Abstract
The inhibition of activated protein C by six different serine protease inhibitors (serpins) that have arginine residues in the P1 position has been investigated. Micromolar concentrations of C1-inhibitor failed to inhibit the enzyme, and it was inhibited only slowly by antithrombin III with an association rate constant (kass.) of 0.15 M-1.s-1. The kass. values for the other serpins tested (protease nexin I, protein C inhibitor, and mutants of alpha 1-antichymotrypsin and alpha 1-antitrypsin with P1 arginine residues) were at least 1000-fold higher, with P1-Arg-alpha 1-antitrypsin (kass. = 7 x 10(4) M-1.s-1) being the most effective inhibitor. The inhibition with these four serpins appeared to be reversible, with inhibition constants in the nanomolar range. The relatively high value of kass. for protease nexin I (5 x 10(3) M-1.s-1) suggested that it may be involved in the control of activated protein C on the surface of platelets where protein nexin I is present at relatively high concentrations. The value of kass. for protease nexin I, protein C inhibitor and antithrombin III showed a bell-shaped dependence on heparin concentration. At optimal concentrations, heparin accelerated the rate of inhibition by protease nexin I, protein C inhibitor and antithrombin III by 44-, 18- and 13-fold respectively. The kinetic constants for the inhibition of thrombin were also determined, and in all cases the serpins were more effective inhibitors of thrombin. Comparison of the sequences of the active-site regions of activated protein C and thrombin suggested that the more hydrophobic active site of thrombin may be more favourable for interactions with serpins.
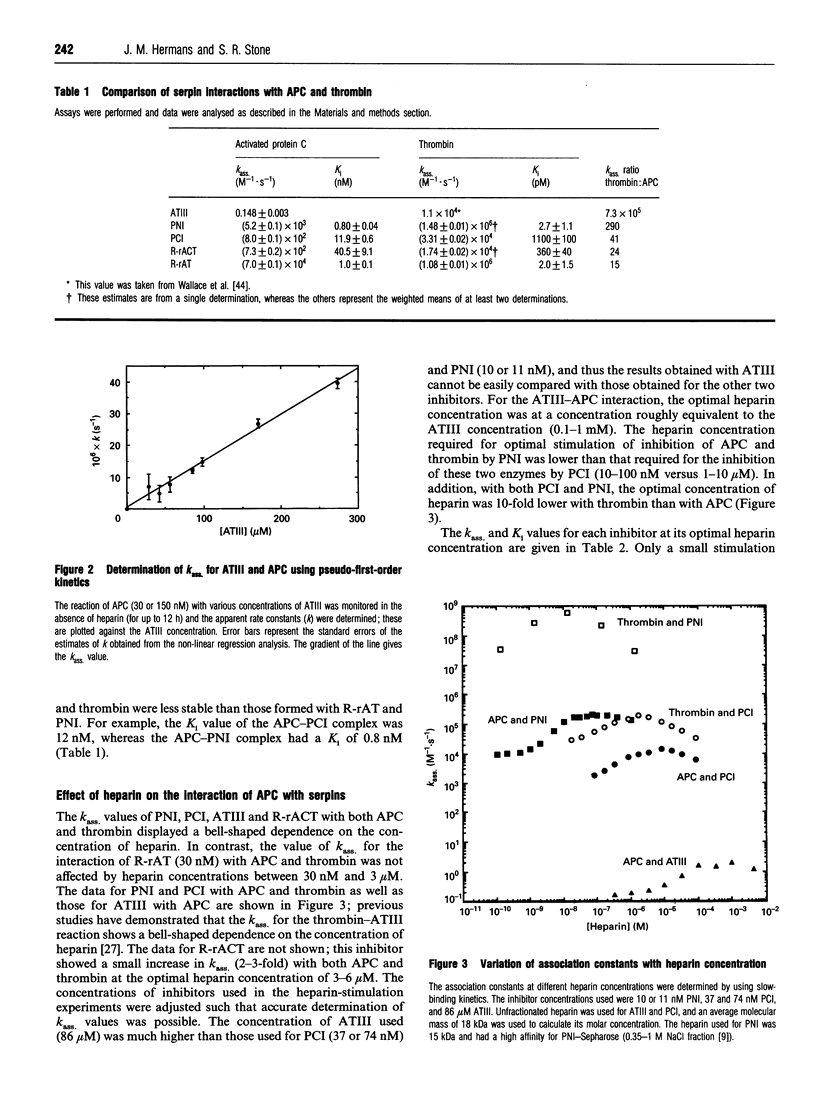
Full text
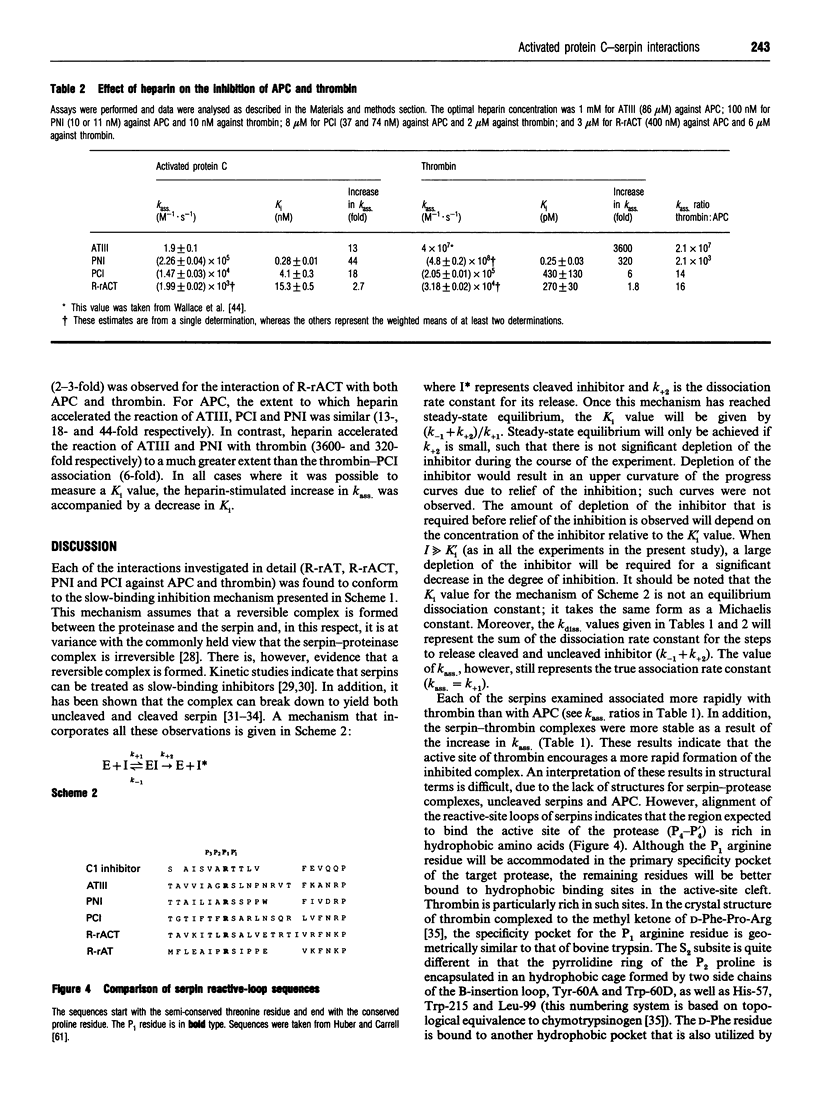
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker J. B., Gronke R. S. Protease nexins and cellular regulation. Semin Thromb Hemost. 1986 Jul;12(3):216–220. doi: 10.1055/s-2007-1003554. [DOI] [PubMed] [Google Scholar]
- Baumann U., Huber R., Bode W., Grosse D., Lesjak M., Laurell C. B. Crystal structure of cleaved human alpha 1-antichymotrypsin at 2.7 A resolution and its comparison with other serpins. J Mol Biol. 1991 Apr 5;218(3):595–606. doi: 10.1016/0022-2836(91)90704-a. [DOI] [PubMed] [Google Scholar]
- Beatty K., Travis J., Bieth J. The effect of alpha 2-macroglobulin on the interaction of alpha 1-proteinase inhibitor with porcine trypsin. Biochim Biophys Acta. 1982 Jun 4;704(2):221–226. doi: 10.1016/0167-4838(82)90149-2. [DOI] [PubMed] [Google Scholar]
- Bode W., Mayr I., Baumann U., Huber R., Stone S. R., Hofsteenge J. The refined 1.9 A crystal structure of human alpha-thrombin: interaction with D-Phe-Pro-Arg chloromethylketone and significance of the Tyr-Pro-Pro-Trp insertion segment. EMBO J. 1989 Nov;8(11):3467–3475. doi: 10.1002/j.1460-2075.1989.tb08511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bode W., Turk D., Karshikov A. The refined 1.9-A X-ray crystal structure of D-Phe-Pro-Arg chloromethylketone-inhibited human alpha-thrombin: structure analysis, overall structure, electrostatic properties, detailed active-site geometry, and structure-function relationships. Protein Sci. 1992 Apr;1(4):426–471. doi: 10.1002/pro.5560010402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branson H. E., Katz J., Marble R., Griffin J. H. Inherited protein C deficiency and coumarin-responsive chronic relapsing purpura fulminans in a newborn infant. Lancet. 1983 Nov 19;2(8360):1165–1168. doi: 10.1016/s0140-6736(83)91216-3. [DOI] [PubMed] [Google Scholar]
- Broekmans A. W., Veltkamp J. J., Bertina R. M. Congenital protein C deficiency and venous thromboembolism. A study of three Dutch families. N Engl J Med. 1983 Aug 11;309(6):340–344. doi: 10.1056/NEJM198308113090604. [DOI] [PubMed] [Google Scholar]
- Danielsson A., Björk I. Mechanism of inactivation of trypsin by antithrombin. Biochem J. 1982 Oct 1;207(1):21–28. doi: 10.1042/bj2070021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielsson A., Björk I. Properties of antithrombin-thrombin complex formed in the presence and in the absence of heparin. Biochem J. 1983 Aug 1;213(2):345–353. doi: 10.1042/bj2130345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danishefsky I., Pixley R. Effect of heparin on the inhibition of thrombin by alpha 1-proteinase inhibitor. Biochem Biophys Res Commun. 1979 Dec 14;91(3):862–870. doi: 10.1016/0006-291x(79)91959-4. [DOI] [PubMed] [Google Scholar]
- Esmon C. T. The roles of protein C and thrombomodulin in the regulation of blood coagulation. J Biol Chem. 1989 Mar 25;264(9):4743–4746. [PubMed] [Google Scholar]
- España F., Estelles A., Griffin J. H., Aznar J. Interaction of plasma kallikrein with protein C inhibitor in purified mixtures and in plasma. Thromb Haemost. 1991 Jan 23;65(1):46–51. [PubMed] [Google Scholar]
- Evans D. L., McGrogan M., Scott R. W., Carrell R. W. Protease specificity and heparin binding and activation of recombinant protease nexin I. J Biol Chem. 1991 Nov 25;266(33):22307–22312. [PubMed] [Google Scholar]
- Griffith M. J. Kinetics of the heparin-enhanced antithrombin III/thrombin reaction. Evidence for a template model for the mechanism of action of heparin. J Biol Chem. 1982 Jul 10;257(13):7360–7365. [PubMed] [Google Scholar]
- Gronke R. S., Bergman B. L., Baker J. B. Thrombin interaction with platelets. Influence of a platelet protease nexin. J Biol Chem. 1987 Mar 5;262(7):3030–3036. [PubMed] [Google Scholar]
- Harris K. W., Esmon C. T. Protein S is required for bovine platelets to support activated protein C binding and activity. J Biol Chem. 1985 Feb 25;260(4):2007–2010. [PubMed] [Google Scholar]
- Harrison R. A. Human C1 inhibitor: improved isolation and preliminary structural characterization. Biochemistry. 1983 Oct 11;22(21):5001–5007. doi: 10.1021/bi00290a019. [DOI] [PubMed] [Google Scholar]
- Heeb M. J., Bischoff R., Courtney M., Griffin J. H. Inhibition of activated protein C by recombinant alpha 1-antitrypsin variants with substitution of arginine or leucine for methionine358. J Biol Chem. 1990 Feb 5;265(4):2365–2369. [PubMed] [Google Scholar]
- Heeb M. J., Griffin J. H. Physiologic inhibition of human activated protein C by alpha 1-antitrypsin. J Biol Chem. 1988 Aug 25;263(24):11613–11616. [PubMed] [Google Scholar]
- Heeb M. J., Gruber A., Griffin J. H. Identification of divalent metal ion-dependent inhibition of activated protein C by alpha 2-macroglobulin and alpha 2-antiplasmin in blood and comparisons to inhibition of factor Xa, thrombin, and plasmin. J Biol Chem. 1991 Sep 15;266(26):17606–17612. [PubMed] [Google Scholar]
- Huber R., Carrell R. W. Implications of the three-dimensional structure of alpha 1-antitrypsin for structure and function of serpins. Biochemistry. 1989 Nov 14;28(23):8951–8966. doi: 10.1021/bi00449a001. [DOI] [PubMed] [Google Scholar]
- Kuhn L. A., Griffin J. H., Fisher C. L., Greengard J. S., Bouma B. N., España F., Tainer J. A. Elucidating the structural chemistry of glycosaminoglycan recognition by protein C inhibitor. Proc Natl Acad Sci U S A. 1990 Nov;87(21):8506–8510. doi: 10.1073/pnas.87.21.8506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurell M., Carlson T. H., Stenflo J. Monoclonal antibodies against the heparin-dependent protein C inhibitor suitable for inhibitor purification and assay of inhibitor complexes. Thromb Haemost. 1988 Oct 31;60(2):334–339. [PubMed] [Google Scholar]
- Laurell M., Christensson A., Abrahamsson P. A., Stenflo J., Lilja H. Protein C inhibitor in human body fluids. Seminal plasma is rich in inhibitor antigen deriving from cells throughout the male reproductive system. J Clin Invest. 1992 Apr;89(4):1094–1101. doi: 10.1172/JCI115689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longstaff C., Gaffney P. J. Serpin-serine protease binding kinetics: alpha 2-antiplasmin as a model inhibitor. Biochemistry. 1991 Jan 29;30(4):979–986. doi: 10.1021/bi00218a014. [DOI] [PubMed] [Google Scholar]
- Lottenberg R., Jackson C. M. Solution composition dependent variation in extinction coefficients for p-nitroaniline. Biochim Biophys Acta. 1983 Feb 15;742(3):558–564. doi: 10.1016/0167-4838(83)90273-x. [DOI] [PubMed] [Google Scholar]
- Low D. A., Baker J. B., Koonce W. C., Cunningham D. D. Released protease-nexin regulates cellular binding, internalization, and degradation of serine proteases. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2340–2344. doi: 10.1073/pnas.78.4.2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maimone M. M., Tollefsen D. M. Structure of a dermatan sulfate hexasaccharide that binds to heparin cofactor II with high affinity. J Biol Chem. 1990 Oct 25;265(30):18263–18271. [PubMed] [Google Scholar]
- Martin P. D., Robertson W., Turk D., Huber R., Bode W., Edwards B. F. The structure of residues 7-16 of the A alpha-chain of human fibrinogen bound to bovine thrombin at 2.3-A resolution. J Biol Chem. 1992 Apr 15;267(11):7911–7920. [PubMed] [Google Scholar]
- McKay E. J. A simple two-step procedure for the isolation of antithrombin III from biological fluids. 1981 Feb 15-Mar 1Thromb Res. 21(4-5):375–382. doi: 10.1016/0049-3848(81)90138-9. [DOI] [PubMed] [Google Scholar]
- Morrison J. F., Walsh C. T. The behavior and significance of slow-binding enzyme inhibitors. Adv Enzymol Relat Areas Mol Biol. 1988;61:201–301. doi: 10.1002/9780470123072.ch5. [DOI] [PubMed] [Google Scholar]
- Murano G., Williams L., Miller-Andersson M., Aronson D. L., King C. Some properties of antithrombin-III and its concentration in human plasma. Thromb Res. 1980 Apr 1;18(1-2):259–262. doi: 10.1016/0049-3848(80)90190-5. [DOI] [PubMed] [Google Scholar]
- Nesheim M. E. A simple rate law that describes the kinetics of the heparin-catalyzed reaction between antithrombin III and thrombin. J Biol Chem. 1983 Dec 10;258(23):14708–14717. [PubMed] [Google Scholar]
- Patston P. A., Roodi N., Schifferli J. A., Bischoff R., Courtney M., Schapira M. Reactivity of alpha 1-antitrypsin mutants against proteolytic enzymes of the kallikrein-kinin, complement, and fibrinolytic systems. J Biol Chem. 1990 Jun 25;265(18):10786–10791. [PubMed] [Google Scholar]
- Pomerantz M. W., Owen W. G. A catalytic role for heparin. Evidence for a ternary complex of heparin cofactor thrombin and heparin. Biochim Biophys Acta. 1978 Jul 21;535(1):66–77. doi: 10.1016/0005-2795(78)90033-8. [DOI] [PubMed] [Google Scholar]
- Pratt C. W., Church F. C. Heparin binding to protein C inhibitor. J Biol Chem. 1992 May 5;267(13):8789–8794. [PubMed] [Google Scholar]
- Pratt C. W., Macik B. G., Church F. C. Protein C inhibitor: purification and proteinase reactivity. Thromb Res. 1989 Mar 15;53(6):595–602. doi: 10.1016/0049-3848(89)90149-7. [DOI] [PubMed] [Google Scholar]
- Pratt C. W., Whinna H. C., Church F. C. A comparison of three heparin-binding serine proteinase inhibitors. J Biol Chem. 1992 May 5;267(13):8795–8801. [PubMed] [Google Scholar]
- Rovelli G., Stone S. R., Guidolin A., Sommer J., Monard D. Characterization of the heparin-binding site of glia-derived nexin/protease nexin-1. Biochemistry. 1992 Apr 7;31(13):3542–3549. doi: 10.1021/bi00128a031. [DOI] [PubMed] [Google Scholar]
- Rubin H., Wang Z. M., Nickbarg E. B., McLarney S., Naidoo N., Schoenberger O. L., Johnson J. L., Cooperman B. S. Cloning, expression, purification, and biological activity of recombinant native and variant human alpha 1-antichymotrypsins. J Biol Chem. 1990 Jan 15;265(2):1199–1207. [PubMed] [Google Scholar]
- Rånby M., Bergsdorf N., Nilsson T. Enzymatic properties of the one- and two-chain form of tissue plasminogen activator. Thromb Res. 1982 Jul 15;27(2):175–183. doi: 10.1016/0049-3848(82)90197-9. [DOI] [PubMed] [Google Scholar]
- Schechter I., Berger A. On the size of the active site in proteases. I. Papain. Biochem Biophys Res Commun. 1967 Apr 20;27(2):157–162. doi: 10.1016/s0006-291x(67)80055-x. [DOI] [PubMed] [Google Scholar]
- Seligsohn U., Berger A., Abend M., Rubin L., Attias D., Zivelin A., Rapaport S. I. Homozygous protein C deficiency manifested by massive venous thrombosis in the newborn. N Engl J Med. 1984 Mar 1;310(9):559–562. doi: 10.1056/NEJM198403013100904. [DOI] [PubMed] [Google Scholar]
- Shieh B. H., Potempa J., Travis J. The use of alpha 2-antiplasmin as a model for the demonstration of complex reversibility in serpins. J Biol Chem. 1989 Aug 15;264(23):13420–13423. [PubMed] [Google Scholar]
- Sommer J., Meyhack B., Rovelli G., Buergi R., Monard D. Synthesis of glia-derived nexin in yeast. Gene. 1989 Dec 28;85(2):453–459. doi: 10.1016/0378-1119(89)90439-3. [DOI] [PubMed] [Google Scholar]
- Stone S. R., Hofsteenge J. Kinetics of the inhibition of thrombin by hirudin. Biochemistry. 1986 Aug 12;25(16):4622–4628. doi: 10.1021/bi00364a025. [DOI] [PubMed] [Google Scholar]
- Stone S. R., Hofsteenge J. Specificity of activated human protein C. Biochem J. 1985 Sep 1;230(2):497–502. doi: 10.1042/bj2300497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone S. R., Nick H., Hofsteenge J., Monard D. Glial-derived neurite-promoting factor is a slow-binding inhibitor of trypsin, thrombin, and urokinase. Arch Biochem Biophys. 1987 Jan;252(1):237–244. doi: 10.1016/0003-9861(87)90028-2. [DOI] [PubMed] [Google Scholar]
- Stubbs M. T., Oschkinat H., Mayr I., Huber R., Angliker H., Stone S. R., Bode W. The interaction of thrombin with fibrinogen. A structural basis for its specificity. Eur J Biochem. 1992 May 15;206(1):187–195. doi: 10.1111/j.1432-1033.1992.tb16916.x. [DOI] [PubMed] [Google Scholar]
- Travis J., Salvesen G. S. Human plasma proteinase inhibitors. Annu Rev Biochem. 1983;52:655–709. doi: 10.1146/annurev.bi.52.070183.003255. [DOI] [PubMed] [Google Scholar]
- Wallace A., Rovelli G., Hofsteenge J., Stone S. R. Effect of heparin on the glia-derived-nexin-thrombin interaction. Biochem J. 1989 Jan 1;257(1):191–196. doi: 10.1042/bj2570191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Agostini A., Lijnen H. R., Pixley R. A., Colman R. W., Schapira M. Inactivation of factor XII active fragment in normal plasma. Predominant role of C-1-inhibitor. J Clin Invest. 1984 Jun;73(6):1542–1549. doi: 10.1172/JCI111360. [DOI] [PMC free article] [PubMed] [Google Scholar]


