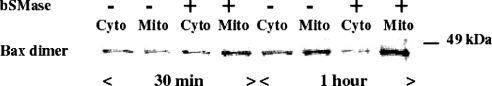Abstract
We recently showed that targeting bSMase (bacterial sphingomyelinase) specifically to mitochondria caused accumulation of ceramide in mitochondria, and induced cytochrome c release and cell death [Birbes, El Bawab, Hannun and Obeid (2001) FASEB J., 15, 2669–2679]. In the present study, we investigated the role of this mitochondrial pool of ceramide in response to a receptor-mediated event, namely TNFα (tumour necrosis factor α), and the involvement of this mitochondrial pool of ceramide in Bax translocation to mitochondria, an event that precedes cytochrome c release. Treatment of MCF7 cells with TNFα caused an increase in ceramide levels in the mitochondrial fraction which accompanied Bax translocation to mitochondria. Targeting bSMase to mitochondria specifically resulted in Bax translocation to mitochondria, suggesting that the mitochondrial ceramide pool is involved in Bax translocation. Moreover, in a reconstituted cell-free system, treatment of isolated mitochondria with bSMase enhanced Bax association with mitochondrial membranes. Collectively, these results suggest that the generation of ceramide in mitochondria in response to TNFα is sufficient to induce Bax translocation to mitochondria and subsequent cytochrome c release and cell death.
Keywords: apoptosis, Bax translocation, ceramide, mitochondrion, sphingomyelinase, tumour necrosis factor α (TNFα)
Abbreviations: DAPI, 4,6-diamidino-2-phenylindole; DGK, diacylglycerol kinase; FBS, foetal bovine serum; GFP, green fluorescent protein; IAP, inhibitor of apoptosis; PBS-T, PBS with Tween; pHi, intracellular pH; PP2A, protein phosphatase 2A; SM, sphingomyelin; SMase, sphingomyelinase; bSMase, bacterial SMase; N-SMase, neutral SMase; TNFα, tumour necrosis factor α
INTRODUCTION
Apoptosis induced by TNFα (tumour necrosis factor α) is a complex event that involves a variety of mediators and regulators, including proteases, the sphingolipid ceramide and Bcl-2 family members. It is now well established that mitochondria have a central role in the control of apoptosis. In fact, many death signals, including TNFα, converge on mitochondria through the activation of pro-apoptotic members of the Bcl-2 family, including Bak, Bad, Bid and Bax. In response to apoptotic stimuli, these proapoptotic proteins redistribute from the cytosol to the mitochondria [1,2]. The translocation of Bax is accompanied by apparent changes in Bax conformation, unmasking its N-terminus [3,4] and resulting in Bax oligomerization, which promote the permeabilization of the outer mitochondrial membrane. Released intermembrane space proteins include cytochrome c, which complexes with Apaf-1 (apoptotic protease-activating factor 1) and caspase 9 to form a post-mitochondrial apoptosome that amplifies effector caspase activation [5,6]. Cytochrome c plays an essential role in apoptosis, since cell lines from mice that have a disrupted gene are refractory to apoptosis induced by stimuli that affect mitochondria [7]. However, cytochrome c release alone does not appear to be sufficient to trigger apoptosis. It has to act in concert with other pro-apoptotic proteins to ensure commitment of cells to death. Thus the mitochondrial intermembrane protein Smac/DIABLO [direct IAP (inhibitor of apoptosis) protein-binding protein with low pI] is also released in the cytosol and facilitates activation of caspases indirectly, by eliminating their inhibition by IAP proteins, a family of proteins which suppress apoptosis by antagonizing caspase 3 activation [8,9]. In addition to pro-caspases and activators of caspases, the mitochondrial intermembrane space contains caspase-independent death effectors, such as the AIF (apoptosis-inducing factor). Thus the mitochondrion has a key role in the control of apoptosis, as it is an integrator of cell death pathways and a death executioner.
Several studies have demonstrated strong links between ceramide and the mitochondrion in the regulation of apoptosis (for a review see [10]). For example, (i) treatment of isolated mitochondria with ceramide led to inhibition of mitochondrial respiratory chain complex III [11], (ii) mitochondrial targeting of bSMase (bacterial sphingomyelinase) induced ceramide accumulation in mitochondria that was sufficient to induce cytochrome c release and apoptosis [12], (iii) ceramide was found to activate a mitochondrial PP2A (protein phosphatase 2A), which rapidly and completely dephosphorylated Bcl-2 and led to cell death [13], and (iv) ceramide-metabolizing enzymes have been localized to mitochondria, such as a bovine liver ceramide synthase [14] and a human ceramidase [15]. Another line of evidence that suggests the possibility that ceramide could interact with the mitochondrial apoptotic machinery is supported by studies on Bax. Ceramide was shown to potentiate synergistically the induction of mitochondrial permeability transition by Bax [16]. Cells lacking Bax were found to be resistant to ceramide-induced apoptosis, whereas cells in which Bax expression was restored regained sensitivity to ceramide [17]. Furthermore, ceramide was shown to induce a transitory increase in pHi (intracellular pH) in relation to the permeability transition pore. This rise in pHi led to conformational changes in Bax which could be responsible for further apoptosis in the ceramide pathway [18].
TNFα is known to increase the intracellular levels of ceramide through the activation of at least two pathways: one is mediated by SMase (sphingomyelinase) and the other is dependent on ceramide synthesis. For instance, in MCF7 cells treated with TNFα, ceramide generation results in an early peak coupled to the activation of N-SMase (neutral SMase), followed by a later peak resulting from the activation of the de novo pathway [19]. A previous study using a newly characterized inhibitor of N-SMase provides evidence for the first time that activation of the N-SMase in response to TNFα leads to cytochrome c release and mitochondrial dysfunction [20]. Moreover, we have demonstrated that targeting of bSMase to mitochondria causes ceramide accumulation that was sufficient to induce mitochondrial dysfunction (cytochrome c release) and cell death [12]. These results suggest that mitochondrial SM (sphingomyelin) and ceramide pools exist and are involved in apoptosis. As N-SMase is thought to be localized in different compartments, such as the plasma membrane, Golgi, nuclei and mitochondria [21], it is likely that TNFα-induced apoptosis could involve several SM pools localized in different compartments. Thus the aim of the present study was to determine if TNFα-induced apoptosis involves a mitochondrial pool of ceramide and whether this pool is involved in Bax translocation. Our results show that treatment of MCF7 cells with TNFα results in an increase in ceramide levels in mitochondria. In addition, only increases in mitochondrial ceramide were associated with Bax translocation to mitochondria. Taken together, these results suggest that a mitochondrial pool of ceramide is involved in TNFα-induced Bax translocation, cytochrome c release and cell death.
EXPERIMENTAL
Materials
TNFα was from Peprotech. The Bradford protein assay was from Bio-Rad. Triton X-100 was from Pierce. TLC plates were from Merck. pEGFP-N1 vector was from Clontech and pCMV/GFP vectors were from Invitrogen. Superfect was from Qiagen. MitoTracker Red CMXRos, annexin-V-conjugated Alexa Fluor 594 and DAPI (4,6-diamidino-2-phenylindole) were from Molecular Probes. Rhodamine-labelled goat anti-mouse IgG and FITC-conjugated goat anti-mouse antibody were from Jackson Immunoresearch Laboratories. [33P]ATP was from Amersham Biosciences. Exogenous bSMase from Staphylococcus aureus was from Sigma.
Cell culture and transient transfection
MCF7 breast cancer cells were maintained in RPMI 1640 medium (Life Technologies) supplemented with 10% FBS (foetal bovine serum) in 5% CO2 at 37 °C in a humidified incubator. Cells were seeded at 105 cells per 60-mm-diameter dish, and, 24 h after plating, they were transiently transfected with control vectors containing GFP (green fluorescent protein) (pCMV/GFP) or vectors containing bSMase–GFP (pCMV/bSMase-GFP) using Superfect and 3.5 μg of each plasmid per dish. After 3–4 h of incubation with the mixture, the cells were washed with PBS, and fresh medium was added. For Bax translocation assays, MCF7 cells were co-transfected with pCMV/bSMase-GFP vectors plus pEGFP-N1 empty vector at a ratio of 10:1 in order to enhance the fluorescence of positively transfected cells. The construction of all of the vectors has been described in detail in our previous study [12].
Preparation of cytosolic and mitochondria-enriched fractions
At the indicated time points, plates were washed once with ice-cold PBS, and floaters in the medium and from the washes were collected by centrifugation at 1300 g for 10 min. Adherent cells were scraped into lysis buffer A (20 mM Tris/HCl, pH 7.4, 150 mM NaCl, 10 mM sodium orthovanadate, 20 mM sodium fluoride, 0.25 M sucrose, 1 mM PMSF, 1 mM dithiothreitol, and 10 μg/ml each of chymostatin, leupeptin, aprotinin and pepstatin). After combining with floaters, cells were lysed by passing them ten times through a 28G 1/2 needle, and then centrifuged at 1300 g for 15 min to remove nuclei and unbroken cells. The supernatant was collected and centrifuged again for 15 min at 5000 g to pellet the mitochondrial fraction. The supernatant was centrifuged for 1 h at 100000 g to obtain cytosolic fractions. After determining the protein concentration, the cytosolic extracts were diluted with 2× sample buffer, boiled for 7 min and stored at −80 °C until Western-blotting analysis.
To perform the in vitro experiments, the mitochondrial fraction (approx. 100 μg of protein) was treated with or without 300 m-units of bSMase for 1 h at 37 °C. After treatment, mitochondria were washed twice in buffer A and then resuspended in a small volume of buffer A, and the protein concentration was measured. A 5 μg sample of control or bSMase-treated mitochondria was then incubated with 25 μg of cytosolic fraction proteins (100000 g, supernatant) for the indicated times. The samples were then subjected to centrifugation at 5000 g for 15 min to pellet the mitochondria, and the resultant supernatant (cytosolic fraction) and the mitochondrial fraction were analysed by Western blotting.
Western blotting
Proteins from cytosolic and mitochondria-enriched fractions were resolved by SDS/12% PAGE for analysis of Bax levels. After transfer to a PVDF membrane (at 80 V for 1 h, at 4 °C), the proteins were blocked for 2 h at room temperature (25 °C) in 5% (v/v) FBS in PBS containing 0.05% (v/v) Tween (PBS-T). After a quick wash with PBS-T, membranes were incubated overnight at 4 °C with anti-Bax monoclonal antibodies (clone IF6/IF2; 1:10 dilution in blocking solution) [1]. After a thorough wash of the membranes, proteins were incubated with secondary anti-mouse antibodies (Santa Cruz Biotechnology; 1:6000 in blocking solution) for 45 min at room temperature. The signal was visualized by ECL® (enhanced chemiluminescence) (Amersham Biosciences).
Immunocytochemistry of Bax
For immunocytochemical analysis, cells were plated at a density of 1.7×106 cells/100-mm-diameter plate that contained 22-mm glass coverslips. Following treatment, cells were washed three times with PBS, followed by fixation in freshly prepared 3.7% (w/v) paraformaldehyde for 10 min. The fixed cells were washed three times with PBS for 15 min each, followed by permeabilization in 0.15% (v/v) Triton X-100 in PBS for 15 min. After washing with PBS, the cells were blocked and treated for 3 h with anti-Bax monoclonal antibodies (clone IF6/IF2; 1:2 dilution in 2% FBS). The cells were washed three times for 10 min with PBS and incubated for 30 min with a rhodamine-labelled goat anti-mouse IgG (1:200) or a FITC-conjugated goat anti-mouse antibody (1:100 in 5% FBS in PBS-T; Jackson Immunoresearch Laboratories). Finally, for some samples, the cells were washed three times for 10 min in PBS, stained with 1 μg/ml DAPI and then photographed with an MTI RC300 video camera mounted on a Nikon eclipse TE200 microscope equipped with fluorescence optics, using a video digitizer (SCION Image 1.62) operated through Adobe Photoshop. In some experiments, cells were incubated with 25 nM MitoTracker Red CMXRos for 20 min before fixation to stain the mitochondria. Cells were then processed as described above and observed with a confocal microscope (Olympus IX 70), PerkinElmer Biosciences Ultraview software, spinning disk, using an Olympus ×40, 1.4 numerical aperture, oil-immersion lens. Fluorescence signals were collected after single-line excitation at 543 nm (red) and 488 nm (green).
Subcellular fractionation and ceramide measurement
Cells seeded at 106 cells/T150 flask were treated with 3 nM TNFα for 18 h. The medium from each flask was collected, and the cells were washed once with 5 ml of PBS, and floaters from the washes were collected by centrifugation at 1300 g for 10 min. Cells were then scraped in 5 ml of PBS, and each flask was washed with an additional 5 ml of PBS. Cells and washes were pooled with the medium and centrifuged for 10 min at 1300 g (4 °C). The cell pellets were homogenized by passing them 20 times through a 28G 1/2 needle in buffer A, and mitochondrial and cytosolic fractions prepared as described above.
Lipids from each fraction were then extracted as described by Bligh and Dyer [21a]. Total endogenous ceramide levels and endogenous ceramide levels form each fraction were measured using the DGK (diacylglycerol kinase) method as described previously [22].
RESULTS
Previous studies in our laboratory showed that ceramide action is compartment-specific in MCF7 cells. In fact, it was observed that ceramide induced cell death specifically when generated in mitochondria, through a mechanism involving the release of cytochrome c from mitochondria [12]. On the other hand, it was also observed that TNFα-induced cytochrome c release involved N-SMase action [20]. Since Bax translocation to mitochondria is known to precede cytochrome c release, we investigated whether TNFα induces the accumulation of ceramide in mitochondria and whether this specific pool of ceramide is involved in the translocation of Bax to mitochondria.
TNFα treatment induces Bax translocation to mitochondria
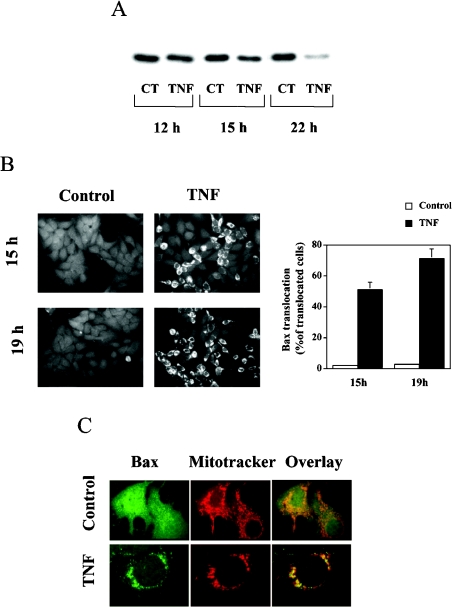
The effect of TNFα on Bax translocation was determined using Western blotting and immunocytochemistry. MCF7 cells were treated with 3 nM TNFα, and, at the indicated times, cells were collected, fractionated, and Western blotting was performed using cytosolic proteins. As shown in Figure 1(A), TNFα treatment induced a decrease in Bax protein in the cytosolic fraction in a time-dependent manner. Concomitant to this cytosolic loss, TNFα induced a relocalization of Bax as analysed by immunocytochemistry, resulting in a change of its staining pattern from a diffuse cytosolic distribution in untreated cells to a punctate mitochondrial profile in TNFα-treated cells (Figure 1B). After 19 h, TNFα induced a marked translocation of Bax, with 70–75% of cells having Bax translocated to mitochondria (Figure 1B). This punctate pattern was confirmed to be mitochondria, as shown by the co-localization of the endogenous Bax signal with MitoTracker signal (Figure 1C). These results demonstrate that TNFα induces the translocation of Bax to mitochondria, in agreement with previous studies [23–25].
Figure 1. TNFα-induced mitochondrial translocation of Bax in MCF7 cells.
(A) Western blot analysis of cytosolic extracts (5 μg) from control (CT) and TNFα-treated (TNF) cells after 12, 15 and 22 h of incubation. Cells (1.7×106) were treated with TNFα (3 nM) for the indicated times, and cytosolic extracts for analysis of Bax levels were obtained as described in the Experimental section. (B) Immunocytochemical analysis of Bax in control and TNFα-treated cells. Cells (1.7×106) were treated with TNFα (3 nM) for the indicated times, and analysis of Bax localization by indirect immunofluorescence was performed as described in the Experimental section. A clear translocation of Bax was observed after 15 h of TNFα treatment. The punctate pattern was more pronounced after 19 h of treatment. Ten to 15 random fields from each experimental condition were counted, and the percentage of cells that showed translocation of Bax over the total number of counted cells is represented in the histogram. Results are means±S.D. for three different experiments. (C) Localization of Bax after TNFα treatment. Cells (1.7×106) were treated with TNFα (3 nM) for 15 h. At 20 min before fixation, the cells were incubated with 25 nM MitoTracker Red, and analysis of Bax localization and mitochondrial patterns were performed as described in the Experimental section.
TNFα induces ceramide generation in mitochondria
It has been clearly established that TNFα-treatment of MCF7 cells results in an accumulation of intracellular ceramide levels. To test whether ceramide accumulation in response to TNFα is, at least in part, occurring in mitochondria, MCF7 cells were treated with TNFα, lysed and fractionated, and ceramide levels were measured in total and mitochondrial fractions. As shown in Table 1, TNFα treatment caused a 2-fold increase of total cellular ceramide levels. A 2-fold increase of ceramide levels was also observed in lipids that were extracted from the mitochondrial fraction of cells treated with TNFα as compared with control cells, indicating that TNFα induces the production of a ceramide pool in mitochondria. Based on our results, the mitochondrial pool of ceramide that increased in response to TNFα would represent approx. 5% of the total increase of ceramide in TNFα-treated MCF7 cells (total ceramide increase represented approx. 4.2 pmol of ceramide/total nmol of phosphate, and mitochondrial ceramide increase represented approx. 0.235 pmol of ceramide/total nmol of phosphate). These results suggest that a receptor-mediated death signal induces ceramide production in mitochondria.
Table 1. TNFα treatment of MCF7 cells induces ceramide generation in the mitochondrial fraction.
MCF7 cells were treated with 3 nM TNFα for 18 h. At the end of the treatment, the mitochondrial fraction was prepared by fractionation as described in the Experimental section, and lipids were then extracted, and ceramide levels were measured using the DGK assay as described in the Experimental section. Results are means±S.D. for four independent experiments.
| Ceramide (pmol/nmol of phosphate) | ||
|---|---|---|
| Control cells | TNFα-treated cells | |
| Total | 4.74±0.5 | 10.44±0.6* |
| Mitochondria | 5.12±0.4 | 11.62±0.8* |
* Significant difference (P<0.05) compared with the control.
Mitochondrial ceramide generation induces Bax translocation
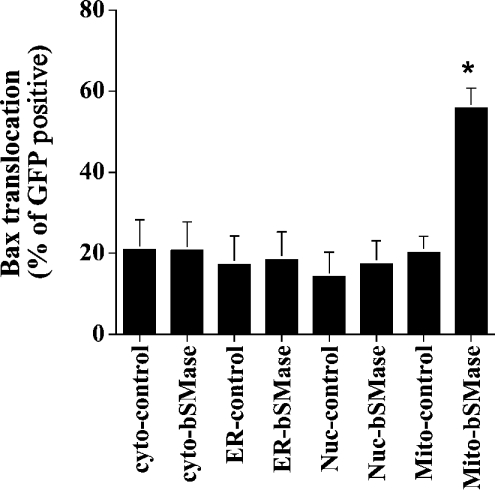
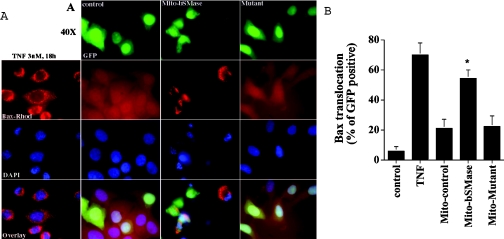
To investigate which pool of ceramide may be involved in Bax translocation, the effect of the overexpression of different bSMase-targeted constructs on Bax translocation was evaluated by immunocytochemistry. As shown in Figure 2, after 48 h of transfection, targeting the bSMase to the cytoplasm, endoplasmic reticulum or nucleus was without any effect on Bax translocation. In contrast, the targeting of bSMase into mitochondria induced a pattern similar to that observed when TNFα induced Bax translocation from the cytosol to mitochondria (Figure 3A) with approx. 55% of transfected cells showing Bax translocation to mitochondria (Figure 3B). Similar results were obtained for all the constructs at 24 and 72 h (results not shown). To ascertain the specificity of the observed translocation pattern, a construct containing an inactive SMase mutant was transfected in MCF7 cells, and Bax translocation was determined. The overexpression of the bSMase-D295G–GFP/Mito mutant (Asp295→Gly) did not affect the distribution of Bax, as it retained the same persistent diffuse staining that was present in control cells (Figure 3). These results suggest that a mitochondrial ceramide pool is selectively involved, at least partly, in Bax translocation into mitochondria.
Figure 2. Mitochondrial ceramide generation induces Bax translocation.
MCF7 cells were co-transfected with 3.5 μg of the different pCMV/GFP controls vector or pCMV/bSMase-GFP vectors plus 0.35 μg of pEGFP-N1 vector. At 48 h after transfection, cells were fixed, immunostained with Bax mouse monoclonal antibody, and visualized with rhodamine-conjugated secondary antibody. Cells were then mounted, and observed by phase-contrast and fluorescent microscopy. In each field, total GFP-positive cells were counted, and the results are expressed as the percentage of GFP-positive cells having Bax translocation to mitochondria. Different fields (at least 300 cells) were counted in each experiment. Data are from one experiment performed in duplicate, representative of at least three separate experiments. Asterisks indicate a significant difference (P<0.05) compared with the control. For statistical analysis, Student's t test for paired sample means was used. Cyto, cytoplasm; ER, endoplasmic reticulum; Nuc, nuclei; Mito, mitochondria.
Figure 3. Overexpression of the mitochondria-targeted bSMase-D295G mutant did not cause Bax translocation.
MCF7 cells were treated with 3 nM TNFα for 18 h or were co-transfected with 3.5 μg of pCMV/GFP/Mito empty vector plus 0.35 μg of pEGFP-N1 vector, or with 3.5 μg of pCMV/bSMase-GFP/Mito vector plus 0.35 μg of pEGFP-N1 vector, or 3.5 μg of pCMV/bSMase-D295G-GFP/Mito mutant vector plus 0.35 μg of pEGFP-N1. At 48 h after transfection, cells were fixed, immunostained with anti-Bax mouse monoclonal antibody, and visualized with rhodamine-conjugated secondary antibody. Analysis and quantification of Bax translocation was performed by indirect immunofluorescence as described in the Experimental section. Data are from one experiment performed in duplicate, representative of at least three separate experiments. Asterisks indicate a significant difference (P<0.05) compared with the control. For statistical analysis, Student's t test for paired sample means was used.
In vitro, Bax associates with mitochondria treated with exogenous bSMase
To determine if ceramide generation is sufficient to induce Bax redistribution, Bax translocation to mitochondria was assessed by measuring the association of Bax to the mitochondrial fraction in a reconstituted cell-free system. Thus an enriched mitochondrial fraction was treated with exogenous bSMase in order to generate ceramide, and Bax association with mitochondrial membranes was then studied by Western blotting. As shown in Figure 4, when the cytosolic fraction was incubated with the mitochondrial fraction treated with bSMase, a time-dependent decrease of Bax protein in the cytosolic fraction was observed with a concomitant increase in Bax association to the mitochondrial fraction. This redistribution did not occur when the cytosolic fraction was incubated with untreated mitochondria. These results strongly suggest that ceramide produced from a mitochondrial pool of SM may be sufficient to induce Bax translocation from the cytosol to mitochondria.
Figure 4. Treatment of mitochondria with bSMase induces Bax association with mitochondria.
Mitochondrial fraction was prepared by fractionation as described in the Experimental section, and treated with or without 300 m-units of bSMase for 1 h at 37 °C. Mitochondrial fraction (5 μg of protein) was then incubated with 25 μg of cytosolic fraction for the indicated times at 37 °C. At the end of incubation, the mitochondrial and cytosolic fractions were separated by centrifugation (5000 g for 15 min) and Bax translocation was evaluated by Western blotting as described in the Experimental section, but in the absence of SDS. Cyto, cytoplasm; Mito, mitochondria.
DISCUSSION
In the present study, we investigated the role of the mitochondrial pool of ceramide in response to a receptor-mediated apoptosis induced by TNFα. Our results demonstrate that the generation of ceramide at the level of mitochondria indeed occurs physiologically in a receptor-mediated event. In fact, fractionation experiments showed that a significant increase in ceramide levels in response to TNFα occurs in a mitochondrial-enriched fraction. Our results also show that the generation of ceramide in mitochondria drives Bax translocation and cytochrome c release, whereas ceramide accumulation in other subcellular compartments does not itself promote mitochondrial activation. Moreover, ceramide generation from bSMase-treated mitochondria in vitro appears to be sufficient for Bax translocation to mitochondria.
TNFα induces cell death through a complex process that involves protein–protein interactions and the participation of several intermediates, including ceramide. Strong support exists for ceramide generation through SMase activation in apoptotic pathways induced by TNFα (for reviews see [26,27]). Several studies have implicated the N-SMase isoform [20,28,29] in apoptosis induced by death receptors. On the other hand, an important role of A-SMase (acid SMase) in TNFα-mediated apoptosis has also been shown [30–32]. It was also shown that in MCF7 cells, ceramide generation in response to TNFα results from de novo formation and N-SMase activation [19]. In addition, we recently showed that the activation of N-SMase in response to TNFα is involved in mitochondrial dysfunction [20], and demonstrated for the first time, by overexpression of bSMase, that mitochondrial SM and ceramide pools are involved in cell death [12]. Our results from this study are in agreement with those of Garcia-Ruiz et al. [33], who demonstrated an accumulation of ceramide in purified mitochondria following treatment with TNFα.
We showed recently that overexpression of bSMase in mitochondria induces cell death through cytochrome c release, but how mitochondrial ceramide induces the release of cytochrome c remains unanswered. Bax translocation to mitochondria is a recognized event that precedes and regulates cytochrome c release. It is known that, after apoptotic stimulation, conformational changes occur in the N- and C-termini of the Bax protein. These events result in Bax translocation, oligomerization and integration into mitochondrial membranes, thus promoting the permeabilization of the outer mitochondrial membrane and leading to the release of intermembrane proteins [34]. In the present study, we show that mitochondrial ceramide (overexpression of Mito–bSMase) enhances the translocation of Bax to the mitochondria. Thus it is highly likely that mitochondrial ceramide induces the release of cytochrome c by first inducing the translocation of Bax.
Cross–talk between ceramide and Bax has been addressed in several studies. One mechanism by which ceramide might induce apoptosis is by increasing the Bax/Bcl-2 or Bax/Bcl-xL ratio [35,36]. It has also been shown that ceramide potentiates the induction of mitochondrial permeability transition by Bax in a synergistic manner [16]. Recently, von Haefen et al. [17] showed in human carcinoma cells that ceramide induces mitochondrial activation via a Bax-dependent and caspase-independent pathway. In addition, they also demonstrated that ceramide treatment induces a conformational change in Bax, and proposed that a possible interaction between ceramide and Bax leads to the N-terminal conformation change in the Bax protein [17]. Furthermore, it has been shown in an in vitro assay using isolated mitochondria, that recombinant Bax opened the mitochondrial permeability transition pore, an event potentiated by exogenous addition of ceramide [16]. Our results using bSMase targeted to mitochondria, coupled with our results from treating isolated mitochondria with bSMase, strongly suggest that enrichment of ceramide in mitochondria is sufficient both in cells as well as in vitro to induce association of Bax to this organelle.
Taken together, these observations argue in favour of an interaction between ceramide and the mitochondrial membrane, thereby facilitating the insertion of Bax into mitochondrial membranes. It should be mentioned that ceramide action on mitochondria might also require dephosphorylation events involving ceramide-dependent phosphatases, such as PP2A, as suggested from studies showing the effect of ceramide on Bcl-2 phosphorylation [13]. On the other hand, as discussed above, in MCF7 cells, TNFα induces the production of ceramide through the de novo and SMase pathways, indicating that TNFα-induced apoptosis involves several ceramide pools, including a mitochondrial pool. If one considers a role of the mitochondrial ceramide pool in the mitochondrial pathway of apoptosis (Bax translocation, cytochrome c release and other effects) then what is the role of the non-mitochondrial ceramide pools in TNFα-induced apoptosis? The answer to this question is not known. Recent data suggested that the protein kinase Akt might be a target of ceramide, thus ceramide could signal mitochondrial apoptosis by inhibiting the Akt [37,38], which has been shown to phosphorylate Bad. Phosphorylation of Bad via growth factor receptor signalling and the Akt kinase releases Bcl-xL to target mitochondria. Thus inhibition of Akt by ceramide leads to inhibition of the anti-apoptotic protein Bcl-xL by Bad.
An important conclusion from the present study is that apoptosis induced by TNFα involves several pools of ceramide, at least in part a mitochondrial ceramide pool, that could have different or common targets in the apoptotic machinery. The identification of these targets requires further investigation.
Acknowledgments
This work was supported by the National Institutes of Health grants AG16583 (L.M.O.), P01-DK59340 (Y.A.H.) and NS40932 (Y.-T.H.). The authors thank Dr Prigent and Professor Lagarde for hosting H.B. in unit INSERM 585, and Kathy Wiita-Fisk for administrative assistance.
References
- 1.Hsu Y. T., Wolter K. G., Youle R. J. Cytosol-to-membrane redistribution of Bax and Bcl-XL during apoptosis. Proc. Natl. Acad. Sci. U.S.A. 1997;94:3668–3672. doi: 10.1073/pnas.94.8.3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gross A., Jockel J., Wei M. C., Korsmeyer S. J. Enforced dimerization of BAX results in its translocation, mitochondrial dysfunction and apoptosis. EMBO J. 1998;17:3878–3885. doi: 10.1093/emboj/17.14.3878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goping I. S., Gross A., Lavoie J. N., Nguyen M., Jemmerson R., Roth K., Korsmeyer S. J., Shore G. C. Regulated targeting of BAX to mitochondria. J. Cell Biol. 1998;143:207–215. doi: 10.1083/jcb.143.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Desagher S., Osen-Sand A., Nichols A., Eskes R., Montessuit S., Lauper S., Maundrell K., Antonsson B., Martinou J. C. Bid-induced conformational change of Bax is responsible for mitochondrial cytochrome c release during apoptosis. J. Cell Biol. 1999;144:891–901. doi: 10.1083/jcb.144.5.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li P., Nijhawan D., Budihardjo I., Srinivasula S. M., Ahmad M., Alnemri E. S., Wang X. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91:479–489. doi: 10.1016/s0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- 6.Thornberry N. A., Lazebnik Y. Caspases: enemies within. Science. 1998;281:1312–1316. doi: 10.1126/science.281.5381.1312. [DOI] [PubMed] [Google Scholar]
- 7.Li K., Li Y., Shelton J. M., Richardson J. A., Spencer E., Chen Z. J., Wang X., Williams R. S. Cytochrome c deficiency causes embryonic lethality and attenuates stress-induced apoptosis. Cell. 2000;101:389–399. doi: 10.1016/s0092-8674(00)80849-1. [DOI] [PubMed] [Google Scholar]
- 8.Deveraux Q. L., Reed J. C. IAP family proteins – suppressors of apoptosis. Genes Dev. 1999;13:239–252. doi: 10.1101/gad.13.3.239. [DOI] [PubMed] [Google Scholar]
- 9.Du C., Fang M., Li Y., Li L., Wang X. Smac, a mitochondrial protein that promotes cytochrome c-dependent caspase activation by eliminating IAP inhibition. Cell. 2000;102:33–42. doi: 10.1016/s0092-8674(00)00008-8. [DOI] [PubMed] [Google Scholar]
- 10.Birbes H., Bawab S. E., Obeid L. M., Hannun Y. A. Mitochondria and ceramide: intertwined roles in regulation of apoptosis. Adv. Enzyme Regul. 2002;42:113–129. doi: 10.1016/s0065-2571(01)00026-7. [DOI] [PubMed] [Google Scholar]
- 11.Gudz T. I., Tserng K. Y., Hoppel C. L. Direct inhibition of mitochondrial respiratory chain complex III by cell-permeable ceramide. J. Biol. Chem. 1997;272:24154–24158. doi: 10.1074/jbc.272.39.24154. [DOI] [PubMed] [Google Scholar]
- 12.Birbes H., El Bawab S., Hannun Y. A., Obeid L. M. Selective hydrolysis of a mitochondrial pool of sphingomyelin induces apoptosis. FASEB J. 2001;15:2669–2679. doi: 10.1096/fj.01-0539com. [DOI] [PubMed] [Google Scholar]
- 13.Ruvolo P. P., Deng X. M., Ito T., Carr B. K., May W. S. Ceramide induces Bcl2 dephosphorylation via a mechanism involving mitochondrial PP2A. J. Biol. Chem. 1999;274:20296–20300. doi: 10.1074/jbc.274.29.20296. [DOI] [PubMed] [Google Scholar]
- 14.Shimeno H., Soeda S., Sakamoto M., Kouchi T., Kowakame T., Kihara T. Partial purification and characterization of sphingosine N-acyltransferase (ceramide synthase) from bovine liver mitochondrion-rich fraction. Lipids. 1998;33:601–605. doi: 10.1007/s11745-998-0246-2. [DOI] [PubMed] [Google Scholar]
- 15.El Bawab S., Roddy P., Qian T., Bielawska A., Lemasters J. J., Hannun Y. A. Molecular cloning and characterization of a human mitochondrial ceramidase. J. Biol. Chem. 2000;275:21508–21513. doi: 10.1074/jbc.M002522200. [DOI] [PubMed] [Google Scholar]
- 16.Pastorino J. G., Tafani M., Rothman R. J., Marcinkeviciute A., Hoek J. B., Farber J. L. Functional consequences of the sustained or transient activation by Bax of the mitochondrial permeability transition pore. J. Biol. Chem. 1999;274:31734–31739. doi: 10.1074/jbc.274.44.31734. [DOI] [PubMed] [Google Scholar]
- 17.von Haefen C., Wieder T., Gillissen B., Starck L., Graupner V., Dorken B., Daniel P. T. Ceramide induces mitochondrial activation and apoptosis via a Bax-dependent pathway in human carcinoma cells. Oncogene. 2002;21:4009–4019. doi: 10.1038/sj.onc.1205497. [DOI] [PubMed] [Google Scholar]
- 18.Belaud-Rotureau M. A., Leducq N., Macouillard Poulletier de Gannes F., Diolez P., Lacoste L., Lacombe F., Bernard P., Belloc F. Early transitory rise in intracellular pH leads to Bax conformation change during ceramide-induced apoptosis. Apoptosis. 2000;5:551–560. doi: 10.1023/a:1009693630664. [DOI] [PubMed] [Google Scholar]
- 19.Dbaibo G. S., El-Assaad W., Krikorian A., Liu B., Diab K., Idriss N. Z., El-Sabban M., Driscoll T. A., Perry D. K., Hannun Y. A. Ceramide generation by two distinct pathways in tumor necrosis factor α-induced cell death. FEBS Lett. 2001;503:7–12. doi: 10.1016/s0014-5793(01)02625-4. [DOI] [PubMed] [Google Scholar]
- 20.Luberto C., Hassler D. F., Signorelli P., Okamoto Y., Sawai H., Boros E., Hazen-Martin D. J., Obeid L. M., Hannun Y. A., Smith G. K. Inhibition of tumor necrosis factor-induced cell death in MCF7 by a novel inhibitor of neutral sphingomyelinase. J. Biol. Chem. 2002;277:41128–41139. doi: 10.1074/jbc.M206747200. [DOI] [PubMed] [Google Scholar]
- 21.Pettus B. J., Chalfant C. E., Hannun Y. A. Ceramide in apoptosis: an overview and current perspectives. Biochim. Biophys. Acta. 2002;1585:114–125. doi: 10.1016/s1388-1981(02)00331-1. [DOI] [PubMed] [Google Scholar]
- 21a.Bligh E. G., Dyer W. J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 22.Dbaibo G. S., Pushkareva M. Y., Rachid R. A., Alter N., Smyth M. J., Obeid L. M., Hannun Y. A. p53-dependent ceramide response to genotoxic stress. J. Clin. Invest. 1998;102:329–339. doi: 10.1172/JCI1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao Y., Li S., Childs E. E., Kuharsky D. K., Yin X. M. Activation of pro-death Bcl-2 family proteins and mitochondria apoptosis pathway in tumor necrosis factor-α-induced liver injury. J. Biol. Chem. 2001;276:27432–27440. doi: 10.1074/jbc.M102465200. [DOI] [PubMed] [Google Scholar]
- 24.Deng Y., Lin Y., Wu X. TRAIL-induced apoptosis requires Bax-dependent mitochondrial release of Smac/DIABLO. Genes Dev. 2002;16:33–45. doi: 10.1101/gad.949602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maianski N. A., Roos D., Kuijpers T. W. Tumor necrosis factor α induces a caspase-independent death pathway in human neutrophils. Blood. 2003;101:1987–1995. doi: 10.1182/blood-2002-02-0522. [DOI] [PubMed] [Google Scholar]
- 26.Hannun Y. A., Luberto C. Ceramide in the eukaryotic stress response. Trends Cell Biol. 2000;10:73–80. doi: 10.1016/s0962-8924(99)01694-3. [DOI] [PubMed] [Google Scholar]
- 27.Hannun Y. A., Luberto C., Argraves K. M. Enzymes of sphingolipid metabolism: from modular to integrative signaling. Biochemistry. 2001;40:4893–4903. doi: 10.1021/bi002836k. [DOI] [PubMed] [Google Scholar]
- 28.Liu B., Andrieu-Abadie N., Levade T., Zhang P., Obeid L. M., Hannun Y. A. Glutathione regulation of neutral sphingomyelinase in tumor necrosis factor-α-induced cell death. J. Biol. Chem. 1998;273:11313–11320. doi: 10.1074/jbc.273.18.11313. [DOI] [PubMed] [Google Scholar]
- 29.Segui B., Cuvillier O., Adam-Klages S., Garcia V., Malagarie-Cazenave S., Leveque S., Caspar-Bauguil S., Coudert J., Salvayre R., Kronke M., Levade T. Involvement of FAN in TNF-induced apoptosis. J. Clin. Invest. 2001;108:143–151. doi: 10.1172/JCI11498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Higuchi M., Singh S., Jaffrezou J. P., Aggarwal B. B. Acidic sphingomyelinase-generated ceramide is needed but not sufficient for TNF-induced apoptosis and nuclear factor-κB activation. J. Immunol. 1996;157:297–304. [PubMed] [Google Scholar]
- 31.Monney L., Olivier R., Otter I., Jansen B., Poirier G. G., Borner C. Role of an acidic compartment in tumor-necrosis-factor-α-induced production of ceramide, activation of caspase-3 and apoptosis. Eur. J. Biochem. 1998;251:295–303. doi: 10.1046/j.1432-1327.1998.2510295.x. [DOI] [PubMed] [Google Scholar]
- 32.Garcia-Ruiz C., Colell A., Mari M., Morales A., Calvo M., Enrich C., Fernandez-Checa J. C. Defective TNF-α-mediated hepatocellular apoptosis and liver damage in acidic sphingomyelinase knockout mice. J. Clin. Invest. 2003;111:197–208. doi: 10.1172/JCI16010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.García-Ruiz C., Colell A., Marí M., Morales A., Fernández-Checa J. C. Direct effect of ceramide on the mitochondrial electron transport chain leads to generation of reactive oxygen species: role of mitochondrial glutathione. J. Biol. Chem. 1997;272:11369–11377. doi: 10.1074/jbc.272.17.11369. [DOI] [PubMed] [Google Scholar]
- 34.Antonsson B., Montessuit S., Sanchez B., Martinou J. C. Bax is present as a high molecular weight oligomer/complex in the mitochondrial membrane of apoptotic cells. J. Biol. Chem. 2001;276:11615–11623. doi: 10.1074/jbc.M010810200. [DOI] [PubMed] [Google Scholar]
- 35.Kim W. H., Ghil K. C., Lee J. H., Yeo S. H., Chun Y. J., Choi K. H., Kim D. K., Kim M. Y. Involvement of p27kip1 in ceramide-mediated apoptosis in HL-60 cells. Cancer Lett. 2000;151:39–48. doi: 10.1016/s0304-3835(99)00402-4. [DOI] [PubMed] [Google Scholar]
- 36.Sawada M., Nakashima S., Banno Y., Yamakawa H., Hayashi K., Takenaka K., Nishimura Y., Sakai N., Nozawa Y. Ordering of ceramide formation, caspase activation, and Bax/Bcl-2 expression during etoposide-induced apoptosis in C6 glioma cells. Cell Death Differ. 2000;7:761–772. doi: 10.1038/sj.cdd.4400711. [DOI] [PubMed] [Google Scholar]
- 37.Zhou H., Summers S. A., Birnbaum M. J., Pittman R. N. Inhibition of Akt kinase by cell-permeable ceramide and its implications for ceramide-induced apoptosis. J. Biol. Chem. 1998;273:16568–16575. doi: 10.1074/jbc.273.26.16568. [DOI] [PubMed] [Google Scholar]
- 38.Zhou H., Li X. M., Meinkoth J., Pittman R. N. Akt regulates cell survival and apoptosis at a postmitochondrial level. J. Cell Biol. 2000;151:483–494. doi: 10.1083/jcb.151.3.483. [DOI] [PMC free article] [PubMed] [Google Scholar]