Abstract
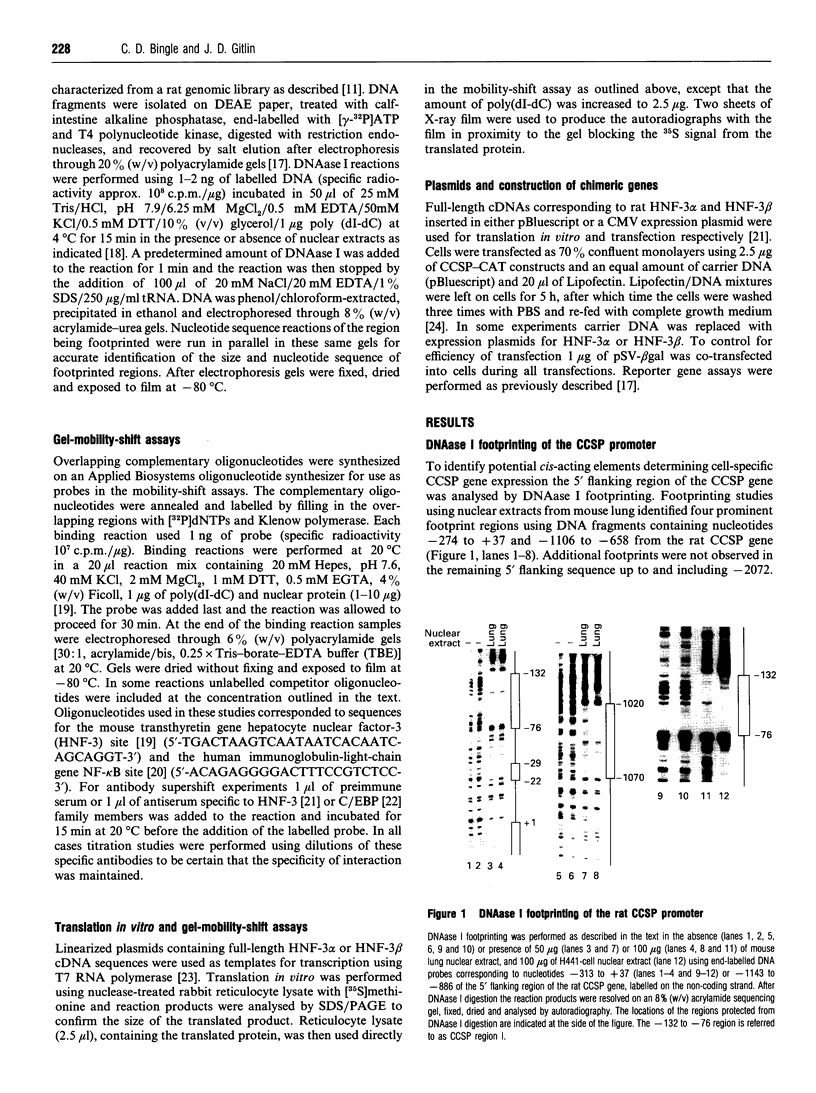
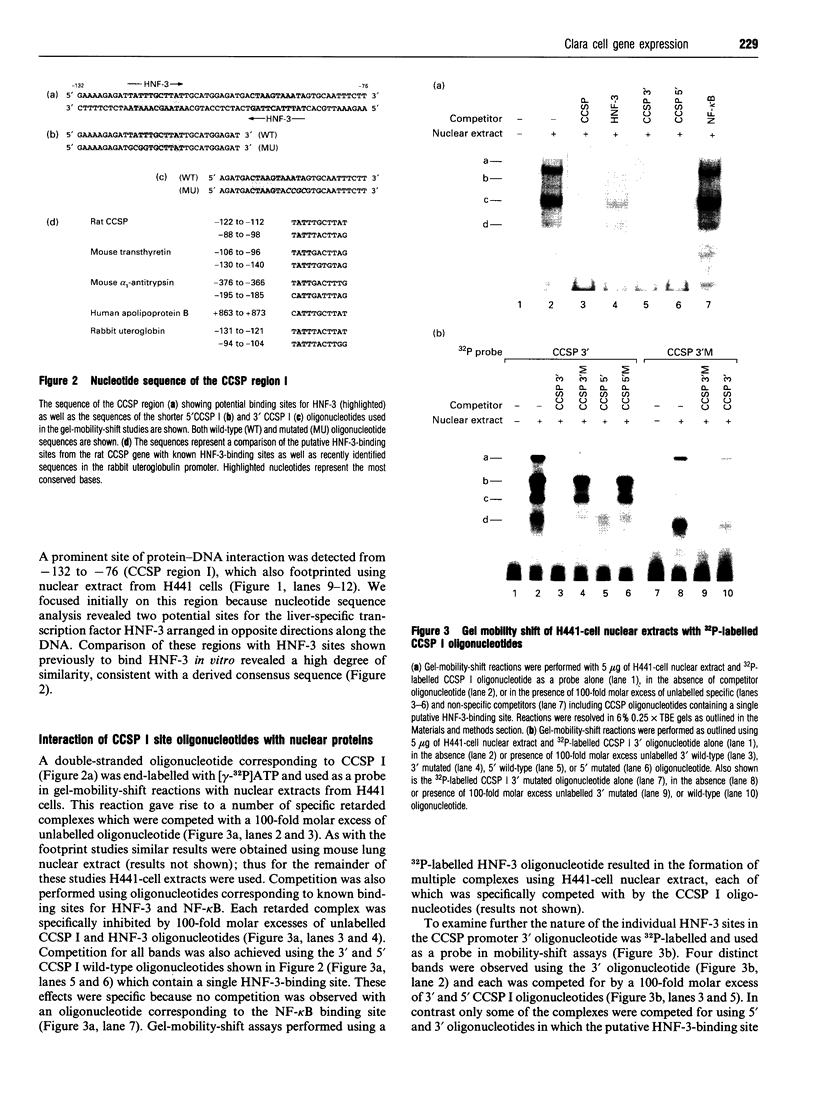
To determine the mechanisms of cell-specific gene expression in the developing pulmonary epithelium the Clara cell secretory protein (CCSP) gene promoter was analysed by DNAase I footprinting. A prominent site of protein-DNA interaction was detected from nucleotides -132 to -76 using nuclear extract from mouse lung and human H441 cells. Mobility shift analysis revealed that an oligonucleotide corresponding to this region interacted with multiple proteins from lung and H441 cell nuclear extracts. Analysis of the nucleotide sequence of this region identified two potential binding sites for hepatocyte nuclear factor 3 (HNF-3), and consistent with this finding binding to this CCSP oligonucleotide was specifically competed for by an oligonucleotide corresponding to the HNF-3-binding site from the mouse transthyretin gene. Mobility shift of the CCSP oligonucleotide was supershifted using antisera specific to HNF-3 alpha and HNF-3 beta, and HNF-3 alpha and HNF-3 beta translated in vitro were found to bind specifically to this same oligonucleotide. Co-transfection of HNF-3 alpha- and HNF-3 beta-expression plasmids increased cell-specific reporter gene activity in H441 cells transfected with a CCSP-CAT gene chimeric construct containing this -132 to -76 region. Taken together, these results suggest a role for HNF-3 in mediating cell-specific CCSP gene expression within the bronchiolar epithelium. These findings support the hypothesis that members of the HNF-3 'forkhead' family of transcription factors determine gene expression and cell fate in multiple cell lineages derived from the primitive gut endoderm.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cao Z., Umek R. M., McKnight S. L. Regulated expression of three C/EBP isoforms during adipose conversion of 3T3-L1 cells. Genes Dev. 1991 Sep;5(9):1538–1552. doi: 10.1101/gad.5.9.1538. [DOI] [PubMed] [Google Scholar]
- Clevidence D. E., Overdier D. G., Tao W., Qian X., Pani L., Lai E., Costa R. H. Identification of nine tissue-specific transcription factors of the hepatocyte nuclear factor 3/forkhead DNA-binding-domain family. Proc Natl Acad Sci U S A. 1993 May 1;90(9):3948–3952. doi: 10.1073/pnas.90.9.3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa R. H., Grayson D. R., Darnell J. E., Jr Multiple hepatocyte-enriched nuclear factors function in the regulation of transthyretin and alpha 1-antitrypsin genes. Mol Cell Biol. 1989 Apr;9(4):1415–1425. doi: 10.1128/mcb.9.4.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirksen M. L., Jamrich M. A novel, activin-inducible, blastopore lip-specific gene of Xenopus laevis contains a fork head DNA-binding domain. Genes Dev. 1992 Apr;6(4):599–608. doi: 10.1101/gad.6.4.599. [DOI] [PubMed] [Google Scholar]
- Felgner P. L., Gadek T. R., Holm M., Roman R., Chan H. W., Wenz M., Northrop J. P., Ringold G. M., Danielsen M. Lipofection: a highly efficient, lipid-mediated DNA-transfection procedure. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7413–7417. doi: 10.1073/pnas.84.21.7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming R. E., Gitlin J. D. Structural and functional analysis of the 5'-flanking region of the rat ceruloplasmin gene. J Biol Chem. 1992 Jan 5;267(1):479–486. [PubMed] [Google Scholar]
- Gazdar A. F., Linnoila R. I. The pathology of lung cancer--changing concepts and newer diagnostic techniques. Semin Oncol. 1988 Jun;15(3):215–225. [PubMed] [Google Scholar]
- Gorski K., Carneiro M., Schibler U. Tissue-specific in vitro transcription from the mouse albumin promoter. Cell. 1986 Dec 5;47(5):767–776. doi: 10.1016/0092-8674(86)90519-2. [DOI] [PubMed] [Google Scholar]
- Hackett B. P., Gitlin J. D. Cell-specific expression of a Clara cell secretory protein-human growth hormone gene in the bronchiolar epithelium of transgenic mice. Proc Natl Acad Sci U S A. 1992 Oct 1;89(19):9079–9083. doi: 10.1073/pnas.89.19.9079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackett B. P., Shimizu N., Gitlin J. D. Clara cell secretory protein gene expression in bronchiolar epithelium. Am J Physiol. 1992 Apr;262(4 Pt 1):L399–L404. doi: 10.1152/ajplung.1992.262.4.L399. [DOI] [PubMed] [Google Scholar]
- Hagen G., Wolf M., Katyal S. L., Singh G., Beato M., Suske G. Tissue-specific expression, hormonal regulation and 5'-flanking gene region of the rat Clara cell 10 kDa protein: comparison to rabbit uteroglobin. Nucleic Acids Res. 1990 May 25;18(10):2939–2946. doi: 10.1093/nar/18.10.2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai Y., Takebe K., Takashina M., Kobayashi S., Takeichi M. Epimorphin: a mesenchymal protein essential for epithelial morphogenesis. Cell. 1992 May 1;69(3):471–481. doi: 10.1016/0092-8674(92)90448-l. [DOI] [PubMed] [Google Scholar]
- Häcker U., Grossniklaus U., Gehring W. J., Jäckle H. Developmentally regulated Drosophila gene family encoding the fork head domain. Proc Natl Acad Sci U S A. 1992 Sep 15;89(18):8754–8758. doi: 10.1073/pnas.89.18.8754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones K. A., Yamamoto K. R., Tjian R. Two distinct transcription factors bind to the HSV thymidine kinase promoter in vitro. Cell. 1985 Sep;42(2):559–572. doi: 10.1016/0092-8674(85)90113-8. [DOI] [PubMed] [Google Scholar]
- Lai E., Prezioso V. R., Tao W. F., Chen W. S., Darnell J. E., Jr Hepatocyte nuclear factor 3 alpha belongs to a gene family in mammals that is homologous to the Drosophila homeotic gene fork head. Genes Dev. 1991 Mar;5(3):416–427. doi: 10.1101/gad.5.3.416. [DOI] [PubMed] [Google Scholar]
- Li C., Lai C. F., Sigman D. S., Gaynor R. B. Cloning of a cellular factor, interleukin binding factor, that binds to NFAT-like motifs in the human immunodeficiency virus long terminal repeat. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7739–7743. doi: 10.1073/pnas.88.17.7739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C., Lusis A. J., Sparkes R., Tran S. M., Gaynor R. Characterization and chromosomal mapping of the gene encoding the cellular DNA binding protein HTLF. Genomics. 1992 Jul;13(3):658–664. doi: 10.1016/0888-7543(92)90138-i. [DOI] [PubMed] [Google Scholar]
- McCray P. B., Jr, Wohlford-Lenane C. L., Snyder J. M. Localization of cystic fibrosis transmembrane conductance regulator mRNA in human fetal lung tissue by in situ hybridization. J Clin Invest. 1992 Aug;90(2):619–625. doi: 10.1172/JCI115901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordlund-Möller L., Andersson O., Ahlgren R., Schilling J., Gillner M., Gustafsson J. A., Lund J. Cloning, structure, and expression of a rat binding protein for polychlorinated biphenyls. Homology to the hormonally regulated progesterone-binding protein uteroglobin. J Biol Chem. 1990 Jul 25;265(21):12690–12693. [PubMed] [Google Scholar]
- Oliver S. G., van der Aart Q. J., Agostoni-Carbone M. L., Aigle M., Alberghina L., Alexandraki D., Antoine G., Anwar R., Ballesta J. P., Benit P. The complete DNA sequence of yeast chromosome III. Nature. 1992 May 7;357(6373):38–46. doi: 10.1038/357038a0. [DOI] [PubMed] [Google Scholar]
- Picard D., Schaffner W. A lymphocyte-specific enhancer in the mouse immunoglobulin kappa gene. Nature. 1984 Jan 5;307(5946):80–82. doi: 10.1038/307080a0. [DOI] [PubMed] [Google Scholar]
- Singh G., Katyal S. L., Gottron S. A. Antigenic, molecular and functional heterogeneity of Clara cell secretory proteins in the rat. Biochim Biophys Acta. 1985 Jun 10;829(2):156–163. doi: 10.1016/0167-4838(85)90184-0. [DOI] [PubMed] [Google Scholar]
- Stripp B. R., Sawaya P. L., Luse D. S., Wikenheiser K. A., Wert S. E., Huffman J. A., Lattier D. L., Singh G., Katyal S. L., Whitsett J. A. cis-acting elements that confer lung epithelial cell expression of the CC10 gene. J Biol Chem. 1992 Jul 25;267(21):14703–14712. [PubMed] [Google Scholar]
- Tao W., Lai E. Telencephalon-restricted expression of BF-1, a new member of the HNF-3/fork head gene family, in the developing rat brain. Neuron. 1992 May;8(5):957–966. doi: 10.1016/0896-6273(92)90210-5. [DOI] [PubMed] [Google Scholar]
- Voss J. W., Rosenfeld M. G. Anterior pituitary development: short tales from dwarf mice. Cell. 1992 Aug 21;70(4):527–530. doi: 10.1016/0092-8674(92)90422-9. [DOI] [PubMed] [Google Scholar]
- Weigel D., Jürgens G., Küttner F., Seifert E., Jäckle H. The homeotic gene fork head encodes a nuclear protein and is expressed in the terminal regions of the Drosophila embryo. Cell. 1989 May 19;57(4):645–658. doi: 10.1016/0092-8674(89)90133-5. [DOI] [PubMed] [Google Scholar]
- Wikenheiser K. A., Wert S. E., Wispé J. R., Stahlman M., D'Amore-Bruno M., Singh G., Katyal S. L., Whitsett J. A. Distinct effects of oxygen on surfactant protein B expression in bronchiolar and alveolar epithelium. Am J Physiol. 1992 Jan;262(1 Pt 1):L32–L39. doi: 10.1152/ajplung.1992.262.1.L32. [DOI] [PubMed] [Google Scholar]