 142, 031004 (2020). [DOI] [PubMed] [Google Scholar]
- 40.Lin W & Klein J Recent progress in cartilage lubrication. Adv. Mater 33, 2005513 (2021). [DOI] [PubMed] [Google Scholar]
- 41.Stribeck R. Kugellager Für Beliebige Belastungen (Buchdruckerei AW Schade, 1901). [Google Scholar]
- 42.Mori S, Naito M & Moriyama S Highly viscous sodium hyaluronate and joint lubrication. Int. Orthop 26, 116–121 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gleghorn JP & Bonassar LJ Lubrication mode analysis of articular cartilage using Stribeck surfaces. J. Biomech 41, 1910–1918 (2008). [DOI] [PubMed] [Google Scholar]
- 44.Bonnevie ED, Galesso D, Secchieri C & Bonassar LJ Frictional characterization of injectable hyaluronic acids is more predictive of clinical outcomes than traditional rheological or viscoelastic characterization. PLoS One 14, e0216702 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lewis PR & McCutchen CW Mechanism of animal joints: experimental evidence for weeping lubrication in mammalian joints. Nature 184, 1285 (1959). [DOI] [PubMed] [Google Scholar]
- 46.Walker PS, Dowson D, Longfield MD & Wright V ‘Boosted lubrication’ in synovial joints by fluid entrapment and enrichment. Ann. Rheum. Dis 27, 512–520 (1968). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hlaváček M. Squeeze-film lubrication of the human ankle joint with synovial fluid filtrated by articular cartilage with the superficial zone worn out. J. Biomech 33, 1415–1422 (2000). [DOI] [PubMed] [Google Scholar]
- 48.Higginson GR & Norman R A model investigation of squeeze-film lubrication in animal joints. Phys. Med. Biol 19, 785 (1974). [DOI] [PubMed] [Google Scholar]
- 49.Dowson D & Jin Z-M Micro-elastohydrodynamic lubrication of synovial joints. Eng. Med 15, 63–65 (1986). [DOI] [PubMed] [Google Scholar]
- 50.Schmidt TA & Sah RL Effect of synovial fluid on boundary lubrication of articular cartilage. Osteoarthritis Cartilage 15, 35–47 (2007). [DOI] [PubMed] [Google Scholar]
- 51.Bellamy N. et al. Viscosupplementation for the treatment of osteoarthritis of the knee. Cochrane Database Syst. Rev 2006, CD005321 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gupta RC, Lall R, Srivastava A & Sinha A Hyaluronic acid: molecular mechanisms and therapeutic trajectory. Front. Vet. Sci 6, 192 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yatabe T. et al. Hyaluronan inhibits expression of ADAMTS4 (aggrecanase-1) in human osteoarthritic chondrocytes. Ann. Rheum. Dis 68, 1051 LP–1051058 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nelson FR et al. The effects of an oral preparation containing hyaluronic acid (Oralvisc®) on obese knee osteoarthritis patients determined by pain, function, bradykinin, leptin, inflammatory cytokines, and heavy water analyses. Rheumatol. Int 35, 43–52 (2015). [DOI] [PubMed] [Google Scholar]
- 55.Gotoh S. et al. Effects of the molecular weight of hyaluronic acid and its action mechanisms on experimental joint pain in rats. Ann. Rheum. Dis 52, 817 LP–817822 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Huang & Le T et al. Intra-articular injections of sodium hyaluronate (Hyalgan®) in osteoarthritis of the knee. A randomized, controlled, double-blind, multicenter trial in the Asian population. BMC Musculoskelet. Disord 12, 1–8 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Priano F. Early efficacy of intra-articular HYADD® 4 (Hymovis®) injections for symptomatic knee osteoarthritis. Joints 5, 79–84 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Petterson SC & Plancher KD Single intra-articular injection of lightly cross-linked hyaluronic acid reduces knee pain in symptomatic knee osteoarthritis: a multicenter, double-blind, randomized, placebo-controlled trial. Knee Surgery. Sport. Traumatol. Arthrosc 27, 1992–2002 (2019). [DOI] [PubMed] [Google Scholar]
- 59.Adams ME et al. The role of viscosupplementation with hylan G-F 20 (Synvisc®) in the treatment of osteoarthritis of the knee: a Canadian multicenter trial comparing hylan G-F 20 alone, hylan G-F 20 with non-steroidal anti-inflammatory drugs (NSAIDs) and NSAIDs alone. Osteoarthritis Cartilage 3, 213–225 (1995). [DOI] [PubMed] [Google Scholar]
- 60.Chevalier X. et al. Single, intra-articular treatment with 6 ml hylan G-F 20 in patients with symptomatic primary osteoarthritis of the knee: a randomised, multicentre, double-blind, placebo controlled trial. Ann. Rheum. Dis 69, 113–119 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xin Y. et al. The efficacy and safety of sodium hyaluronate injection (Adant®) in treating degenerative osteoarthritis: a multi-center, randomized, double-blind, positive-drug parallel-controlled and non-inferiority clinical study. Int. J. Rheum. Dis 19, 271–278 (2016). [DOI] [PubMed] [Google Scholar]
- 62.Gadek A, Miśkowiec K, Wordliczek J & Lekarski HL Effectiveness and safety of intra-articular use of hyaluronic acid (Suplasyn) in the treatment of knee osteoarthritis. Przegl. Lek 68, 307–310 (2011). [PubMed] [Google Scholar]
- 63.Pavelka K & Uebelhart D Efficacy evaluation of highly purified intra-articular hyaluronic acid (Sinovial®) vs Hylan G-F20 (Synvisc®) in the treatment of symptomatic knee osteoarthritis. A double-blind, controlled, randomized, parallel-group non-inferiority study. Osteoarthritis Cartilage 19, 1294–1300 (2011). [DOI] [PubMed] [Google Scholar]
- 64.Bronstone A, Neary JT, Lambert TH & Dasa V Supartz (Sodium Hyaluronate) for the treatment of knee osteoarthritis: a review of efficacy and safety. Clin. Med. Insights Arthritis Musculoskelet. Disord 12, 1179544119835221 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Neustadt D, Caldwell J, Bell M, Wade J & Gimbel J Clinical effects of intraarticuLar injection of high molecular weight hyaluronan (Orthovisc) in osteoarthritis of the knee: a randomized, controlled, multicenter trial. J. Rheumatol 32, 1928–1936 (2005). [PubMed] [Google Scholar]
- 66.Altman RD, Rosen JE, Bloch DA, Hatoum HT & Korner P A double-blind, randomized, saline-controlled study of the efficacy and safety of EUFLEXXA® for treatment of painful osteoarthritis of the knee, with an open-label safety extension (The FLEXX Trial). Semin. Arthritis Rheum 39, 1–9 (2009). [DOI] [PubMed] [Google Scholar]
- 67.Strand V, Baraf HSB, Lavin PT, Lim S & Hosokawa H A multicenter, randomized controlled trial comparing a single intra-articular injection of Gel-200, a new cross-linked formulation of hyaluronic acid, to phosphate buffered saline for treatment of osteoarthritis of the knee. Osteoarthritis Cartilage 20, 350–356 (2012). [DOI] [PubMed] [Google Scholar]
- 68.Altman RD et al. Efficacy and safety of a single intra-articular injection of non-animal stabilized hyaluronic acid (NASHA) in patients with osteoarthritis of the knee. Osteoarthritis Cartilage 12, 642–649 (2004). [DOI] [PubMed] [Google Scholar]
- 69.Kolasinski SL et al. 2019 American College of Rheumatology/Arthritis Foundation guideline for the management of osteoarthritis of the hand, hip, and knee. Arthritis Rheumatol. 72, 220–233 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.American Academy of Orthopaedic Surgeons. Viscosupplementation; cannot recommend. AAOS; http://www.orthoguidelines.org/guideline-detail?id=1214 (2015). [Google Scholar]
- 71.Kirchner M & Marshall D A double-blind randomized controlled trial comparing alternate forms of high molecular weight hyaluronan for the treatment of osteoarthritis of the knee. Osteoarthritis Cartilage 14, 154–162 (2006). [DOI] [PubMed] [Google Scholar]
- 72.Wobig M, Dickhut A, Maier R & Vetter G Viscosupplementation with Hylan G-F 20: a 26-week controlled trial of efficacy and safety in the osteoarthritic knee. Clin. Ther 20, 410–423 (1998). [DOI] [PubMed] [Google Scholar]
- 73.Shah RP et al. T1Rho magnetic resonance imaging at 3t detects knee cartilage changes after viscosupplementation. Orthopedics 38, e604–e610 (2015). [DOI] [PubMed] [Google Scholar]
- 74.Kolarz G, Kotz R & Hochmayer I Long-term benefits and repeated treatment cycles of intra-articular sodium hyaluronate (Hyalgan) in patients with osteoarthritis of the knee. Semin. Arthritis Rheum 32, 310–319 (2003). [DOI] [PubMed] [Google Scholar]
- 75.Waddell DD & Bricker DWC Total knee replacement delayed with Hylan G-F 20 use in patients with grade IV osteoarthritis. J. Managed Care Pharm 13, 113–121 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rutjes AWS et al. Viscosupplementation for osteoarthritis of the knee: a systematic review and meta-analysis. Ann. Intern. Med 157, 180–191 (2012). [DOI] [PubMed] [Google Scholar]
- 77.Berenbaum F. et al. A randomised, double-blind, controlled trial comparing two intra-articular hyaluronic acid preparations differing by their molecular weight in symptomatic knee osteoarthritis. Ann. Rheum. Dis 71, 1454–1460 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gigis I, Fotiadis E, Nenopoulos A, Tsitas K & Hatzokos I Comparison of two different molecular weight intra-articular injections of hyaluronic acid for the treatment of knee osteoarthritis. Hippokratia 20, 26–31 (2016). [PMC free article] [PubMed] [Google Scholar]
- 79.Wang Y. et al. Effects of Hylan G-F 20 supplementation on cartilage preservation detected by magnetic resonance imaging in osteoarthritis of the knee: a two-year single-blind clinical trial. BMC Musculoskelet. Disord 12, 1–9 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kul-Panza E & Berker N Is hyaluronate sodium effective in the management of knee osteoarthritis? A placebo-controlled double-blind study. Minerva Med. 101, 63–72 (2010). [PubMed] [Google Scholar]
- 81.Karlsson J. Comparison of two hyaluronan drugs and placebo in patients with knee osteoarthritis. A controlled, randomized, double-blind, parallel-design multicentre study. Rheumatology 41, 1240–1248 (2002). [DOI] [PubMed] [Google Scholar]
- 82.Pereira TV et al. Viscosupplementation for knee osteoarthritis: systematic review and meta-analysis. Br. Med. J 378, e069722 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.van der Weegen W, Wullems JA, Bos E, Noten H & van Drumpt RAM No difference between intra-articular injection of hyaluronic acid and placebo for mild to moderate knee osteoarthritis: a randomized, controlled, double-blind trial. J. Arthroplast 30, 754–757 (2015). [DOI] [PubMed] [Google Scholar]
- 84.Jevsevar D, Donnelly P, Brown GA & Cummins DS Viscosupplementation for osteoarthritis of the knee: a systematic review of the evidence. J. Bone Joint Surg. Am 97, 2047–2060 (2014). [DOI] [PubMed] [Google Scholar]
- 85.Cooper BG, Catalina Bordeianu, Nazarian A, Snyder BD & Grinstaff MW Active agents, biomaterials, and technologies to improve biolubrication and strengthen soft tissues. Biomaterials 181, 210–226 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pontes-Quero GM et al. Active viscosupplements for osteoarthritis treatment. Semin. Arthritis Rheumatism 49, 171–183 (2019). [DOI] [PubMed] [Google Scholar]
- 87.Hohmann E, Tetsworth K & Glatt V Is platelet-rich plasma effective for the treatment of knee osteoarthritis? A systematic review and meta-analysis of level 1 and 2 randomized controlled trials. Eur. J. Orthop. Surg. Traumatol 30, 955–967 (2020). [DOI] [PubMed] [Google Scholar]
- 88.Chouhan DK et al. Multiple platelet-rich plasma injections versus single platelet-rich plasma injection in early osteoarthritis of the knee: an experimental study in a guinea pig model of early knee osteoarthritis. Am. J. Sports Med 47, 2300–2307 (2019). [DOI] [PubMed] [Google Scholar]
- 89.Yang F. et al. Autophagy is independent of the chondroprotection induced by platelet-rich plasma releasate. Biomed. Res. Int 2018, 9726703 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Xue Y. et al. Pure platelet-rich plasma facilitates the repair of damaged cartilage and synovium in a rabbit hemorrhagic arthritis knee model. Arthritis Res. Ther 22, 1–15 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sánchez M. et al. Platelet-rich plasma injections delay the need for knee arthroplasty: a retrospective study and survival analysis. Int. Orthop 45, 401–410 (2021). [DOI] [PubMed] [Google Scholar]
- 92.Dallari D. et al. Ultrasound-guided injection of platelet-rich plasma and hyaluronic acid, separately and in combination, for hip osteoarthritis. Am. J. Sports Med 44, 664–671 (2016). [DOI] [PubMed] [Google Scholar]
- 93.Saturveithan C. et al. Intra-articular hyaluronic acid (HA) and platelet rich plasma (PRP) injection versus hyaluronic acid (HA) injection alone in patients with grade III and IV knee osteoarthritis (OA): a retrospective study on functional outcome. Malays. Orthop. J 10, 35 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kon E. et al. Platelet-rich plasma intra-articular injection versus hyaluronic acid viscosupplementation as treatments for cartilage pathology: from early degeneration to osteoarthritis. Arthrosc. J. Arthrosc. Relat. Surg 27, 1490–1501 (2011). [DOI] [PubMed] [Google Scholar]
- 95.Cole BJ et al. Hyaluronic acid versus platelet-rich plasma: a prospective, double-blind randomized controlled trial comparing clinical outcomes and effects on intra-articular biology for the treatment of knee osteoarthritis. Am. J. Sports Med 45, 339–346 (2016). [DOI] [PubMed] [Google Scholar]
- 96.Sánchez M. et al. A randomized clinical trial evaluating plasma rich in growth factors (PRGF-Endoret) versus hyaluronic acid in the short-term treatment of symptomatic knee osteoarthritis. Arthrosc. J. Arthrosc. Relat. Surg 28, 1070–1078 (2012). [DOI] [PubMed] [Google Scholar]
- 97.Spaková T, Rosocha J, Lacko M, Harvanová D & Gharaibeh A Treatment of knee joint osteoarthritis with autologous platelet-rich plasma in comparison with hyaluronic acid. Am. J. Phys. Med. Rehabil 91, 411–417 (2012). [DOI] [PubMed] [Google Scholar]
- 98.Patel S, Dhillon MS, Aggarwal S, Marwaha N & Jain A Treatment with platelet-rich plasma is more effective than placebo for knee osteoarthritis: a prospective, double-blind, randomized trial. Am. J. Sports Med 41, 356–364 (2013). [DOI] [PubMed] [Google Scholar]
- 99.Filardo G. et al. Platelet-rich plasma vs hyaluronic acid to treat knee degenerative pathology: study design and preliminary results of a randomized controlled trial. BMC Musculoskelet. Disord 13, 229 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Raynauld J-P et al. Safety and efficacy of long-term intraarticular steroid injections in osteoarthritis of the knee: a randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 48, 370–377 (2003). [DOI] [PubMed] [Google Scholar]
- 101.Savvidou O. et al. Glucocorticoid signaling and osteoarthritis. Mol. Cell. Endocrinol 480, 153–166 (2019). [DOI] [PubMed] [Google Scholar]
- 102.Hangody L. et al. Intraarticular injection of a cross-linked sodium hyaluronate combined with triamcinolone hexacetonide (cingal) to provide symptomatic relief of osteoarthritis of the knee: a randomized, double-blind, placebo-controlled multicenter clinical trial. Cartilage 9, 276–283 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kaderli S. et al. A novel oxido-viscosifying Hyaluronic Acid-antioxidant conjugate for osteoarthritis therapy: biocompatibility assessments. Eur. J. Pharm. Biopharm 90, 70–79 (2015). [DOI] [PubMed] [Google Scholar]
- 104.Borrás-Verdera A, Calcedo-Bernal V, Ojeda-Levenfeld J & Clavel-Sainz C Efficacy and safety of a single intra-articular injection of 2% hyaluronic acid plus mannitol injection in knee osteoarthritis over a 6-month period. Rev. Esp. Cir. Ortop. Ed. Traumatol 56, 274–280 (2012). [DOI] [PubMed] [Google Scholar]
- 105.Frobenius K. A new high-dose treatment with intra-articular hyaluronic acid facilitates the management of osteoarthritis. shopify; https://cdn.shopify.com/s/files/1/0630/0467/2149/files/Frebenius_2009_English_Only_Ostenil_Plus.pdf?v=1671531691 (2009). [Google Scholar]
- 106.Lertwanich P & Lamsam C Efficacy of a single intra-articular injection of 2% sodium hyaluronate plus 0.5% mannitol in patients with symptomatic osteoarthritis of the knee: a preliminary report. J. Med. Assoc. Thai 99, 1094–1101 (2016). [PubMed] [Google Scholar]
- 107.Dernek B. et al. Efficacy of single-dose hyaluronic acid products with two different structures in patients with early-stage knee osteoarthritis. J. Phys. Ther. Sci 28, 3036–3040 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Maheu E, Avouac B, Dreiser RL & Bardin T A single intra-articular injection of 2.0% non-chemically modified sodium hyaluronate vs 0.8% hylan G-F 20 in the treatment of symptomatic knee osteoarthritis: a 6-month, multicenter, randomized, controlled non-inferiority trial. PLoS One 14, e0226007 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Conrozier T. et al. Safety and efficacy of intra-articular injections of a combination of hyaluronic acid and mannitol (HAnOX-M) in patients with symptomatic knee osteoarthritis: results of a double-blind, controlled, multicenter, randomized trial. Knee 23, 842–848 (2016). [DOI] [PubMed] [Google Scholar]
- 110.Matthieu M, Bozgan A-M & Conrozier T Safety and efficacy of single intra-articular injection of a cross-linked hyaluronic acid/mannitol formulation (Happycross™) in knee osteoarthritis results of a prospective observational study in daily practice conditions. Ortho. Rheum. Open Access. J 5, 555664 (2017). [Google Scholar]
- 111.Conrozier T, Bossert M, Balblanc J, Sondag M & Walliser-Lohse A Viscosupplementation with HANOX-M-XL is effective in moderate hip osteoarthritis but is not an alternative to hip joint surgery in patients with severe disease. Results of a clinical survey in 191 patients treated in daily practice. Eur. J. Musculoskelet. Dis 3, 49–55 (2014). [Google Scholar]
- 112.Cortet B, Lombion S, Naissant B, Vidovic E & Bruyère O Non-inferiority of a single injection of sodium hyaluronate plus sorbitol to hylan G-F20: a 6-month randomized controlled trial. Adv. Ther 38, 2271–2283 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bali JP, Cousse H & Neuzil E Biochemical basis of the pharmacologic action of chondroitin sulfates on the osteoarticular system. Semin. Arthritis Rheum 31, 58–68 (2001). [DOI] [PubMed] [Google Scholar]
- 114.Rivera F. et al. Effectiveness of intra-articular injections of sodium hyaluronate-chondroitin sulfate in knee osteoarthritis: a multicenter prospective study. J. Orthop. Traumatol 17, 27–33 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Vincent P. Intra-articular hyaluronic acid in knee osteoarthritis: clinical data for a product family (ARTHRUM), with comparative meta-analyses. Curr. Ther. Res 95, 100637 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.McNary SM, Athanasiou KA & Reddi AH Engineering Lubrication in articular cartilage. Tissue Eng. Part. B Rev 18, 88–100 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Dedinaite A. Biomimetic Lubrication. Soft Matter 8, 273–284 (2012). [Google Scholar]
- 118.Chen M, Briscoe WH, Armes SP & Klein J Lubrication at physiological pressures by polyzwitterionic brushes. Science 323, 1698–1701 (2009). [DOI] [PubMed] [Google Scholar]
- 119.Pradal C, Yakubov GE, Williams MAK, McGuckin MA & Stokes JR Lubrication by biomacromolecules: mechanisms and biomimetic strategies. Bioinspiration Biomim. 14, 51001 (2019). [DOI] [PubMed] [Google Scholar]
- 120.Schmidt TA, Gastelum NS, Nguyen QT, Schumacher BL & Sah RL Boundary Lubrication of articular cartilage: role of synovial fluid constituents. Arthritis Rheum. 56, 882–891 (2007). [DOI] [PubMed] [Google Scholar]
- 121.Zhao T, Wei Z, Zhu W & Weng X Recent developments and current applications of hydrogels in osteoarthritis. Bioengineering 9, 132 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lawson TB, Mäkelä JTA, Klein T, Snyder BD & Grinstaff MW Nanotechnology and osteoarthritis. part 1: clinical landscape and opportunities for advanced diagnostics. J. Orthop. Res 39, 465–472 (2020). [DOI] [PubMed] [Google Scholar]
- 123.Samaroo KJ, Tan M, Putnam D & Bonassar LJ Binding and Lubrication of biomimetic boundary lubricants on articular cartilage. J. Orthop. Res 35, 548–557 (2017). [DOI] [PubMed] [Google Scholar]
- 124.Wang X-B & Liu W-M Nanoparticle-based Lubricant additives. Encycl. Tribol 2369–2376 (2013). [Google Scholar]
- 125.Chen M, Briscoe WH, Armes SP, Cohen H & Klein J Polyzwitterionic brushes: extreme Lubrication by design. Eur. Polym. J 47, 511–523 (2011). [Google Scholar]
- 126.Chen H. et al. Cartilage matrix-inspired biomimetic superlubricated nanospheres for treatment of osteoarthritis. Biomaterials 242, 119931 (2020). [DOI] [PubMed] [Google Scholar]
- 127.Zheng Y. et al. Bioinspired hyaluronic acid/phosphorylcholine polymer with enhanced Lubrication and anti-inflammation. Biomacromolecules 20, 4135–4142 (2019). [DOI] [PubMed] [Google Scholar]
- 128.Gonçalves C, Carvalho DN, Silva TH, Reis RL & Oliveira JM Engineering of viscosupplement biomaterials for treatment of osteoarthritis: a comprehensive review. Adv. Eng. Mater 24, 2101541 (2022). [Google Scholar]
- 129.Xie R. et al. Biomimetic cartilage-lubricating polymers regenerate cartilage in rats with early osteoarthritis. Nat. Biomed. Eng 5, 1189–1201 (2021). [DOI] [PubMed] [Google Scholar]
- 130.Wathier M. et al. A synthetic polymeric biolubricant imparts chondroprotection in a rat meniscal tear model. Biomaterials 182, 13–20 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wathier M. et al. A large-molecular-weight polyanion, synthesized via ring-opening metathesis polymerization, as a lubricant for human articular cartilage. J. Am. Chem. Soc 135, 4930–4933 (2013). [DOI] [PubMed] [Google Scholar]
- 132.Lakin BA et al. A synthetic bottle-brush polyelectrolyte reduces friction and wear of intact and previously worn cartilage. ACS Biomater. Sci. Eng 5, 3060–3067 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Gleghorn JP, Jones ARC, Flannery CR & Bonassar LJ Boundary mode lubrication of articular cartilage by recombinant human lubricin. J. Orthop. Res 27, 771–777 (2009). [DOI] [PubMed] [Google Scholar]
- 134.Abubacker S. et al. Full-length recombinant human proteoglycan 4 interacts with hyaluronan to provide cartilage boundary lubrication. Ann. Biomed. Eng 44, 1128–1137 (2016). [DOI] [PubMed] [Google Scholar]
- 135.Jay GD et al. Prevention of cartilage degeneration and restoration of chondroprotection by lubricin tribosupplementation in the rat following anterior cruciate ligament transection. Arthritis Rheum. 62, 2382–2391 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Waller KA et al. Intra-articular recombinant human proteoglycan 4 mitigates cartilage damage after destabilization of the medial meniscus in the Yucatan minipig. Am. J. Sports Med 45, 1512–1521 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Shurer CR et al. Stable recombinant production of codon-scrambled lubricin and mucin in human cells. Biotechnol. Bioeng 116, 1292–1303 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Flannery CR et al. Prevention of cartilage degeneration in a rat model of osteoarthritis by intraarticular treatment with recombinant lubricin. Arthritis Rheum. 60, 840–847 (2009). [DOI] [PubMed] [Google Scholar]
- 139.Lambiase A. et al. A two-week, randomized, double-masked study to evaluate safety and efficacy of Lubricin (150 μg/mL) eye drops versus sodium hyaluronate (HA) 0.18% eye drops (Vismed®) in patients with moderate dry eye disease. Ocul. Surf 15, 77–87 (2017). [DOI] [PubMed] [Google Scholar]
- 140.Cooper BG et al. A polymer network architecture provides superior cushioning and Lubrication of soft tissue compared to a Linear architecture. Biomater. Sci 11, 7339–7345 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Cai Z, Zhang H, Wei Y, Wu M & Fu A Shear-thinning hyaluronan-based fluid hydrogels to modulate viscoelastic properties of osteoarthritis synovial fluids. Biomater. Sci 7, 3143–3157 (2019). [DOI] [PubMed] [Google Scholar]
- 142.Chen M. et al. Gellan gum modified hyaluronic acid hydrogels as viscosupplements with lubrication maintenance and enzymatic resistance. J. Mater. Chem. B 10, 4479–4490 (2022). [DOI] [PubMed] [Google Scholar]
- 143.Mou D. et al. Intra-articular injection of chitosan-based supramolecular hydrogel for osteoarthritis treatment. Tissue Eng. Regen. Med 18, 113–125 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kivitz A. et al. A randomized, open-label, single-dose study to assess safety and systemic exposure of triamcinolone acetonide extended-release in patients with hip osteoarthritis. Rheumatol. Ther 9, 679–691 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Conaghan PG et al. Effects of a single intra-articular injection of a microsphere formulation of triamcinolone acetonide on knee osteoarthritis pain: a double-blinded, randomized, placebo-controlled, multinational study. J. Bone Joint Surg. Am 100, 666–677 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Conaghan PG et al. Brief report: a phase IIb trial of a novel extended-release microsphere formulation of triamcinolone acetonide for intraarticular injection in knee osteoarthritis. Arthritis Rheumatol. 70, 204–211 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Lawson TB, Mäkelä JTA, Klein T, Snyder BD & Grinstaff MW Nanotechnology and osteoarthritis. part 2: opportunities for advanced devices and therapeutics. J. Orthop. Res 39, 473–484 (2021). [DOI] [PubMed] [Google Scholar]
- 148.Zhao W. et al. Dopamine/phosphorylcholine copolymer as an efficient joint lubricant and ROS scavenger for the treatment of osteoarthritis. ACS Appl. Mater. Interfaces 12, 51236–51248 (2020). [DOI] [PubMed] [Google Scholar]
- 149.Lin W, Kampf N, Goldberg R, Driver MJ & Klein J Poly-phosphocholinated liposomes form stable superlubrication vectors. Langmuir 35, 6048–6054 (2019). [DOI] [PubMed] [Google Scholar]
- 150.Lin W, Goldberg R & Klein J Poly-phosphocholination of liposomes leads to highly-extended retention time in mice joints. J. Mater. Chem. B 10, 2820–2827 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Charlesworth J, Fitzpatrick J, Perera NKP & Orchard J Osteoarthritis — a systematic review of Long-term safety implications for osteoarthritis of the knee. BMC Musculoskelet. Disord 20, 151 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Yang L, Sun L, Zhang H, Bian F & Zhao Y Ice-inspired lubricated drug delivery particles from microfluidic electrospray for osteoarthritis treatment. ACS Nano 15, 20600–20606 (2021). [DOI] [PubMed] [Google Scholar]
- 153.Han Z. et al. Nanofat functionalized injectable super-lubricating microfluidic microspheres for treatment of osteoarthritis. Biomaterials 285, 121545 (2022). [DOI] [PubMed] [Google Scholar]
- 154.Grünherz L, Sanchez-Macedo N, Frueh FS, McLuckie M & Lindenblatt N Nanofat applications: from clinical esthetics to regenerative research: potential applications of nanofat in tissue regeneration with a focus on wound healing and vascularization. Curr. Opin. Biomed. Eng 10, 174–180 (2019). [Google Scholar]
- 155.Burzio LO, Burzio VA, Silva T, Burzio LA & Pardo J Environmental bioadhesion: themes and applications. Curr. Opin. Biotechnol 8, 309–312 (1997). [DOI] [PubMed] [Google Scholar]
- 156.Feinberg H & Hanks TW Polydopamine: a bioinspired adhesive and surface modification platform. Polym. Int 71, 578–582 (2022). [Google Scholar]
- 157.Yang J. et al. Ball-bearing-inspired polyampholyte-modified microspheres as bio-lubricants attenuate osteoarthritis. Small 16, 2004519 (2020). [DOI] [PubMed] [Google Scholar]
- 158.Lei Y. et al. Injectable hydrogel microspheres with self-renewable hydration layers alleviate osteoarthritis. Sci. Adv 8, eabl6449 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Trujillo RJ, Tam AT, Bonassar LJ & Putnam D Effective viscous Lubrication of cartilage with low viscosity microgels. Materialia 33, 102000 (2024). [Google Scholar]
- 160.Jahn S, Seror J & Klein J Lubrication of articular cartilage. Annu. Rev. Biomed. Eng 18, 235–258 (2016). [DOI] [PubMed] [Google Scholar]
- 161.Chawla K, Ham HO, Nguyen T & Messersmith PB Molecular resurfacing of cartilage with proteoglycan 4. Acta Biomater. 6, 3388–3394 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Abubacker S, Ham HO, Messersmith PB & Schmidt TA Cartilage boundary lubricating ability of aldehyde modified proteoglycan 4 (PRG4-CHO). Osteoarthritis Cartilage 21, 186–189 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Morgese G, Ramakrishna SN, Simic R, Zenobi-Wong M & Benetti EM Hairy and slippery polyoxazoline-based copolymers on model and cartilage surfaces. Biomacromolecules 19, 680–690 (2018). [DOI] [PubMed] [Google Scholar]
- 164.Morgese G, Cavalli E, Rosenboom J-G, Zenobi-Wong M & Benetti EM Cyclic polymer grafts that Lubricate and protect damaged cartilage. Angew. Chem 130, 1637–1642 (2018). [DOI] [PubMed] [Google Scholar]
- 165.Morgese G, Cavalli E, Müller M, Zenobi-Wong M & Benetti EM Nanoassemblies of tissue-reactive, polyoxazoline graft-copolymers restore the lubrication properties of degraded cartilage. ACS Nano 11, 2794–2804 (2017). [DOI] [PubMed] [Google Scholar]
- 166.Singh A. et al. Enhanced lubrication on tissue and biomaterial surfaces through peptide-mediated binding of hyaluronic acid. Nat. Mater 13, 988–995 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Sun Z. et al. Boundary mode Lubrication of articular cartilage with a biomimetic diblock copolymer. Proc. Natl Acad. Sci. USA 116, 12437–12441 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Nemirov D. et al. Effect of Lubricin mimetics on the inhibition of osteoarthritis in a rat anterior cruciate Ligament transection model. Am. J. Sports Med 48, 624–634 (2020). [DOI] [PubMed] [Google Scholar]
- 169.Barthold JE et al. Particulate ECM biomaterial ink is 3D printed and naturally crosslinked to form structurally-layered and lubricated cartilage tissue mimics. Biofabrication 14, 025021 (2022). [DOI] [PubMed] [Google Scholar]
- 170.Mancipe Castro LM, Sequeira A, García AJ & Guldberg RE Articular cartilage- and synoviocyte-binding poly(ethylene glycol) nanocomposite microgels as intra-articular drug delivery vehicles for the treatment of osteoarthritis. ACS Biomater. Sci. Eng 6, 5084–5095 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Stewart HL et al. A missed opportunity: a scoping review of the effect of sex and age on osteoarthritis using large animal models. Osteoarthritis Cartilage 32, 501–513 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]


