Abstract
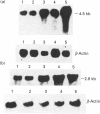
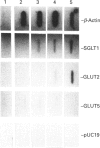
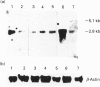
Dietary sugars are known to stimulate intestinal glucose transport activity, but the specific signals involved are unknown. The Na(+)-dependent glucose co-transporter (SGLT1), the liver-type facilitative glucose transporter (GLUT2) and the intestinal-type facilitative glucose transporter (GLUT5) are all expressed in rat jejunum [Miyamoto, Hase, Taketani, Minami, Oka, Nakabou and Hagihira (1991) Biochem. Biophys. Res. Commun. 181, 1110-1117]. In the present study we have investigated the effects of dietary sugars on these glucose transporter genes. A high-glucose diet stimulated glucose transport activity and increased the levels of SGLT1 and GLUT2 mRNAs in rat jejunum. 3-O-Methylglucose, D-galactose, D-fructose, D-mannose and D-xylose can mimic the regulatory effect of glucose on the SGLT1 mRNA level in rat jejunum. However, only D-galactose and D-fructose increased the levels of GLUT2 mRNA. The GLUT5 mRNA level was increased significantly only by D-fructose. Our results suggest that the increase in intestinal transport activity in rats caused by dietary glucose is due to an increase in the levels of SGLT1 and GLUT2 mRNAs, and that these increases in mRNA may be caused by an enhancement of the transcriptional rate. Furthermore, for expression of the SGLT1 gene, the signal need not be a metabolizable or transportable substrate whereas, for expression of the GLUT2 gene, metabolism of the substrate in the liver may be necessary for signalling. Only D-fructose is an effective signal for expression of the GLUT5 gene.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asano T., Katagiri H., Tsukuda K., Lin J. L., Ishihara H., Yazaki Y., Oka Y. Upregulation of GLUT2 mRNA by glucose, mannose, and fructose in isolated rat hepatocytes. Diabetes. 1992 Jan;41(1):22–25. doi: 10.2337/diab.41.1.22. [DOI] [PubMed] [Google Scholar]
- Bell G. I., Kayano T., Buse J. B., Burant C. F., Takeda J., Lin D., Fukumoto H., Seino S. Molecular biology of mammalian glucose transporters. Diabetes Care. 1990 Mar;13(3):198–208. doi: 10.2337/diacare.13.3.198. [DOI] [PubMed] [Google Scholar]
- Bode C., Eisenhardt J. M., Haberich F. J., Bode J. C. Influence of feeding fructose on fructose and glucose absorption in rat jejunum and ileum. Res Exp Med (Berl) 1981;179(2):163–168. doi: 10.1007/BF01851984. [DOI] [PubMed] [Google Scholar]
- Brot-Laroche E., Serrano M. A., Delhomme B., Alvarado F. Temperature sensitivity and substrate specificity of two distinct Na+-activated D-glucose transport systems in guinea pig jejunal brush border membrane vesicles. J Biol Chem. 1986 May 15;261(14):6168–6176. [PubMed] [Google Scholar]
- Burant C. F., Takeda J., Brot-Laroche E., Bell G. I., Davidson N. O. Fructose transporter in human spermatozoa and small intestine is GLUT5. J Biol Chem. 1992 Jul 25;267(21):14523–14526. [PubMed] [Google Scholar]
- Cheeseman C. I., Harley B. Adaptation of glucose transport across rat enterocyte basolateral membrane in response to altered dietary carbohydrate intake. J Physiol. 1991 Jun;437:563–575. doi: 10.1113/jphysiol.1991.sp018611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheeseman C. I., Maenz D. D. Rapid regulation of D-glucose transport in basolateral membrane of rat jejunum. Am J Physiol. 1989 May;256(5 Pt 1):G878–G883. doi: 10.1152/ajpgi.1989.256.5.G878. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Csáky T. Z., Fischer E. Effects of ketohexosemia on the ketohexose transport in the small intestine of rats. Biochim Biophys Acta. 1984 May 30;772(3):259–263. doi: 10.1016/0005-2736(84)90142-1. [DOI] [PubMed] [Google Scholar]
- Davidson N. O., Hausman A. M., Ifkovits C. A., Buse J. B., Gould G. W., Burant C. F., Bell G. I. Human intestinal glucose transporter expression and localization of GLUT5. Am J Physiol. 1992 Mar;262(3 Pt 1):C795–C800. doi: 10.1152/ajpcell.1992.262.3.C795. [DOI] [PubMed] [Google Scholar]
- Debnam E. S. Adaptation of hexose uptake by the rat jejunum induced by the perfusion of sugars into the distal ileum. Digestion. 1985;31(1):25–30. doi: 10.1159/000199173. [DOI] [PubMed] [Google Scholar]
- Debnam E. S. Effect of sodium concentration and plasma sugar concentration on hexose absorption by the rat jejunum in vivo. Further evidence of two transport mechanisms. Pflugers Arch. 1982 Mar;393(1):104–108. doi: 10.1007/BF00582401. [DOI] [PubMed] [Google Scholar]
- Diamond J. M., Karasov W. H., Cary C., Enders D., Yung R. Effect of dietary carbohydrate on monosaccharide uptake by mouse small intestine in vitro. J Physiol. 1984 Apr;349:419–440. doi: 10.1113/jphysiol.1984.sp015165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferraris R. P., Diamond J. M. Use of phlorizin binding to demonstrate induction of intestinal glucose transporters. J Membr Biol. 1986;94(1):77–82. doi: 10.1007/BF01901015. [DOI] [PubMed] [Google Scholar]
- Ferraris R. P., Diamond J. Crypt-villus site of glucose transporter induction by dietary carbohydrate in mouse intestine. Am J Physiol. 1992 Jun;262(6 Pt 1):G1069–G1073. doi: 10.1152/ajpgi.1992.262.6.G1069. [DOI] [PubMed] [Google Scholar]
- Fukumoto H., Kayano T., Buse J. B., Edwards Y., Pilch P. F., Bell G. I., Seino S. Cloning and characterization of the major insulin-responsive glucose transporter expressed in human skeletal muscle and other insulin-responsive tissues. J Biol Chem. 1989 May 15;264(14):7776–7779. [PubMed] [Google Scholar]
- Hallfrisch J. Metabolic effects of dietary fructose. FASEB J. 1990 Jun;4(9):2652–2660. doi: 10.1096/fasebj.4.9.2189777. [DOI] [PubMed] [Google Scholar]
- Hediger M. A., Coady M. J., Ikeda T. S., Wright E. M. Expression cloning and cDNA sequencing of the Na+/glucose co-transporter. 1987 Nov 26-Dec 2Nature. 330(6146):379–381. doi: 10.1038/330379a0. [DOI] [PubMed] [Google Scholar]
- Karasov W. H., Pond R. S., 3rd, Solberg D. H., Diamond J. M. Regulation of proline and glucose transport in mouse intestine by dietary substrate levels. Proc Natl Acad Sci U S A. 1983 Dec;80(24):7674–7677. doi: 10.1073/pnas.80.24.7674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayano T., Burant C. F., Fukumoto H., Gould G. W., Fan Y. S., Eddy R. L., Byers M. G., Shows T. B., Seino S., Bell G. I. Human facilitative glucose transporters. Isolation, functional characterization, and gene localization of cDNAs encoding an isoform (GLUT5) expressed in small intestine, kidney, muscle, and adipose tissue and an unusual glucose transporter pseudogene-like sequence (GLUT6). J Biol Chem. 1990 Aug 5;265(22):13276–13282. [PubMed] [Google Scholar]
- Kayano T., Fukumoto H., Eddy R. L., Fan Y. S., Byers M. G., Shows T. B., Bell G. I. Evidence for a family of human glucose transporter-like proteins. Sequence and gene localization of a protein expressed in fetal skeletal muscle and other tissues. J Biol Chem. 1988 Oct 25;263(30):15245–15248. [PubMed] [Google Scholar]
- Lamers W. H., Hanson R. W., Meisner H. M. cAMP stimulates transcription of the gene for cytosolic phosphoenolpyruvate carboxykinase in rat liver nuclei. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5137–5141. doi: 10.1073/pnas.79.17.5137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maenz D. D., Cheeseman C. I. Effect of hyperglycemia on D-glucose transport across the brush-border and basolateral membrane of rat small intestine. Biochim Biophys Acta. 1986 Aug 21;860(2):277–285. doi: 10.1016/0005-2736(86)90524-9. [DOI] [PubMed] [Google Scholar]
- Miyamoto K., Hase K., Taketani Y., Minami H., Oka T., Nakabou Y., Hagihira H. Developmental changes in intestinal glucose transporter mRNA levels. Biochem Biophys Res Commun. 1992 Mar 16;183(2):626–631. doi: 10.1016/0006-291x(92)90528-s. [DOI] [PubMed] [Google Scholar]
- Miyamoto K., Hase K., Taketani Y., Minami H., Oka T., Nakabou Y., Hagihira H. Diabetes and glucose transporter gene expression in rat small intestine. Biochem Biophys Res Commun. 1991 Dec 31;181(3):1110–1117. doi: 10.1016/0006-291x(91)92053-m. [DOI] [PubMed] [Google Scholar]
- Miyamoto K., Takagi T., Fujii T., Matsubara T., Hase K., Taketani Y., Oka T., Minami H., Nakabou Y. Role of liver-type glucose transporter (GLUT2) in transport across the basolateral membrane in rat jejunum. FEBS Lett. 1992 Dec 21;314(3):466–470. doi: 10.1016/0014-5793(92)81528-t. [DOI] [PubMed] [Google Scholar]
- Nakabou Y., Ishikawa Y., Misake A., Hagihira H. Effect of food intake on intestinal absorption and mucosal hydrolases in alloxan diabetic rats. Metabolism. 1980 Feb;29(2):181–185. doi: 10.1016/0026-0495(80)90144-4. [DOI] [PubMed] [Google Scholar]
- Rancour N. J., Hawkins E. D., Wells W. W. Galactose oxidation in liver. Arch Biochem Biophys. 1979 Mar;193(1):232–241. doi: 10.1016/0003-9861(79)90027-4. [DOI] [PubMed] [Google Scholar]
- Schibler U., Hagenbüchle O., Wellauer P. K., Pittet A. C. Two promoters of different strengths control the transcription of the mouse alpha-amylase gene Amy-1a in the parotid gland and the liver. Cell. 1983 Jun;33(2):501–508. doi: 10.1016/0092-8674(83)90431-2. [DOI] [PubMed] [Google Scholar]
- Shows T. B., Eddy R. L., Byers M. G., Fukushima Y., Dehaven C. R., Murray J. C., Bell G. I. Polymorphic human glucose transporter gene (GLUT) is on chromosome 1p31.3----p35. Diabetes. 1987 Apr;36(4):546–549. doi: 10.2337/diab.36.4.546. [DOI] [PubMed] [Google Scholar]
- Solberg D. H., Diamond J. M. Comparison of different dietary sugars as inducers of intestinal sugar transporters. Am J Physiol. 1987 Apr;252(4 Pt 1):G574–G584. doi: 10.1152/ajpgi.1987.252.4.G574. [DOI] [PubMed] [Google Scholar]
- Stein E. D., Chang S. D., Diamond J. M. Comparison of different dietary amino acids as inducers of intestinal amino acid transport. Am J Physiol. 1987 May;252(5 Pt 1):G626–G635. doi: 10.1152/ajpgi.1987.252.5.G626. [DOI] [PubMed] [Google Scholar]
- Thorens B., Charron M. J., Lodish H. F. Molecular physiology of glucose transporters. Diabetes Care. 1990 Mar;13(3):209–218. doi: 10.2337/diacare.13.3.209. [DOI] [PubMed] [Google Scholar]
- Thorens B., Cheng Z. Q., Brown D., Lodish H. F. Liver glucose transporter: a basolateral protein in hepatocytes and intestine and kidney cells. Am J Physiol. 1990 Dec;259(6 Pt 1):C279–C285. doi: 10.1152/ajpcell.1990.259.2.C279. [DOI] [PubMed] [Google Scholar]
- Thorens B., Sarkar H. K., Kaback H. R., Lodish H. F. Cloning and functional expression in bacteria of a novel glucose transporter present in liver, intestine, kidney, and beta-pancreatic islet cells. Cell. 1988 Oct 21;55(2):281–290. doi: 10.1016/0092-8674(88)90051-7. [DOI] [PubMed] [Google Scholar]
- Wright E. M., Turk E., Zabel B., Mundlos S., Dyer J. Molecular genetics of intestinal glucose transport. J Clin Invest. 1991 Nov;88(5):1435–1440. doi: 10.1172/JCI115451. [DOI] [PMC free article] [PubMed] [Google Scholar]