Abstract
Objectives: To determine the proportion of people who achieve minimal clinically important differences (MCID) with centre-based or home-based pulmonary rehabilitation and to synthesise data on adverse events.
Methods: Cochrane reviews and electronic databases were searched to identify randomised trials comparing centre-based to home-based pulmonary rehabilitation, or either model to usual care, in people with chronic respiratory disease. Primary outcomes were the proportion of participants achieving MCIDs in exercise capacity and disease-specific quality of life. Secondary outcomes were symptoms and adverse events. Cochrane Risk of Bias 1.0 and GRADE were used to assess the risk of bias and certainty of evidence respectively.
Results: Forty-nine trials were eligible. Compared to usual care, a higher proportion of pulmonary rehabilitation participants achieved the MCID for exercise capacity (6MWT: 47% vs 20%, p = 0.11), dyspnoea (43% vs 29%, p = 0.0001), fatigue (48% vs 27%, p = 0.0002) and emotional function (37% vs 25%, p = 0.02), with all of these between group differences statistically significant except for exercise capacity. There were no differences between centre-based and home-based pulmonary rehabilitation in the proportion of participants who achieved MCIDs (34%- 58% across studies). Ninety percent of trials reported no adverse events. Certainty of evidence was low-to- moderate with all outcomes except for CRQ-mastery (centre-based vs home-based pulmonary rehabilitation, or pulmonary rehabilitation vs usual care in COPD), ESWT (pulmonary rehabilitation vs usual care in COPD) and 6MWT (pulmonary rehabilitation vs usual care in bronchiectasis) where evidence was very uncertain.
Discussion: Clinically meaningful outcomes are achieved by similar proportions of participants in centre-based and home-based pulmonary rehabilitation, with few adverse events. Reporting of trial outcomes according to MCIDs is necessary for informed decision making regarding pulmonary rehabilitation models.
Keywords: Chronic obstructive pulmonary disease, bronchiectasis, interstitial lung disease, exercise training, rehabilitation
Introduction
Chronic respiratory diseases including bronchiectasis, chronic obstructive pulmonary disease (COPD) and interstitial lung diseases (ILD) are leading causes of morbidity and mortality1,2 and frequently present with increased dyspnoea, fatigue, reduced exercise tolerance and health-related quality of life.3–5
Pulmonary rehabilitation is a cornerstone of treatment for people with chronic respiratory disease 6 and has traditionally consisted of supervised exercise training, self-management strategies, and support delivered to groups of people in-person by a multidisciplinary team. 7 There is level one evidence supporting the effectiveness of pulmonary rehabilitation for people with COPD,8,9 and increasing evidence in bronchiectasis and ILD.10,11
Despite compelling evidence for the benefits of pulmonary rehabilitation, access to this treatment remains limited worldwide and barriers are multifactorial.12–18 Referrals by health care professionals are influenced by their knowledge and perceptions regarding the benefits and harms of the program, and referral uptake by patients is influenced by their beliefs, expectations, and the physical challenges of attendance. 19 International policy statements 20 have called for novel pulmonary rehabilitation program models that are accessible and acceptable to people with chronic respiratory disease. Alternative models, such as telerehabilitation; home-based models; and web-enabled pulmonary rehabilitation are reported to provide comparable outcomes to traditional centre-based pulmonary rehabilitation.21–23
Offering a range of pulmonary rehabilitation models is aligned with contemporary principles of person-centred care in which treatment choices are informed by an individual’s characteristics, preferences, and the likelihood of personal success. 7 With multiple effective models of pulmonary rehabilitation available, it is important to measure what patients perceive as a valuable change. The minimal clinically important difference (MCID) is defined as the “smallest difference in score in the domain of interest which patients perceive as beneficial”. 24 National COPD audit programs (UK) have reported on the number of people who achieve the MCID in exercise capacity, health-related quality of life and symptoms with pulmonary rehabilitation. 16 Previous systematic reviews of clinical trials have only reported the absolute mean changes in these continuous outcome measures.8–11 To our knowledge, no systematic review has attempted to collate all the available evidence on the number of patients achieving MCID in key outcomes with pulmonary rehabilitation, including head-to-head comparisons of different pulmonary rehabilitation models or comparisons to usual care. Although the incidence of adverse events has been reported in some systematic reviews, an up-to-date synthesis of studies in a wider range of settings and comparisons of novel program models is necessary. A full understanding of the benefits and safety of different models will help health professionals and patients make informed decisions on pulmonary rehabilitation. 25
The aims of this systematic review were to compare the proportion of people with chronic respiratory disease who achieve clinically significant changes in exercise capacity, health-related quality of life and symptoms with pulmonary rehabilitation versus usual care, and centre-based versus home-based pulmonary rehabilitation; and collate all the available evidence on the number and type of adverse events during pulmonary rehabilitation.
Methods
The protocol for this study was pre-specified (International Prospective Register of Systematic Reviews, PROSPERO: CRD42020185841).
Participants/population
Studies were included where more than 90% of adult participants had a diagnosis of bronchiectasis, COPD or ILD. Studies with participants who were stable and post-acute exacerbation (except where mechanically ventilated) were included.
Intervention
Eligible interventions were any centre-based or home-based rehabilitation program of at least 4 weeks’ duration that included exercise training (that was aerobically demanding) with or without any form of education and/or psychological support. Centre-based pulmonary rehabilitation was defined as any in-patient, out-patient, or community-based program. Home-based rehabilitation was defined as any program where the majority (>75%) of the program took place in the home or primary residential setting (nursing home/supported accommodation), including tele-rehabilitation.
Comparator
Eligible comparisons were pulmonary rehabilitation versus usual care, and centre-based versus home-based pulmonary rehabilitation. Usual care was defined as conventional care. This may have included verbal advice about physical activity, education, and self-management, but no supervised exercise or self-management training.
Outcomes
Primary outcomes were the proportion of patients achieving the MCID for exercise capacity (e.g. 6-min walk test (6MWT), incremental shuttle walk test (ISWT), endurance shuttle walk test (ESWT), cycle endurance test (CET), incremental cycle ergometry) and disease-specific health-related quality of life (e.g. chronic respiratory disease questionnaire (CRQ); St Georges Respiratory Questionnaire (SGRQ)).
Secondary outcome measures were symptom control (modified Medical Research Council dyspnoea scale (mMRC), COPD Assessment Test (CAT)) and adverse events categorised as mild or serious, and documented as the number of participants experiencing one or more event.
Studies were only included for exercise capacity, health-related quality of life and symptom control if they reported data according to the MCID of outcome measures.
Study design
Studies were eligible if they adopted a trial design with allocation of participants by individual or cluster randomisation, or via a quasi-randomised method.
Search strategy
Firstly, we searched for existing Cochrane systematic reviews of exercise training and/or pulmonary rehabilitation in Bronchiectasis, COPD or ILD in the Cochrane Database of Systematic Reviews that had evaluated eligible comparisons. The following bibliographic databases were searched from the last search date of the final Cochrane review to August 2023: MEDLINE, CINAHL, EMBASE, Cochrane Central Register of Controlled Trials (CENTRAL), Physiotherapy Evidence Database (PEDro). No limits were set on publication or language. Search strategies for all bibliographic databases are presented in Table S1-5. Search results were imported into Covidence (Veritas Health Innovation, Melbourne, Australia). Following removal of duplicate citations, titles and abstracts were screened independently by two reviewers. For studies that were not excluded based on title/abstract, full text papers were retrieved and independently assessed by two reviewers for eligibility. Any discrepancies in decisions of study eligibility were resolved through discussion.
Data extraction and quality appraisal
Data extraction was completed within Covidence using a form based on the Cochrane data extraction template for randomised controlled trials. Study characteristics were extracted in accordance with the pre-specified protocol. The number of participants achieving the MCID for exercise capacity, health-related quality of life and symptoms and total number per group were extracted from the studies and entered into Review Manager 5.4. Data on adverse events were compiled in Microsoft Excel. One reviewer undertook data extraction for each study, with the accuracy of this extraction cross-checked by a second reviewer.
Risk of bias (quality) assessment
Two reviewers independently assessed the risk of bias of included studies using the Cochrane Tool for Risk of Bias 1.0. The protocol was registered prior to publication of Risk of Bias 2.0. The domains evaluated were random sequence generation, allocation concealment, blinding of outcome assessment, incomplete outcome data, selective outcome reporting and other bias. Due to the nature of the intervention, blinding of participants and personnel to treatment allocation was not expected, and hence this was not considered during the assessments. Each of the domains were categorised as having high, low or unclear risk of bias, with the overall risk of bias for each study then determined as high (more than two ‘unclear’ or more than one ‘high’ risk domain), moderate (two ‘unclear’ or one ‘high’ risk domain), or low (no ‘unclear’ or ‘high’ risk domains). Any disagreements in risk of bias assessments were resolved through discussion.
Strategy for data synthesis
All meta-analyses were performed using Review Manager version 5.4. For exercise capacity, health-related quality of life and symptoms, the measure of effect was the number and proportion of people with chronic respiratory disease who achieved the MCID (relative risk). Where possible the following MCID thresholds were used: 30 m on 6MWD, 26 36 m on ISWT, 27 between 174 and 279 s on ESWT, 28 100 s on cycle endurance time, 29 4 W on peak cycle exercise capacity, 30 4 points on SGRQ, 31 0.5 points on CRQ, 32 1 grade on mMRC dyspnoea scale, 33 2 points on CAT. 34 Analysis for each of the chronic respiratory diseases was considered separately. We contacted study authors to obtain any missing numerical outcome data or confirm data only reported in conference abstracts. Where studies were clinically homogeneous (in terms of population, intervention, and comparison), we combined individual study estimates in a meta-analysis. Studies including stable patients and those post exacerbation were included in the same analysis, as we were not aware of evidence indicating MCID achievement differed across these groups, however we considered this as a potential source of heterogeneity for exploration in subgroup analysis where indicated. We used random-effects models to incorporate between-study heterogeneity (i.e. assumption that the studies may have heterogeneous, but related, intervention effect estimates due to the clinical nature of the intervention). A single numerical estimate of effect (relative risk) of achieving the MCID in those who attended pulmonary rehabilitation versus those who did not, and in those attending centre versus home-based pulmonary rehabilitation were calculated. Adverse events were intended to be interpreted as the number of participants experiencing one or more event (relative risk) but there was limited homogenous data to consider meta-analysis. Statistical heterogeneity in all meta-analyses was interpreted by the I2 value. In meta-analyses where I2 was greater than 40%, potential sources of the statistical heterogeneity were explored. We used the five GRADE considerations (study limitations, consistency of effort, imprecision, indirectness and publication bias) to assess the quality of a body of evidence as it relates to studies that contributed data to the meta-analyses for primary outcomes. Summary of findings tables were produced using GRADEpro software to document the certainty of evidence assessments with all explanations to downgrade the evidence reported in footnotes.
Results
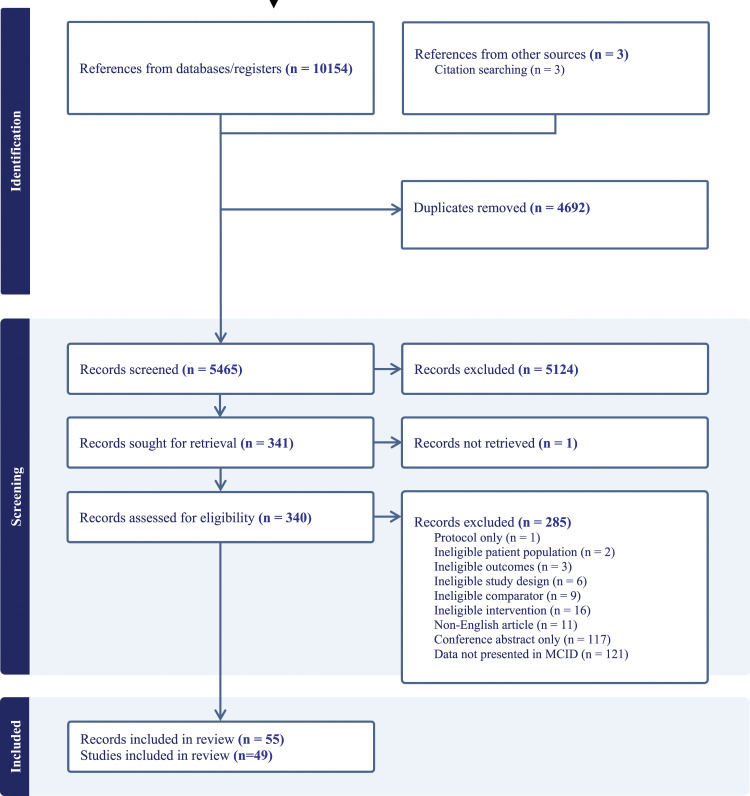
After duplicates were removed, searches identified 5465 records for screening, of which 5124 records were excluded based on title or abstract. Full texts were sought for the remaining 341 records of which 49 trials (55 reports) met the eligibility criteria (Figure 1). See supplementary appendix for full list of included studies (Table S6). Of the 49 included trials, 42 reported adverse event data and only 16 reported exercise capacity, health related quality of life or symptom control data according to MCID for meta-analyses.22,23,35–48
Figure 1.
PRISMA flow diagram.
Characteristics of included studies
The 49 included trials were published between 2004 and 2023 (Tables 1 and 2), with a total of 4921 participants randomised. Of the included studies, 35 focused on COPD, 11 on ILD, 2 on bronchiectasis and 1 a mixed population including all three diseases. For the studies that recruited COPD participants only, 31 were during stable disease (mild to very severe) and 4 were following acute exacerbation. Study sample sizes varied between 12 and 375 participants.
Table 1.
Characteristics of studies comparing pulmonary rehabilitation to usual care.
| Author | Participants | Exercise training component | Additional | ||||
|---|---|---|---|---|---|---|---|
| n | Diagnosis | Frequencydays/week | Intensity | Time session; total | Type | ||
| Centre-based pulmonary rehabilitation versus usual care | |||||||
| Baumann 2012 | 100 | COPD | 1 | Borg 4-6 | 20-60 min; 8-18 weeks | Endurance/resistance | Br, Bc, Ed, Ps, R |
| Bernocchi 2016 | 112 | COPD | 3-7 | Moderate- high Borg | 45-105 min | Endurance/resistance | Am, Ed, Ts |
| Casaburi 2014 | 53 | COPD | 3 | 60%–80% of 1RM | 3-4 sets of 5 exercises; 10 weeks | Resistance | Ed |
| Casey 2013 | 350 | COPD | 2 | Borg 4 | 60 min; 8 weeks | Endurance/resistance | Am, Br, Ed, Sm, Ts |
| Dale 2014 | 35 | ILD | 3 | 80% 6MWT; 60% peak workload | ≥30 min; 8 weeks | Endurance/resistance | None |
| Deepak 2014 | 60 | AECOPD | Not specified | Not specified | 120 min; 12 weeks | Endurance/resistance | Ac, Br, Ed, Ps, Sm |
| Dowman 2017 | 142 | ILD | 2 | 70%–80% peak workload; Borg RPE 12-14 | ≥30 min; 8 weeks | Endurance/resistance | Ed, Ox, Sm |
| Eaton 2009 | 97 | AECOPD | 2-7 | Not specified | 60 min; 8 weeks | Endurance/resistance | Ed, Ox, Sm |
| Faulkner 2010 | 20 | COPD | 1 | Not specified | Not specified; 8 weeks | Endurance/resistance | Am, Ed, Sm |
| Gottlieb 2011 | 61 | COPD | 2 | Bore RPE 16-17 | 7-31 weeks | Endurance/resistance | Ed, Br, Bc, Ns, Sc |
| Hoff 2007 | 12 | COPD | 3 | 85%–90% 1RM | 8 weeks | Resistance | None |
| Holland 2008 | 57 | ILD | 2 | 80% of 6MWT speed | ≥30 min; 8 weeks | Endurance/resistance | Ox |
| Kataoka 2023 | 88 | ILD | 2 | 80% peak workload; 80% of 6MWT speed | ≥30 min; 12 weeks | Endurance/resistance | None |
| Ku 2017 | 40 | ILD | 2 | Not specified | 120 min; 8 weeks | Endurance/resistance | Am, Br, Ed, Ox |
| Lee 2022 | 17 | COPD | 5 | 40%–60% peak VO2; 60%–80% 1RM | 60 min; 8 weeks | Endurance/resistance | None |
| Maglakelidze 2022 | 60 | COPD | 2 | 65%–85% of their maximal ISWT | 90 min; 8 weeks | Endurance/resistance | Ed, Sm |
| Majewska-Pulsakowska 2016 | 43 | COPD | 3 | Not specified | 23-45 min; 8 weeks | Endurance | None |
| Man 2004 | 42 | AECOPD | 2 | Not specified | 60 min; 8 weeks | Endurance/Resistance | Ed, Sm |
| Naz 2018 | 18 | ILD | 2 | Borg 4-6 | Not specified; 12 weeks | Endurance/resistance | Am, Br, Ox |
| Nyberg 2014 | 44 | COPD | 3 | Borg ≥4- | 60 min; 8 weeks | Resistance | Ed |
| O’Shea 2007 | 54 | COPD | 1 | Not specified | 12 weeks | Resistance | None |
| Perez-Bogerd 2018 | 60 | ILD | 2-3 | 60%–75% peak workload | 24 weeks | Endurance/resistance | Bc, Ed, Ot, Ps |
| Vainshelboim 2014 | 34 | ILD | 3 | Borg 3-5 | 60 min; 12 weeks | Endurance/resistance | Br, Ox, Sm |
| Vasilopoulou 2017 | 150 | COPD | 3 | 100% peak workload | 45 min (intervals); 8 weeks | Endurance/resistance | Br, Ed, Sm |
| Wallaert 2020 | 38 | ILD | 3 | Not specified | ≥30 min; 8 weeks | Endurance/resistance | Ed, Sm, Ps |
| Wootton 2014 | 143 | COPD | 3 | 80% of 6MWT speed; Borg 3-4 | 30-45 min; 8 weeks | Endurance | None |
| Home-based pulmonary rehabilitation versus usual care | |||||||
| Benzo 2016 | 215 | COPD | 7 | Not specified | 20 min; not specified | Endurance/resistance | Br, Sm, Ts |
| Benzo 2021 | 154 | COPD | 6 | Not specified | 20 min; 8 weeks | Endurance | Am, Sm Tm, Ts |
| Benzo 2022 | 375 | COPD | 6 | Not specified | 12 weeks | Endurance | Am, Sm, Tm, Ts, R |
| Bourne 2022 | 193 | COPD | Not specified | Not specified | Not specified; 5 months | Endurance/resistance | Am, Ed, Sm |
| Cedeño de Jesús 2022 | 34 | Bronchiectasis | 3-5 | Borg ≥4- | ≥20 min; 8 weeks | Endurance/resistance | Ac, Ed, Sm, Ts |
| Cerdan-de-las-Heras 2021 | 29 | ILD | 3-5 | Not specified | 10-20 min; 12 weeks | Endurance/resistance | Ed, Sm, Tm |
| Cerdan-de-las-Heras 2022 | 30 | ILD | 3-5 | Not specified | 10-20 min; 12 weeks | Endurance/resistance | Ed, Sm, Tm |
| Chen 2018 | 55 | COPD | 3 | Not specified | 20-30 min; 12 weeks | Resistance | Ed, Ps, Sm |
| Johnson-Warrington 2016 | 78 | AECOPD | 7 | Not specified | Not specified | Endurance/resistance | Ed, Sm, Ts |
| Jose 2021 | 63 | Bronchiectasis | 3 | 60%–80% MIST cadence; 70% peak isometric force | 50 min; 8 weeks | Endurance/resistance | Am, Ed, Ts |
| Lahham 2020 | 58 | COPD | 5 | 80% 6MWT speed | ≥30 min; 8 weeks | Endurance/resistance | Am, Ed, Sm, Ts |
| Mitchell 2014 | 184 | COPD | 7 | Not specified | Not specified; 6 weeks | Endurance/resistance | Am, Ed Sm, Ts |
| Tsai 2017 | 37 | COPD | 3 | Borg 3-4 | ≥20 min; 8 weeks | Endurance/resistance | Ed |
| Zanaboni 2023 | 120 | COPD | 3-5 | 80% 6MWT speed; Borg 4-6 | ≥30 min; 2 years | Endurance/resistance | Am, Ed, Sm, Tm |
Ac: airway clearance; Am: activity monitoring; Bc: behavioural counselling; Br: breathing techniques; Ed: education; Ns: Nutritional support; Ot: occupational therapy; Ox: oxygen supplementation; Ps: Psychosocial support; R: Relaxation techniques; Sc: smoking cessation; Sm: self-management training; Ts: telephone support; Tm: telemonitoring.
Table 2.
Characteristics of studies comparing home-based pulmonary rehabilitation to centre-based pulmonary rehabilitation.
| Author | Population | Home-based (exercise training and additional components) | Centre-based (exercise training and additional components) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Diagnosis | Frequencydays/week | Intensity | Timesession; total | Type | Additional components | Frequencydays/week | Intensity | TimeSession; total | Type | Additional components | ||
| Bourne 2017 | 90 | COPD | 2-5 | Not specified | 12-42 min; 6 weeks | Endurance/resistance | Ed, Sm | 2 | Not specified | 12-42 min; 6 weeks | Endurance/resistance | Ed, Sm | |
| Cerdan-de-las-Heras 2022 | 54 | COPD | 3-5 | Not specified | 10-20 min; 8 weeks | Endurance/resistance | Ed, Tm | 2 | Not specified | 60 min; 8 weeks | Not specified | Ed | |
| Cox 2022 | 142 | Mixed | 2 | Borg 3-4 | 30 min; 8 weeks | Endurance/resistance | Am, Ed, Sm | 2 | Borg 3-4 | 30 min; 8 weeks | Endurance/resistance | Am, Ed, Sm | |
| Ercin 2020 | 55 | COPD | 3 | Not specified | Not specified; 8 weeks | Endurance | Br, Ed, Sm | 7 | 50%–70% peak workload | 30 min; 8 weeks | Endurance | Br, Ed | |
| Hansen 2020/2023 | 134 | COPD | 3 | Not specified | 30 min; 8 weeks | Endurance/resistance | Ed, Sm | 2 | Borg 4-7; 40%–80% 1RM | 40-60 min; 10-12 weeks | Endurance/resistance | Ed, Sm | |
| Holland 2017 | 166 | COPD | 5 | 80% of 6MWT speed | 30 min; 8 weeks | Endurance/resistance | Am, Ed, Sm, Ts | 2 | 80% of 6MWT speed | ≥30 min; 8 weeks | Endurance/resistance | Ed, Sm | |
| Horton 2018 | 287 | COPD | 7 | 85% of predicted peak oxygen uptake | 30 min; 7 weeks | Endurance/resistance | Am, Ed, Sm, Ts | 3 | 85% of predicted peak oxygen uptake | ≥30 min; 7 weeks | Endurance/resistance | Am, Ed, Sm | |
| Maltais 2008 | 252 | COPD | 3 | 60% of peak workload | 70 min; 8 weeks | Endurance/resistance | Ed, Sm | 3 | 80% of peak workload | 55-60 min; 8 weeks | Endurance/resistance | Ed, Sm | |
| Widyastuti 2018 | 40 | COPD | 7 | Fastest step pace possible | 30 min; 6 weeks | Endurance | Am | 3 | 80% of 6MWT speed | 30 min; 6 weeks | Endurance | Am | |
Am: activity monitoring; Br: breathing techniques; Ed: education; Sm: self-management training. Tm: telemonitoring. Ts: telephone support.
All trials, except one cluster randomised trial, 49 comprised of allocation at an individual level. Included comparisons were centre-based pulmonary rehabilitation versus usual care (n = 26 studies), home-based pulmonary rehabilitation versus usual care (n = 14), or home-based pulmonary rehabilitation versus centre-based pulmonary rehabilitation (n = 9). Exercise sessions varied in frequency, from once weekly to daily exercise. Desired exercise intensity was not reported in all studies, but where reported varied between moderate and high intensity (Table 1). The most common duration of rehabilitation programs was 8 weeks (range 6 weeks to 2 years). Duration of exercise sessions varied from 10 to 120 min. The exercise modality in studies included endurance training only (n = 7) resistance training only (n = 6) or both (n = 36). For the studies comparing centre versus home rehabilitation, the exercise component of home programs were reported to be bi-weekly supervised sessions at home (n = 1), one or two supervised face-to-face introductory or supervised sessions and unsupervised thereafter with or without telephone follow-up (n = 5), a home visit followed by remote supervision via videoconferencing (n = 1) or entirely unsupervised with use of written or online resources and/or telephone calls (Table 2). The components delivered alongside exercise training for centre or home-based rehabilitation are reported in Table 2. The usual care group of eligible comparisons received advice, counselling or instructions on physical activity, education, airway clearance, breathing techniques and/or structured phone call follow-up.
The primary outcome measures most reported according to MCID were 6MWD and CRQ. All included studies used and MID of 0.5 points on the CRQ22,23,36,39,42–45 however, the MCID thresholds for the 6MWD varied across studies including 54 m38, 25 m,37,47 26 m41, 30 m22,23,44,46,48, and 29-34 m39. Thirty-seven of the included trials reported safety data. These studies covered the entire range of eligible study populations and comparisons, but the unit of classification (combined study population, at group level, for rehabilitation groups only) and type of event (serious adverse events only, any adverse events) varied in the reported data.
Risk of bias
Risk of bias assessments of studies included in the meta-analyses are presented alongside forest plots (Figures 2–4, S1-S4). The domains most assessed as unclear or high risk were allocation concealment and blinding of outcome assessment.
Figure 2.
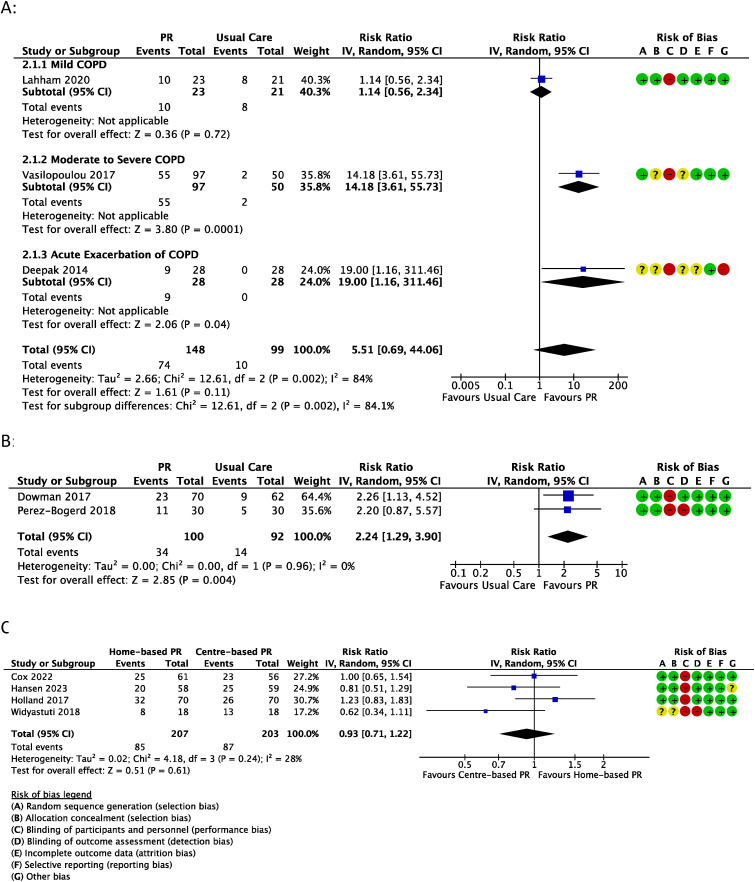
Meta-analysis of the number of participants to achieve MCID in 6MWT following A) pulmonary rehabilitation vs usual care in COPD; B) pulmonary rehabilitation vs usual care in ILD; or C) home-based pulmonary rehabilitation vs centre-based pulmonary rehabilitation. Events = number of participants in group to achieve MCID; Totals = total number of participants in group.
Figure 4.
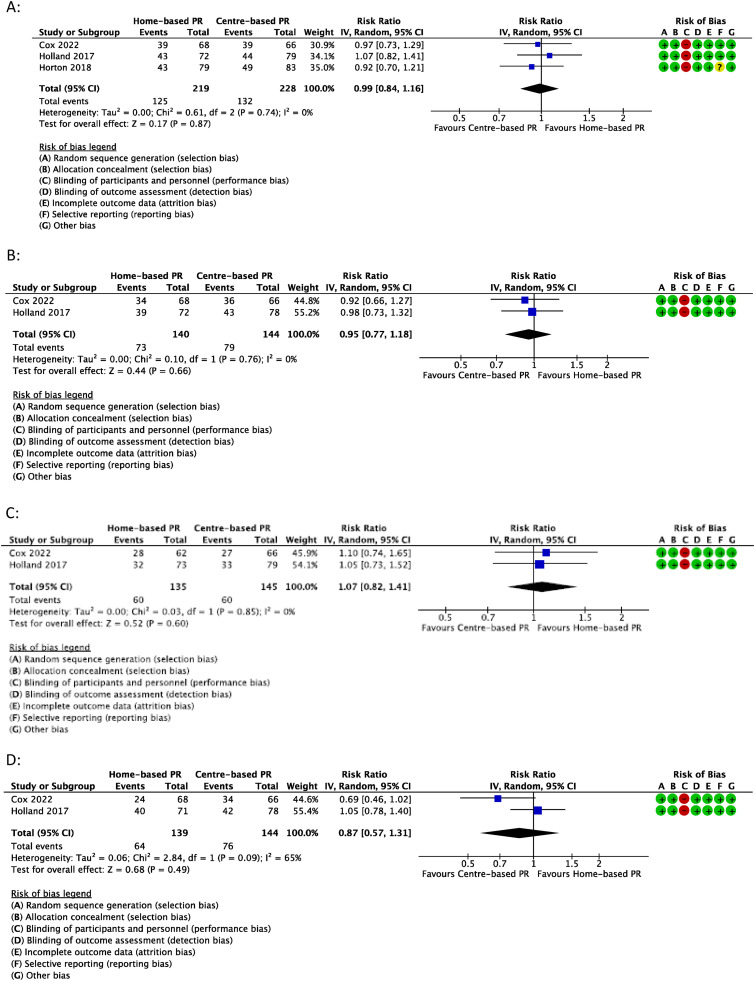
Meta-analysis of the number of participants to achieve MCID following home-based pulmonary rehabilitation vs centre-based pulmonary rehabilitation in A) CRQ-Dyspnoea B) CRQ-Fatigue C) CRQ-Emotional Function; or D) CRQ-Mastery. Events = number of participants in group to achieve MCID; Totals = total number of participants in group.
Data synthesis
Primary outcomes
One trial 35 did not have MCID data available (CRQ domains) in a format that could be combined with other study level estimates, resulting in 15 trials with data on the number of participants achieving MCID in clinical outcomes for comparisons of pulmonary rehabilitation versus usual care or centre-based pulmonary rehabilitation versus home-based pulmonary rehabilitation. All study level data of primary outcomes including full range of reported proportions of people achieving MCIDs with centre-based rehabilitation, home-based rehabilitation and usual care are presented in Table 3, with meta-analyses undertaken according to type of chronic respiratory disease and comparison.
Table 3.
Percentage of participants achieving minimal clinical important differences in exercise capacity and health-related quality of life.
| Pulmonary rehabilitation (PR) vs. usual care (UC) | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | 6MWD | ISWT | ESWT | CRQ-D | CRQ-F | CRQ-E | CRQ-M | SGRQ | ||||||||
| PR | UC | PR | UC | PR | UC | PR | UC | PR | UC | PR | UC | PR | UC | PR | UC | |
| Benzo 2022 | 30 | 18 | 38 | 18 | 33 | 16 | 44 | 27 | ||||||||
| Cedeno de Jesus 2022 | 75 | 47 | ||||||||||||||
| Deepak 2014 | 32 | 0 | 82 | 4 | ||||||||||||
| Dowman 2017 | 33 | 15 | 42 | 30 | 59 | 23 | 45 | 38 | 57 | 33 | ||||||
| Gottlieb 2011 | 23 | 35 | ||||||||||||||
| Johnson-Warrington 2016 | 50 | 41 | 50 | 47 | 63 | 37 | 92 | 57 | 63 | 57 | 73 | 73 | ||||
| Lahham 2020 | 43 | 38 | 44 | 42 | 44 | 42 | 32 | 23 | 56 | 54 | ||||||
| Mitchell 2014 | 20 | 10 | 35 | 17 | 63 | 45 | 47 | 33 | 32 | 25 | 35 | 29 | ||||
| Perez-Bogerd 2018 | 37 | 15 | ||||||||||||||
| Vasilopoulou 2017 | 57 | 4 | ||||||||||||||
6MWD: six-minute walk distance, ISWT: incremental shuttle walk test, ESWT: endurance shuttle walk test, CRQ-D: chronic respiratory questionnaire-dyspnoea; CRQ-F: chronic respiratory questionnaire-fatigue; CRQ-E: chronic respiratory questionnaire-emotional function; CRQ-M: chronic respiratory questionnaire-mastery; SGRQ: St georges respiratory questionnaire.
Exercise capacity
Pulmonary rehabilitation versus usual care
6MWD
Six trials reported the proportion of participants achieving the MCID for 6MWD.37–39,44,46,47 In COPD, a higher proportion of participants achieved the MCID for 6MWD with pulmonary rehabilitation (50%) compared to usual care (10%), but this was not statistically significant (risk ratio (RR) = 5.51, 95% confidence intervals (95% CI) 0.69 to 44.06 (3 studies, 247 participants) (Figure 2(A)). There was evidence of substantial heterogeneity (I2 = 84%; p = 0.002). Stratified analysis according to severity of COPD (Figure 2(A)), revealed a greater number of participants achieving the MCID with pulmonary rehabilitation (vs usual care) in trials including moderate-severe COPD (57% vs 5%) (14.18, 3.61 to 55.73) or following AECOPD (32% vs 0%) (19.00, 1.16 to 311.46) but not mild COPD (43% vs 38%) (1.14, 0.56 to 2.34). Certainty of evidence in COPD was low due to risk of bias and imprecision (Table S8). In ILD39,46 a greater number of pulmonary rehabilitation participants achieved the MCID compared to usual care (34% vs 15%) (2.24, 1.29 to 3.90, 2 studies, 192 participants, I2 = 0%) (Figure 2(B)). Certainty of evidence was moderate (Table S9). A single trial in bronchiectasis 37 favoured an effect of home-based pulmonary rehabilitation compared to usual care (75% vs 47%) but this was not statistically significant (1.61, 0.87 to 2.96, 1 trial, 31 participants). Certainty of evidence in bronchiectasis was downgraded to very low due to risk of bias, indirectness and imprecision (Table S10).
ISWT
Meta-analysis of two COPD trials43,45 revealed a greater number of pulmonary rehabilitation participants achieving the MCID compared to usual care (26% vs 16%), but this effect was not statistically significant (1.50, 0.92 to 2.45, I2 = 0%, Figure S1). Certainty of evidence was low due to indirectness and imprecision (Table S8).
ESWT
Meta-analysis of two COPD trials43,45 also favoured a greater number of rehabilitation participants achieving the MCID compared to usual care (37% vs 22%), but this effect was not statistically significant and there was substantial heterogeneity (1.53, 0.80 to 2.91, I2 = 56%, Figure S2). Certainty of evidence was downgraded to very low due to inconsistency, indirectness and imprecision (Table S8).
Centre-based pulmonary rehabilitation vs home-based pulmonary rehabilitation
Four trials22,23,41,48 reported the proportion of participants achieving the MCID for 6MWD. There was no statistically significant difference in the number of participants achieving MCID for those who completed centre-based programs (43%) and those who completed home-based programs (41%) (0.93, 0.71 to 1.22, I2 = 28%, 4 trials, 410 participants) (Figure 2(C)). Certainty of evidence was low due to risk of bias and imprecision (Table S11).
Health-related quality of life
Pulmonary rehabilitation versus usual care
CRQ-dyspnoea
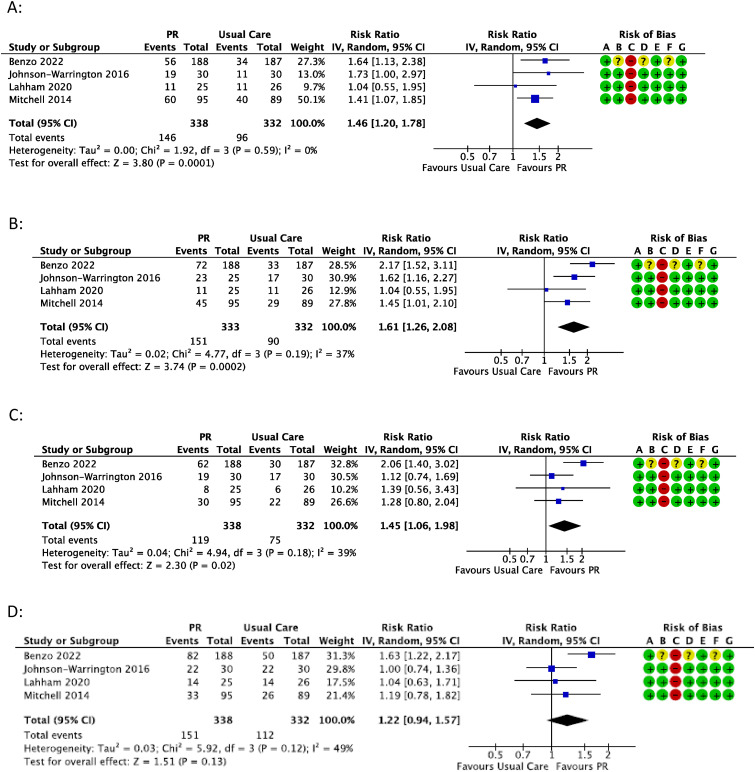
Five trials reported the proportion of participants achieving the MCID for CRQ-Dyspnoea score.36,39,43–45 In COPD,36,43–45 a greater number of participants achieved the MCID with pulmonary rehabilitation compared to usual care (43% vs 29%), which was statistically significant with no apparent heterogeneity (1,46, 1.20 to 1.78, 4 trials, 670 participants) (Figure 3(A)). Certainty of evidence was moderate (Table S8). One trial in ILD 39 had a similar estimate of effect for number of participants achieving the MCID with pulmonary rehabilitation compared to usual care (42% vs 30%), but this trial alone was not statistically significant (1,42, 0.89 to 2.26, 1 trial, 133 participants). Certainty of evidence was low due to indirectness and imprecision (Table S9).
Figure 3.
Meta-analysis of the number of participants to achieve MCID following pulmonary rehabilitation vs usual care in COPD in A) CRQ-Dyspnea B) CRQ-Fatigue C) CRQ-emotional function; or D) CRQ-Mastery. Events = number of participants in group to achieve MCID; Totals = total number of participants in group.
CRQ-fatigue
Five trials reported the proportion of participants achieving the MCID for CRQ-Fatigue score.36,39,43–45 In COPD 36,43–45 a greater number of participants achieved the MCID with pulmonary rehabilitation compared to usual care (45% vs 27%), which was statistically significant with no apparent heterogeneity (1.61, 1.26 to 2.08, I2 = 37%, 4 trials, 665 participants) (Figure 3(B)). Certainty of evidence was moderate (Table S8). One trial in ILD 39 had a larger estimate of effect than the COPD trials for the number of participants achieving the MCID with pulmonary rehabilitation compared to usual care (59% vs 23%), with this trial alone also being statistically significant (2.53, 1.56 to 4.12, 1 trial, 128 participants). Certainty of evidence was moderate (Table S9).
CRQ-emotional function
Five trials reported the proportion of participants achieving the MCID for CRQ-Emotional Function score.36,39,43–45 In COPD,36,43–45 a greater number of participants achieved the MCID with pulmonary rehabilitation compared to usual care (35% vs 23%), which was statistically significant with no apparent heterogeneity (1.45, 1.06 to 1.98, I2 = 39%, 4 trials, 670 participants) (Figure 3(C)). Certainty of evidence was moderate (Table S8). One trial in ILD 39 had a similar estimate of effect for number of participants achieving the MCID with pulmonary rehabilitation compared to usual care (45% vs 38%), but this trial alone was not statistically significant (1.20, 0.79 to 1.81, 1 trial, 128 participants). Certainty of evidence was low due to indirectness and imprecision (Table S9).
CRQ-mastery
Five trials reported the proportion of participants achieving the MCID for CRQ-Mastery score.36,39,43–45 In COPD 36,43–45 a greater number of pulmonary rehabilitation participants achieved the MCID compared to usual care (45% vs 34%) but this effect was not statistically significant and there was evidence of moderate heterogeneity (1.22, 0.94 to 1.57, I2 = 49%, 4 trials, 670 participants) (Figure 3(D)). Certainty of evidence was very low due to inconsistency, indirectness and imprecision (Table S8). One trial in ILD 39 showed a greater number of participants achieving the MCID with pulmonary rehabilitation compared to usual care (57% vs 33%), which was statistically significant (1.70, 1.13 to 2.54, 1 trial, 128 participants). Certainty of evidence was moderate (Table S9).
SGRQ-total
Two trials38,40 reported the proportion of participants achieving the MCID for SGRQ for those that completed pulmonary rehabilitation (82% and 23%) and those in usual care (4% and 35%). One trial included participants following AECOPD and another included stable, moderate COPD. The contrasting estimates of effect in these trials meant that meta-analysis was deemed inappropriate.
Centre-based pulmonary rehabilitation versus home-based pulmonary rehabilitation
CRQ-dyspnoea
Three trials reported the proportion of participants who achieved the MCID in CRQ-Dyspnoea score.22,23,42 There was no statistically significant difference in the number of participants achieving MCID for those that completed centre-based programs (58%) and those that completed home-based programs (57%) (0.99, 0.84 to 1.16, I2 = 0%, 3 trials, 447 participants) (Figure 4(A)). Certainty of evidence was moderate (Table S11).
CRQ-fatigue
Two trials reported the proportion of participants who achieved the MCID in CRQ-Fatigue score.22,23 There was no statistically significant difference in the number of participants achieving MCID for those that completed centre-based programs (55% and 55%) and those that completed home-based programs (50% and 54%) (0.95, 0.77 to 1.18, I2 = 0%, 2 trials, 284 participants) (Figure 4(B)). Certainty of evidence was low due to indirectness and imprecision (Table S11).
CRQ-emotional function
Two trials reported the proportion of participants who achieved the MCID in CRQ-Emotional Function score 22,23 There was no statistically significant difference in the number of participants achieving MCID for those that completed centre-based programs (41% and 42%) and those that completed home-based programs (41% and 44%) (1.07, 0.82 to 1.41, I2 = 0%, 2 trials, 280 participants) (Figure 4(C)). Certainty of evidence was low due to indirectness and imprecision (Table S11).
CRQ-mastery
Two trials reported the proportion of participants who achieved the MCID in CRQ-Mastery.22,23 There was no statistically significant difference in the number of participants achieving MCID for those that completed centre-based programs (52% and 54%) and those that completed home-based programs (35% and 56%) (0.87, 0.57 to 1.31, I2 = 65%, 2 trials, 283 participants), however there was evidence of a substantial heterogeneity with one trial favouring an effect of centre-based pulmonary rehabilitation (Figure 4(D)). Certainty of evidence was downgraded to very low due to inconsistency, indirectness and imprecision (Table S11).
Secondary outcomes
Symptom control
Pulmonary rehabilitation versus usual care
No trials comparing pulmonary rehabilitation to usual care reported symptom data according to achievement of MCID.
Centre-based pulmonary rehabilitation versus home-based pulmonary rehabilitation
mMRC
Two trials reported the proportion of participants who achieved the MCID for mMRC.22,48 There was no statistically significant difference in the number of participants achieving MCID for those that completed centre-based programs (9% and 61%) and those that completed home-based programs (10% and 28%) (0.67, 0.28 to 1.61, I2 = 43%, 2 trials, 171 participants) (Figure S4). The estimate of effect did favour centre-based pulmonary rehabilitation but there was moderate heterogeneity between the two trials.
CAT
Two trials reported the proportion of participants who achieved the MCID for CAT.41,48 There was no statistically significant difference in the number of participants achieving MCID for those that completed centre-based programs (27% and 83%) and those that completed home-based programs (36% and 72%) (1.01, 0.69 to 1.48, I2 = 35%, 2 trials, 157 participants) (Figure S5).
Adverse events
Of the 42 trials that reported safety data, 38 (90%) reported zero adverse events or serious adverse events related to pulmonary rehabilitation (Table S7).
Pulmonary rehabilitation vs. usual care
Two trials49,50 reported mild events related to exercise testing and 2 trials51,52 reported mild temporary events during delivery of rehabilitation (Table S7).
Centre-based pulmonary rehabilitation versus home-based pulmonary rehabilitation
Two trials21,53 reported mild events, with 1 trial 21 reporting such events in both groups whilst the other trial 44 only observing events in the centre-based rehabilitation group.
Discussion
Pulmonary rehabilitation is a fundamental treatment with well-accepted improvements in important outcomes for people with chronic respiratory diseases that remains underused.7–11 In an era of personalised medicine and the emergence of choice in newer pulmonary rehabilitation models, it is a high priority to identify who is most likely to achieve success in different models.7,20
This systematic review included 49 trials conducted in 4921 people with stable COPD, AECOPD, ILD or bronchiectasis. To the best of our knowledge, this is the first review to provide single numerical estimates of effect of the number of people to achieve MCID in functional capacity, quality of life or respiratory symptoms with pulmonary rehabilitation compared to usual care and demonstrate that current estimates suggest achievement of MCID is similar between centre and home-based pulmonary rehabilitation models. However, the proportion of participants who achieved the MCIDs was broad (32% to 75% for exercise capacity, 23% to 92% for health-related quality of life). Adverse events are uncommon in pulmonary rehabilitation trials and when they do occur are mild and temporary.
The findings of this review provide evidence for informed decisions in clinical practice during referral and enrolment to pulmonary rehabilitation by identifying the proportion of people who will achieve a meaningful change in outcomes. This concept may be easier to consider and provides a more personalised understanding of the benefits of pulmonary rehabilitation. Shared decision-making interventions that can utilise such evidence in chronic respiratory disease are limited but have demonstrated potential in improving health-related outcomes, 54 and development of these interventions for pulmonary rehabilitation is progressing.55–57
National clinical audits of pulmonary rehabilitation services in the UK have previously reported ∼60% of people with stable COPD achieve improvements in functional capacity,16,58–60 quality of life and symptoms that meet the MCID. Such estimates have become benchmarks against which individual health services can compare their outcomes to. 60 This review determined a similar number of people with stable COPD (∼50%–60%) achieve MCID in outcomes, but only for functional capacity, dyspnoea, and fatigue. For functional capacity outcomes in our analyses of pulmonary rehabilitation versus usual care we did not find statistically significant between-group differences. This differs from previous analyses 8–11 and likely reflects the loss of statistical power when using a dichotomous outcome, as well as heterogeneity across trials. This limitation did not affect analyses of dyspnoea and fatigue, where heterogeneity was lower. This evidence was judged to be of low-to-moderate certainty in COPD, as was the evidence for these outcomes when estimating the effect of pulmonary rehabilitation in ILD and when comparing centre-based to home-based pulmonary rehabilitation. The evidence was very uncertain, however, on the effect of pulmonary rehabilitation on the number of people achieving a MCID in disease mastery and ESWT in COPD, or 6MWT in bronchiectasis.
Our data demonstrate that in the context of randomised controlled trials, some improvement can be seen with usual care alone. This may be attributed to behaviour changes associated with clinical trial participation for which patient reported outcomes are particularly susceptible. For functional capacity, it is possible that performing a baseline exercise test may influence exercise behaviour and thereby confound control outcome data. 61 All trials in this review included a practice exercise test at baseline except one 53. It is worthy to note that MCID data reporting in randomised trials is substantially lower than the total number of trials reported in previous reviews. The amount of trial data available in emerging areas in stable COPD (e.g. home-based vs centre-based pulmonary rehabilitation) was comparable to the evidence base of more established areas (e.g. pulmonary rehabilitation vs usual care). Given the timespan of the evidence synthesised, the MCID thresholds varied across the trials, and future updates of the evidence could consider other approaches such as individual patient meta-analysis whereby original data are sought directly from the researchers responsible for the randomised controlled trials. However, the fact that many of trials were published more than 10 years ago may make this difficult. Future trials can add new evidence by reporting clinical outcomes according to the number of participants achieving the MCID in addition to mean measures of effect for both established and newer outcomes measures such as the mMRC and CAT tool. This recommendation will improve the certainty of evidence in achieving MCID and is also supported by a recent multistakeholder consensus process that identified exercise capacity, health-related quality of life and symptoms within a core outcome set to increase consistency among clinical trials of pulmonary rehabilitation and help to benchmark service delivery. 62 The estimates of effect presented in this review and their 95% confidence intervals can help inform sample size calculations for future trials. The relatively low proportion of participants who achieved the MCID for 6MWT (50%) suggests that optimisation of exercise training strategies is required, and should be addressed by future research.
Pulmonary rehabilitation is considered a safe treatment for chronic respiratory disease, however previous reviews have infrequently collated data on adverse events 8 or were not able to identify trials that reported adverse event data. 11 The variation in the level of reporting safety data restricted any formal statistical analysis in this review, however the available data indicates that adverse events are uncommon and temporary across rehabilitation models and disease categories. It is recommended that future trials measure and report on all adverse events (intervention related or not) at an individual group level so the likelihood of any harms in conventional or emerging models of pulmonary rehabilitation are accurate. Like previous systematic reviews of pulmonary rehabilitation,8–11 allocation concealment and blinding of outcome assessment were the common domains at high risk of bias. Ensuring the highest quality of trial conduct and reporting in all other domains is paramount irrespective of the pulmonary rehabilitation model being investigated.
We note the following limitations. Our data synthesis on achievement of MCID in clinical outcomes was largely based on trials in stable COPD, which limits generalising the estimates of effect to other contexts, particularly in the comparison of centre and home-based pulmonary rehabilitation where there were no trials focused on people with a primary diagnosis of ILD or bronchiectasis or an acute exacerbation setting. We did not include trials where the majority of participants had a diagnosis of asthma due to weak recommendations in international guidelines 63 and limited implementation in clinical practice. Specific thresholds for MCIDs of some outcomes varied between studies and the exact MCID are likely to evolve over time. Hence, we would recommend use of both absolute number of participants and relative differences between comparison groups when interpreting the number of people achieving MCIDs. Not all included trials met the definition of pulmonary rehabilitation, however all trials included in our analysis incorporated the core components in their intervention protocols, except two that involved endurance training only. 36,48 It should be acknowledged that 40% and 60% of the trials included in the comparison of pulmonary rehabilitation versus usual care and centre and home-based pulmonary rehabilitation respectively involved members of the review team. However, no authors were involved in data extraction or risk of bias assessment on their own trials. We did not search bibliographic databases from inception and instead first collated the evidence from the most recent Cochrane reviews of pulmonary rehabilitation or exercise training within the Cochrane database of systematic reviews and updated searches from the last search date of these reviews. We are confident our approach did not miss out any available evidence given that these reviews followed best-practice methodology and we were able to verify both the included and excluded trials from these reviews. Our protocol was registered before the availability of Cochrane’s Risk of Bias 2.0 tool, hence the risk of bias judgements were not focused on a specific result from the included studies.
Conclusion
The available evidence suggests that centre-based and home-based pulmonary rehabilitation models are safe and lead to comparable number of people with chronic respiratory disease achieving clinically meaningful changes in important outcomes. Future trials, which are likely to be head-to-head comparisons of different pulmonary rehabilitation models, should look to report outcomes according to minimal clinically important thresholds so that treatment decisions on pulmonary rehabilitation can be fully informed and personalised across all chronic respiratory diseases.
Supplemental Material
Supplemental Material for Clinically important changes and adverse events with centre-based or home-based pulmonary rehabilitation in chronic respiratory disease: A systematic review and meta-analysis by Janet Bondarenko, Simone Dal Corso, Michael P Dillon, Sally Singh, Belinda R Miller, Caroline Kein, Anne E Holland and Arwel W Jones in Chronic Respiratory Disease.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Supplemental Material: Supplemental material for this article is available online.
ORCID iDs
Janet Bondarenko https://orcid.org/0000-0002-2332-4417
Arwel W Jones https://orcid.org/0000-0003-1689-8065
Data availability statement
All study data used for analyses are visible in tables and figures. Template Cochrane data extraction form is available at https://training.cochrane.org/data-collection-form-rcts.
References
- 1.GBD Chronic Respiratory Disease Collaborators . Prevalence and attributable health burden of chronic respiratory diseases, 1990-2017: a systematic analysis for the global burden of disease study 2017. Lancet Respir Med 2020; 8(6): 585–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.GBD 2017 DALYs and HALE Collaborators . Global, regional, and national disability-adjusted life-years (DALYs) for 359 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990-2017: a systematic analysis for the global burden of disease study 2017. Lancet 2018; 392(10159): 1859–1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonini M, Fiorenzano G. Exertional dyspnoea in interstitial lung diseases: the clinical utility of cardiopulmonary exercise testing. Eur Respir Rev 2017; 26(143): 160099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Global Initiative for Chronic Obstructive Lung Disease (GOLD) . Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease 2023. https://goldcopd.org/2023-gold-report-2/(Accessed 5 January 2023).
- 5.Polverino E, Goeminne PC, McDonnell MJ, et al. European Respiratory Society guidelines for the management of adult bronchiectasis. Eur Respir J 2017; 50(3): 1700629. [DOI] [PubMed] [Google Scholar]
- 6.Spruit MA, Singh SJ, Garvey C, ATS/ERS Task Force on Pulmonary Rehabilitation , et al. An official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med 2013; 188: e13–e64. [DOI] [PubMed] [Google Scholar]
- 7.Holland AE, Cox NS, Houchen-Wolloff L, et al. Defining modern pulmonary rehabilitation. An official American thoracic society workshop report. Ann Am Thorac Soc 2021; 18(5): e12–e29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McCarthy B, Casey D, Devane D, et al. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2015; 2: CD003793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Puhan MA, Gimeno-Santos E, Cates CJ, et al. Pulmonary rehabilitation following exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2016; 12: CD005305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dowman L, Hill CJ, Holland AE, et al. Pulmonary rehabilitation for interstitial lung disease. Cochrane Database Syst Rev 2021; 2(2): CD006322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee AL, Gordon CS, Osadnik CR. Exercise training for bronchiectasis. Cochrane Database Syst Rev 2021; 4(4): CD013110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brooks D, Sottana R, Bell B, et al. Characterization of pulmonary rehabilitation programs in Canada in 2005. Cancer Res J 2007; 14(2): 87–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Camp PG, Hernandez P, Bourbeau J, et al. Pulmonary rehabilitation in Canada: a report from the Canadian thoracic society COPD clinical assembly. Cancer Res J 2015; 22(3): 147–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marks G, Reddel H, Guevara-Rattray E, et al. Monitoring pulmonary rehabilitation and long-term oxygen therapy for people with chronic obstructive pulmonary disease (COPD) in Australia: a discussion paper. Canberra: Australian Institute of Health and Welfare, 2013. https://www.aihw.gov.au/reports/chronic-respiratory-conditions/monitoring-pulmonary-rehabilitation-and-long-term/contents/table-of-contents (Accessed 4 June 2021). [Google Scholar]
- 15.Nishi SP, Zhang W, Kuo YF, et al. Pulmonary rehabilitation utilization in older adults with chronic obstructive pulmonary disease, 2003 to 2012. J Cardiopulm Rehabil Prev 2016; 36(5): 375–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Steiner M, Holzhauer-Barrie J, Lowe D, et al. Pulmonary rehabilitation: steps to breathe better. National chronic obstructive pulmonary disease (COPD) audit programme: clinical audit of pulmonary rehabilitation services in England and Wales 2015. In: National clinical audit report, London, UK: RCP. Accessed 4 August 2023. Available from: https://www.nacap.org.uk/nacap/welcome.nsf/reportsPR.html [Google Scholar]
- 17.Yohannes AM, Connolly MJ. Pulmonary rehabilitation programmes in the UK: a national representative survey. Clin Rehabil 2004; 18(4): 444–449. [DOI] [PubMed] [Google Scholar]
- 18.Keating A, Lee A, Holland AE. What prevents people with chronic obstructive pulmonary disease from attending pulmonary rehabilitation? A systematic review. Chron Respir Dis 2011; 8: 89–99. [DOI] [PubMed] [Google Scholar]
- 19.Cox NS, Oliveira CC, Lahham A, et al. Pulmonary rehabilitation referral and participation are commonly influenced by environment, knowledge, and beliefs about consequences: a systematic review using the theoretical domains framework. J Physiother 2017; 63(2): 84–93. [DOI] [PubMed] [Google Scholar]
- 20.Rochester CL, Vogiatzis I, Holland AE, ATS/ERS Task Force on Policy in Pulmonary Rehabilitation , et al. An official American thoracic society/European respiratory society policy statement: enhancing implementation, use, and delivery of pulmonary rehabilitation. Am J Respir Crit Care Med 2015; 192(11): 1373–1386. [DOI] [PubMed] [Google Scholar]
- 21.Bourne S, DeVos R, North M, et al. Online versus face-to-face pulmonary rehabilitation for patients with chronic obstructive pulmonary disease: randomised controlled trial. BMJ Open 2017; 7(7): e014580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cox NS, McDonald CF, Mahal A, et al. Telerehabilitation for chronic respiratory disease: a randomised controlled equivalence trial. Thorax 2022; 77(7): 643–651. [DOI] [PubMed] [Google Scholar]
- 23.Holland AE, Mahal A, Hill CJ, et al. Home-based rehabilitation for COPD using minimal resources: a randomised, controlled equivalence trial. Thorax 2017; 72(1): 57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jaeschke R, Singer J, Guyatt GH. Measurement of health status: ascertaining the minimal clinically important difference. Contr Clin Trials 1989; 10(4): 407–415, ISSN 0197-2456. [DOI] [PubMed] [Google Scholar]
- 25.Barradell AC, Bourne C, Alkhathlan B, et al. A qualitative assessment of the pulmonary rehabilitation decision-making needs of patients living with COPD. NPJ Prim Care Respir Med 2022; 32(1): 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holland AE, Spruit MA, Troosters T, et al. An official European respiratory society/american thoracic society technical standard: field walking tests in chronic respiratory disease. Eur Respir J 2014; 44(6): 1428–1446. [DOI] [PubMed] [Google Scholar]
- 27.Evans RA, Singh SJ. Minimum important difference of the incremental shuttle walk test distance in patients with COPD. Thorax 2019; 74(10): 994–995. [DOI] [PubMed] [Google Scholar]
- 28.Zatloukal J, Ward S, Houchen-Wolloff L, et al. The minimal important difference for the endurance shuttle walk test in individuals with chronic obstructive pulmonary disease following a course of pulmonary rehabilitation. Chron Respir Dis 2019; 16: 147997311985382. DOI: 10.1177/1479973119853828, PMCID: PMC6732856. [DOI] [Google Scholar]
- 29.Laviolette L, Bourbeau J, Bernard S, et al. Assessing the impact of pulmonary rehabilitation on functional status in COPD. Thorax 2008; 63(2): 115–121. [DOI] [PubMed] [Google Scholar]
- 30.Puhan MA, Chandra D, Mosenifar Z, National Emphysema Treatment Trial NETT Research Group , et al. The minimal important difference of exercise tests in severe COPD. Eur Respir J 2011; 37(4): 784–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jones PW. St. George’s respiratory questionnaire: MCID. COPD 2005; 2(1): 75–79. [DOI] [PubMed] [Google Scholar]
- 32.Schünemann HJ, PuhanGoldsteinJaeschke MRR, Guyatt GH, et al. Measurement properties and interpretability of the chronic respiratory disease questionnaire (CRQ). COPD 2005; 2(1): 81–89. [DOI] [PubMed] [Google Scholar]
- 33.Spruit MA, Augustin IM, Vanfleteren LE, et al. Differential response to pulmonary rehabilitation in COPD: multidimensional profiling. Eur Respir J 2015; 46: 1625–1635. [DOI] [PubMed] [Google Scholar]
- 34.Kon SS, Canavan JL, Jones SE, et al. Minimum clinically important difference for the COPD assessment test: a prospective analysis. Lancet Respir Med 2014; 2(3): 195–203. [DOI] [PubMed] [Google Scholar]
- 35.Benzo R, Vickers K, Novotny PJ, et al. Health coaching and chronic obstructive pulmonary disease rehospitalization. A randomized study. Am J Respir Crit Care Med 2016; 194(6): 672–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Benzo R, Hoult J, McEvoy C, et al. Promoting chronic obstructive pulmonary disease wellness through remote monitoring and health coaching: a clinical trial. Ann Am Thorac Soc 2022; 19(11): 1808–1817. DOI: 10.1513/AnnalsATS.202203-214OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cedeño de Jesús S, Almadana Pacheco V, Valido Morales A, et al. Exercise capacity and physical activity in non-cystic fibrosis bronchiectasis after a pulmonary rehabilitation home-based programme: a randomised controlled trial. Int J Environ Res Publ Health 2022; 19(17): 11039. DOI: 10.3390/ijerph191711039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Deepak TH, Mohapatra PR, Janmeja AK, et al. Outcome of pulmonary rehabilitation in patients after acute exacerbation of chronic obstructive pulmonary disease. Indian J Chest Dis Allied Sci 2014; 56(1): 7–12. [PubMed] [Google Scholar]
- 39.Dowman LM, McDonald CF, Hill CJ, et al. The evidence of benefits of exercise training in interstitial lung disease: a randomised controlled trial. Thorax 2017; 72(7): 610–619. [DOI] [PubMed] [Google Scholar]
- 40.Gottlieb V, Lyngsø AM, Nybo B, et al. Pulmonary rehabilitation for moderate COPD (GOLD 2)--does it have an effect? COPD 2011; 8(5): 380–386. [DOI] [PubMed] [Google Scholar]
- 41.Hansen H, Torre A, Kallemose T, et al. Pulmonary telerehabilitation vs. conventional pulmonary rehabilitation - a secondary responder analysis. Thorax 2023; 78: 1039, thorax-2023-220065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Horton EJ, Mitchell KE, Johnson-Warrington V, et al. Comparison of a structured home-based rehabilitation programme with conventional supervised pulmonary rehabilitation: a randomised non-inferiority trial. Thorax 2018; 73(1): 29–36. [DOI] [PubMed] [Google Scholar]
- 43.Johnson-Warrington V, Rees K, Gelder C, et al. Can a supported self-management program for COPD upon hospital discharge reduce readmissions? A randomized controlled trial. Int J Chronic Obstr Pulm Dis 2016; 11: 1161–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lahham A, McDonald CF, Moore R, et al. The impact of home-based pulmonary rehabilitation on people with mild chronic obstructive pulmonary disease: a randomised controlled trial. Clin Res J 2020; 14(4): 335–344. [DOI] [PubMed] [Google Scholar]
- 45.Mitchell KE, Johnson-Warrington V, Apps LD, et al. A self-management programme for COPD: a randomised controlled trial. Eur Respir J 2014; 44(6): 1538–1547. [DOI] [PubMed] [Google Scholar]
- 46.Perez-Bogerd S, Wuyts W, Barbier V, et al. Short and long-term effects of pulmonary rehabilitation in interstitial lung diseases: a randomised controlled trial. Respir Res 2018; 19(1): 182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vasilopoulou M, Papaioannou AI, Kaltsakas G, et al. Home-based maintenance tele-rehabilitation reduces the risk for acute exacerbations of COPD, hospitalisations and emergency department visits. Eur Respir J 2017; 49(5): 1602129. [DOI] [PubMed] [Google Scholar]
- 48.Widyastuti K, Makhabah DN, Setijadi AR, et al. Benefits and costs of home pedometer assisted physical activity in patients with COPD. A preliminary randomized controlled trial. Pulmonology 2018; 24(4): 211–218. [DOI] [PubMed] [Google Scholar]
- 49.Casey D, Murphy K, Devane D, et al. The effectiveness of a structured education pulmonary rehabilitation programme for improving the health status of people with moderate and severe chronic obstructive pulmonary disease in primary care: the PRINCE cluster randomised trial. Thorax 2013; 68(10): 922–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen Y, Niu M, Zhang X, et al. Effects of home-based lower limb resistance training on muscle strength and functional status in stable chronic obstructive pulmonary disease patients. J Clin Nurs 2018; 27(5-6): e1022–e1037. [DOI] [PubMed] [Google Scholar]
- 51.Nyberg A, Lindström B, Rickenlund A, et al. Low-load/high-repetition elastic band resistance training in patients with COPD: a randomized, controlled, multicenter trial. Clin Res J 2015; 9(3): 278–288. [DOI] [PubMed] [Google Scholar]
- 52.O'Shea SD, Taylor NF, Paratz JD. A predominantly home-based progressive resistance exercise program increases knee extensor strength in the short-term in people with chronic obstructive pulmonary disease: a randomised controlled trial. Aust J Physiother 2007; 53(4): 229–237. [DOI] [PubMed] [Google Scholar]
- 53.Hansen H, Bieler T, Beyer N, et al. Supervised pulmonary tele-rehabilitation versus pulmonary rehabilitation in severe COPD: a randomised multicentre trial. Thorax 2020; 75(5): 413–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Barradell AC, Gerlis C, Houchen-Wolloff L, et al. Systematic review of shared decision-making interventions for people living with chronic respiratory diseases. BMJ Open 2023; 13(5): e069461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Barradell AC, Singh SJ, Houchen-Wolloff L, et al. A pulmonary rehabilitation shared decision-making intervention for patients living with COPD: PReSent: protocol for a feasibility study. ERJ Open Res 2022; 8(2): 00645–2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jiang Y, Guo J, Chen M, et al. Development of a pulmonary rehabilitation patient decision aid for patients with chronic obstructive pulmonary disease: mixed methods study. Int J Chronic Obstr Pulm Dis 2023; 18: 1377–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jiang Y, Nuerdawulieti B, Chen Z, et al. Effectiveness of patient decision aid supported shared decision-making intervention in in-person and virtual hybrid pulmonary rehabilitation in older adults with chronic obstructive pulmonary disease: a pilot randomized controlled trial. J Telemed Telecare. 2023; 1357633X231156631. [DOI] [PubMed] [Google Scholar]
- 58.Singh S, Latchem S, Andrews R, et al. National asthma and chronic obstructive pulmonary disease audit programme (NACAP). Pulmonary rehabilitation audit report 2019. Combined clinical and organisational audit of pulmonary rehabilitation services in England, Scotland and Wales. RCP (Royal College of Physicians) 2020, London: Accessed 4 August 2023. Available from: https://www.nacap.org.uk/nacap/welcome.nsf/reportsPR.html [Google Scholar]
- 59.Steiner M, McMillan V, Lowe D, et al. Pulmonary rehabilitation: an exercise in improvement. National chronic obstructive pulmonary disease (COPD) audit programme: clinical and organisational audits of pulmonary rehabilitation services in England and Wales 2017. In: National report. London, UK: RCP, 2018. : Accessed 4 August 2023. Available from: https://www.nacap.org.uk/nacap/welcome.nsf/reportsPR.html [Google Scholar]
- 60.Singh S, Legg M, Garnavos N, et al. National asthma and chronic obstructive pulmonary disease audit programme (NACAP). Pulmonary rehabilitation clinical audit 2019. Clinical audit of pulmonary rehabilitation services in England, Scotland and Wales. Patients assessed between 1 March and 31 May and discharged by 31 August 2019. Interim report. RCP 2020;London, UK: Accessed 4 August 2023. Available from: https://www.nacap.org.uk/nacap/welcome.nsf/reportsPR.html
- 61.Stenson A, ChaplinHouchen-Wolloff EJL, Singh SJ, et al. The impact of a baseline exercise test on confidence to perform subsequent incremental shuttle walk tests, walk at home, and manage breathlessness. J Cardiopulm Rehabil Prev 2022; 42(2): 128–132. DOI: 10.1097/HCR.0000000000000616. [DOI] [PubMed] [Google Scholar]
- 62.Souto-Miranda S, Saraiva I, Spruit MA, et al. Core outcome set for pulmonary rehabilitation of patients with COPD: results of a modified Delphi survey. Thorax. 2023; 78(12): 1240–1247. [DOI] [PubMed] [Google Scholar]
- 63.Salison JA, McKeough ZJ, Johnston K, et al. Lung foundation Australia and the thoracic society of Australia and New Zealand. Australian and New Zealand pulmonary rehabilitation guidelines. Respirology 2017; 22(4): 800–819. DOI: 10.1111/resp.13025, Epub 2017 Mar 24. PMID: 28339144.ep 27:thorax-2023-220522. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material for Clinically important changes and adverse events with centre-based or home-based pulmonary rehabilitation in chronic respiratory disease: A systematic review and meta-analysis by Janet Bondarenko, Simone Dal Corso, Michael P Dillon, Sally Singh, Belinda R Miller, Caroline Kein, Anne E Holland and Arwel W Jones in Chronic Respiratory Disease.
Data Availability Statement
All study data used for analyses are visible in tables and figures. Template Cochrane data extraction form is available at https://training.cochrane.org/data-collection-form-rcts.