Abstract
Manganese is an essential, but potentially toxic, trace metal in biological systems. Overexposure to manganese is known to cause neurological deficits in humans, but the pathways that lead to manganese toxicity are largely unknown. We have employed the bakers' yeast Saccharomyces cerevisiae as a model system to identify genes that contribute to manganese-related damage. In a genetic screen for yeast manganese-resistance mutants, we identified S. cerevisiae MAM3 as a gene which, when deleted, would increase cellular tolerance to toxic levels of manganese and also increased the cell's resistance towards cobalt and zinc. By sequence analysis, Mam3p shares strong similarity with the mammalian ACDP (ancient conserved domain protein) family of polypeptides. Mutations in human ACDP1 have been associated with urofacial (Ochoa) syndrome. However, the functions of eukaryotic ACDPs remain unknown. We show here that S. cerevisiae MAM3 encodes an integral membrane protein of the yeast vacuole whose expression levels directly correlate with the degree of manganese toxicity. Surprisingly, Mam3p contributes to manganese toxicity without any obvious changes in vacuolar accumulation of metals. Furthermore, through genetic epistasis studies, we demonstrate that MAM3 operates independently of the well-established manganese-trafficking pathways in yeast, involving the manganese transporters Pmr1p, Smf2p and Pho84p. This is the first report of a eukaryotic ACDP family protein involved in metal homoeostasis.
Keywords: Mam3p, manganese, Saccharomyces cerevisiae, toxicity, urofacial syndrome, vacuole
Abbreviations: ACD, ancient conserved domain; ACDP, ACD protein; COX4, cytochrome oxidase subunit IV; DAPI, 4,6-diamidino-2-phenylindole; GFP, green fluorescent protein; SD, minimal synthetic dextrose medium supplemented with the required amino acids; UFS, urofacial syndrome; YPD, yeast extract and peptone-based medium supplemented with 2% glucose; YPEG, yeast extract and peptone-based medium with 2% ethanol and 2% glycerol
INTRODUCTION
Manganese is an essential trace element in biological systems. Manganese-dependent enzymes are found within diverse locations in the cell, including the Golgi, mitochondria and cytoplasm. However, high concentrations of manganese are potentially toxic. In humans, exposure to this metal can lead to manganism, a Parkinson's disease-like neurological disorder with characteristic syndromes of mental difficulties and impairments in motor skills [1,2]. Although the importance of manganese in health and disease is widely recognized, very little is known regarding the factors that contribute to toxicity from this metal.
Saccharomyces cerevisiae, the bakers' yeast, has proven to be an excellent model system for studying manganese homoeostasis [3]. A number of players have been successfully identified through genetic studies and virtually all have homologues in humans. Two Nramp (natural resistance-associated macrophage protein) family members, Smf1p and Smf2p, contribute to high-affinity manganese transport in S. cerevisiae. The role of Smf1p is to transfer manganese across the cell surface [4,5]. Smf2p is localized at intracellular vesicles and is required to deliver manganese to secretory pathway enzymes and to mitochondrial superoxide dismutase 2 [6]. Except under manganese starvation conditions, Smf1p and Smf2p levels are maintained at a low level to prevent high intracellular accumulation of manganese and other metals. This down-regulation of Smf1p and Smf2p under manganese surplus conditions involves targeting these proteins to the vacuole via a mechanism involving Bsd2p and ubiquitination in S. cerevisiae [5,7–9].
Under manganese toxicity conditions, pathways other than Smf1p/Smf2p mediate uptake of manganese into the cell [3]. We recently found that the cell surface phosphate transporter in yeast, Pho84p, has an additional role as a low-affinity manganese transporter [10]. Pho84p is responsible for manganese or manganese/phosphate complex uptake under manganese toxicity conditions. Strains containing a deletion in PHO84 exhibit low manganese accumulation and resistance to manganese toxicity [10]. Detoxification of manganese in yeast is largely controlled by Pmr1p, a Golgi-localized P-type ATPase transporter for manganese and calcium [11–13]. This transporter helps to eliminate toxic manganese from the cell by pumping it into the Golgi, where manganese is then exported to the cell surface via the secretory pathway [12,14]. pmr1Δ mutants are highly sensitive to manganese and accumulate high levels of the metal [15]. But PMR1 is also important for normal manganese homoeostasis and is required to deliver manganese, as well as calcium, into the secretory pathway where these ions are required for protein processing and secretion [11–14]. Mutations in the human homologue of PMR1 gene cause Hailey–Hailey disease [16,17], which is an inherited skin blistering disorder.
Protection against manganese toxicity in S. cerevisiae can also be facilitated by sequestration of the metal in the vacuole. The yeast vacuole participates in both the storage and detoxification of manganese and the metal appears concentrated in the vacuole compared with cytosol [18]. Yeast cells with mutations that affect vacuolar structure or H+-ATPase are sensitive to a wide spectrum of metal ions. In addition to manganese [19], zinc [20], cobalt [21] and copper [22,23] are also affected by such mutations.
Golgi and vacuolar sequestration of manganese are effective at guarding against metal toxicity to a certain extent. But under manganese surplus conditions, these detoxification pathways become overwhelmed and metal damage ensues. Other than Pho84p operating at the cell surface, virtually nothing is known regarding the pathways that lead to manganese toxicity under these conditions. To this end, we conducted a search for S. cerevisiae genes which, when deleted, would result in increased resistance to manganese toxicity; such genes should represent factors that contribute to manganese damage. One gene identified in this regard was S. cerevisiae MAM3. Mutations in MAM3 confer very high levels of manganese resistance on yeast and also increase resistance to zinc and cobalt. Mam3p is a member of the ACDP (ancient conserved domain protein) family of proteins that are conserved from bacteria to humans [24]. Human ACDP1 has been linked to a genetic disease UFS (urofacial syndrome) or Ochoa syndrome [25,26], although the function of ACDP1 is not known. We show here that yeast Mam3p functions at the site of the vacuolar membrane to affect manganese toxicity through a novel mechanism that does not involve vacuolar sequestration of the metal or the manganese homoeostasis pathways engaged by Pho84p, Pmr1p or Smf2p. Studies on Mam3p provide the first evidence for a eukaryotic ACDP being involved in metal homoeostasis.
EXPERIMENTAL
Yeast strains, plasmids, media and culture conditions
Most of the strains used in this study were derived from parental strain BY4741 (MATa, leu2Δ0, met15Δ0, ura3Δ0, his3Δ1). The pho84Δ::kanMX4 (6524), pmr1Δ::kanMX4 (4534) and smf2Δ::kanMX4 (1878) mutant derivatives of BY4741 and the homozygous diploid pool of yeast deletion strains were purchased from ResGen. Disruptions of MAM3 in strains BY4741, pho84Δ, pmr1Δ and smf2Δ were generated with the mam3Δ::URA3 plasmid pMY001, resulting in strain MY001 (mam3Δ), MY003 (pho84Δmam3Δ), MY006 (pmr1Δmam3Δ) and MY002 (smf2Δmam3Δ). Gene deletions were verified by PCR. Strain MY010 (Rho−) was obtained by growing BY4741 on enriched YPD (yeast extract and peptone-based medium supplemented with 2% glucose) medium containing ethidium bromide (80 mg/l) and by monitoring for formation of petite colonies that failed to grow on medium containing ethanol and glycerol as carbon sources. The pep4Δ (XL126) and its isogenic wild-type strain YR98 have been described previously [9]. The strains SM2188 (MATa his4 leu2 ura3 bar1-1) and SM2186 (SM2188 end3ts) were gifts from Dr S. Michaelis (Department of Cell Biology, Johns Hopkins University School of Medicine, Baltimore, MD, U.S.A.) [27].
Yeast transformations were performed using the lithium acetate procedure [28]. Cells were propagated at 30 °C (unless specified otherwise) either in an enriched yeast extract, YPD or YPEG (yeast extract and peptone-based medium with 2% ethanol and 2% glycerol), or in SD (minimal synthetic dextrose medium supplemented with the required amino acids).
To construct the MAM3 disruption plasmid pMY001, MAM3 upstream sequences (−853 to −257) and downstream sequences (+2102 to +2701) were amplified by PCR using primers introducing BamHI and HindIII or HindIII and SalI sites respectively. The MAM3 PCR products were digested with the indicated enzymes and ligated in a trimolecular reaction to the BamHI- and SalI-digested URA3 integrating vector pRS306, resulting in pMY001. Transformation of yeast strains with pMY001 linearized with HindIII, resulted in deletion of chromosomal MAM3 sequences from −256 to +2101. Plasmid pMY003 (multicopy MAM3) contains MAM3 sequences −694 to +2701 amplified by PCR and inserted at the BamHI and HindIII sites of pRS425, a LEU2 2-micron based vector. The Mam3–GFP (green fluorescent protein) fusion plasmid pAP003 (given by A. Pledge and E. Luk, Department of Biochemistry and Molecular Biology, Johns Hopkins University Bloomberg School of Public Health, Baltimore, MD, U.S.A.) was constructed in the pAA1-based vector (LEU2-CEN) [29], and contains MAM3 coding sequences and promoter up to −847 fused at the Mam3p C-terminus to GFP. The plasmid pHS12 [30] (COX4–GFP) was a gift from Dr R. Jensen (Department of Cell Biology, Johns Hopkins University School of Medicine, Baltimore, MD, U.S.A.) and is a LEU2-CEN plasmid containing one copy of GFP fused at the N-terminus to the pre-sequence of mitochondria COX4 (cytochrome oxidase subunit IV).
Screen for manganese-resistant mutants of S. cerevisiae
A frozen aliquot of the ResGen homozygous diploid deletion collection pool was thawed and cultured in liquid YPD medium for 5 h to a D600 of 0.5 and spread at a density of 107 cells/plate on to solid YPD medium supplemented with 10 mM MnCl2. After incubating at 30 °C for three days, manganese-resistant colonies were isolated and the genes affected in twenty independent colonies were identified by standard PCR and DNA sequencing analysis [31]. Each colony was subjected to two PCR reactions. The primers used in each reaction were either the pair U1 (5′-GAT GTC CAC GAG GTC TCT-3′) and KanB (5′-CTG CAG CGA GGA GCC GTA AT-3′), giving a 300-bp PCR product, or the pair D1 (5′-CGG TGT CGG TCT CGT AG-3′) and KanD (5′-TCG CCT CGA CAT CAT CTG C-3′), giving a 188-bp PCR product. The unique 20-bp tags integrated into the ORF (open reading frame) disruption cassettes were identified by DNA sequencing analysis of the PCR products (Johns Hopkins Genetic Resources Core Facility) using KanB and KanD as sequencing primers. Gene deletions were identified by matching the sequence results of the tags to the yeast deletion database (http://www-sequence.stanford.edu/group/yeast_deletion_project/deletions3.htm).
Fluorescence microscopy, cell fractionation and immunodetection techniques
For fluorescence microscopy studies, strains BY4741 (wild-type parent), MY001 (mam3Δ), YR98 (PEP4+), XL126 (pep4Δ), SM2186 (end3ts) and SM2188 (END3+) were transformed with pHS12 (COX4–GFP), pAP003 (Mam3–GFP CEN plasmid) or pRS415 (vector control). The transformants were grown under aerobic conditions to a D600 of 1.0 in selective SD medium. Cells were washed or, where indicated, further fixed with 37% formaldehyde and stained with DAPI (4,6-diamidino-2-phenylindole). Cells (live or fixed) were monitored by fluorescence and Normarski DIC (differential interference contrast) microscopy on a Zeiss Axiovert 135TV microscope (microscopy facility, Johns Hopkins Medical Institutions) at a magnification of ×1000.
To monitor the membrane localization of Mam3–GFP, cell lysates were prepared as described previously [32] from 500 ml of cells grown in liquid SD medium to a D600 of 2.0; 1 ml aliquots of cell lysates were supplemented with 0.5 M NaCl, 0.1 M Na2CO3 or 1% Triton X-100, followed by separation of membrane and soluble fractions by centrifugation at 100000 g as described previously [32]. Each pellet fraction was resuspended in 1 ml of lysis buffer (50 mM Tris/HCl, pH 7.5, 200 mM sorbitol, 5 mM MgCl2, 100 mM NaCl, 500 μM PMSF, 1:100 protease inhibitor cocktails) containing 1% Triton X-100. All fractions were then subjected SDS/PAGE (12% gel) and analysed by Western blotting using an antibody directed again GFP (1:1000 dilution; Molecular Probes) and a secondary anti-rabbit IgG (1:125000 dilution; Amersham). Detection employed the enhanced chemiluminescence (ECL®) kit (Amersham) according to the manufacturer's instructions.
Vacuoles were prepared according to published methods [18] from 300 ml of cells grown to a D600 of 2.0 in YPD liquid medium or YPD medium supplemented with MnCl2. Cells were washed twice with TE buffer (10 mM Tris/HCl and 1 mM EDTA, pH 8) and once with de-ionized water prior to generation of spheroplasts and preparation of cell lysates as described previously [18]. Vacuoles were isolated by Ficoll gradient centrifugation [18], and vacuolar purity was monitored by Western blot analysis using antibodies directed against CPY (carboxypeptidase Y) and PGK1 (phosphoglycerate kinase 1), which are markers for the vacuole and cytosol respectively.
Metal measurements
For analysis of metal contents in whole cell, vacuolar or cytosolic fractions, cells were grown in liquid YPD medium, or the same medium supplemented with 500 μM MnCl2 for either 16 or 1.5 h. Analysis of whole-cell manganese content was carried out as described previously [10] using a PerkinElmer Life Sciences AAnalyst 600 graphite furnace atomic absorption spectrophotometer according to manufacturer's instructions. In the case of cell fractions, 0.05–0.15 μg of proteins from vacuolar or cytosolic lysate fractions (generated as described above) were subjected to analysis.
Sequence alignments and topology
Alignments of Mam3p against human ACDP1 (NP_065081), ACDP2 (NP_951058), ACDP3 (NP_06093) and ACDP4 (NP_064569) were obtained using the ClustalW application (http://www.ch.embnet.org/software/ClustalW.html). Topology of Mam3p was predicted using TopPred 2 (http://www.sbc.su.se/~erikw/toppred2).
RESULTS
Manganese resistance in S. cerevisiae is associated with mutations in MAM3, encoding a member of the ACDP family of proteins
To identify yeast mutants that are resistant to manganese toxicity, the ResGen homozygous diploid collection of mutants was screened for mutants that survived on medium supplemented with 10 mM MnCl2. Manganese-resistant mutants occurred at a frequency of approx. 1×10−4. A sampling of these revealed that more than half of the manganese-resistant mutants harbour mutations in S. cerevisiae MAM3.
S. cerevisiae Mam3p is a member of the ACDP family of proteins that contain an ACD (‘ancient conserved domain’) [24]. Whereas S. cerevisiae appears to express a single ACD containing protein (Mam3p), humans express four such proteins (ACDP1–ACDP4) [24].
ACDP proteins have received recent attention because human ACDP1 has been linked to UFS, a rare genetic disorder that affects both facial expression and the urinary tract [33]. The role of human ACDP1 in UFS is not understood, largely because the function of ACDP family members remains elusive. Our identification of S. cerevisiae mam3Δ as a manganese-resistant mutant suggests a possible role for eukaryotic ACDP proteins in metal homoeostasis.
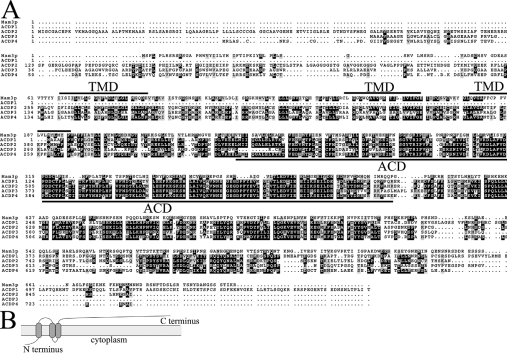
As seen in Figure 1(A), amino acids 241–402 of Mam3p represent the ACD sharing extensive similarity with human ACDPs [24]. Yet the similarity is not limited to this previously reported ACD region, and extends upstream to Mam3p amino acid ∼70. Overall, the region spanning residues 71–402 shares 39% identity and 59% similarity to human ACDP2 and ACDP4, and 27% homology and 47% similarity to ACDP3, whereas Mam3p residues 192–402 share 35% identity and 58% similarity to human ACDP1 (Figure 1A). Mam3p is predicated to harbour three potential transmembrane domains (amino acids 66–86, 144–164 and 177–197) (Figure 1A and illustrated in Figure 1B), suggesting that Mam3p may be a membrane protein. The three potential transmembrane domains are also predicted for human ACDP2–ACDP4.
Figure 1. S. cerevisiae Mam3p is similar to human ACDPs.
(A) The complete amino acid sequence alignments of S. cerevisiae Mam3p compared with human ACDP1, ACDP2, ACDP3 and ACDP4. Three potential transmembrane domains (TMD) of Mam3p and the ACDs are underlined. Sequences were aligned using ClustalW software. Identical residues are highlighted in black and similar residues are outlined in grey. (B) A hydropathy plot of Mam3p predicts three transmembrane domains (amino acids 66–86, 144–164 and 177–197) with the N-terminus predicted to be on the cytosolic face of the membrane (TopPred2 program).
MAM3 gene dosage and resistance to metal toxicity in S. cerevisiae
To confirm that MAM3 alone was responsible for affecting manganese resistance, we engineered a mam3Δ gene deletion in a haploid strain (BY4741), and found that, like the diploid mutants, the mam3Δ haploid was manganese resistant on both minimal medium (Figure 2A) and enriched medium (Figure 2B). Whereas deletion of MAM3 caused manganese resistance, over-expression of MAM3 was associated with increased manganese sensitivity compared with wild-type, as shown in Figure 2(A). There is a good correlation between MAM3 gene dosage and sensitivity towards manganese toxicity.
Figure 2. MAM3 gene dosage affects resistance to a variety of heavy metals.
The indicated strains were tested for growth by applying 105, 104 and 103 cells on (A) SD plates supplemented where indicated with 40 mM MnCl2 (+Mn) or (B) YPD plates supplemented where indicated with the designated concentrations of MnCl2, CoSO4, ZnCl2, or CdCl2. Cells were grown aerobically at 30 °C for 3 days. Strains used: wild-type BY4741 (WT), MY001 (mam3Δ) and BY4741 expressing MAM3 from plasmid pMY003 (WT+2μ MAM3).
We tested whether a mam3Δ mutation affected resistance towards other metal ions. As shown in Figure 2(B), disruption of MAM3 also conferred resistance to cobalt and zinc. However, there was no increased resistance to toxicity from copper (results not shown) and cadmium (Figure 2B). If anything, mam3Δ mutants were associated with increased sensitivity towards cadmium (Figure 2B). Therefore, mam3Δ mutations affect resistance to a specific subclass of heavy metal ions.
mam3Δ mutants are not defective in mitochondrial morphology or function
MAM3 was originally identified as an S. cerevisiae mutant exhibiting mitochondrial aberrant morphology. Through a systemic functional analysis of yeast gene-deletion mutants, mam3Δ mutants were reported to have short and round mitochondria and exhibit growth defects on non-fermentable carbon sources [34]. It was curious as to how a mitochondrial defect of this type could be associated with resistance to metal toxicity.
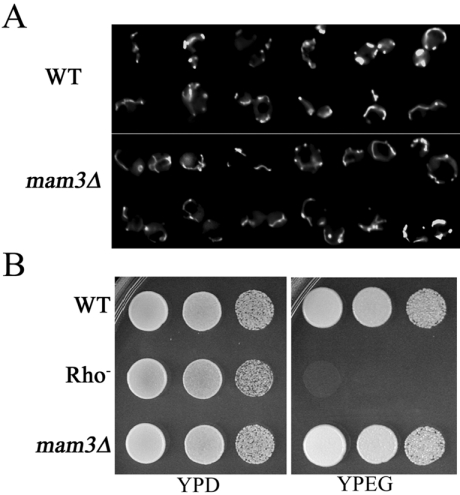
To examine mitochondrial morphology in our mam3Δ::URA3 haploid strain, we employed COX4–GFP as a fluorescence marker for the mitochondria [35]. Contrary to previous reports [34], we failed to detect any major differences in mitochondrial morphology between a mam3Δ mutant and its isogenic wild-type control, and both exhibited long tubular-like mitochondria (Figure 3A). Furthermore, respiratory chain function appears normal in mam3Δ mutants, as these strains exhibited no growth defect on glycerol/ethanol plates (Figure 3B). Therefore, the reported mitochondrial defect of mam3Δ mutants is not reproducible in other strain backgrounds.
Figure 3. mam3Δ mutants exhibit no obvious defect in mitochondrial morphology or function.
(A) Strains BY4741 (WT, wild-type) and MY001 (mam3Δ) transformed with COX4–GFP were prepared and examined by fluorescence microscopy as described in the Experimental section. (B) The indicated strains were tested for growth, as described in Figure 2, on YPD or YPEG plates at 30 °C for 3 days. Strains utilized: wild-type BY4741 (WT), MY010 (Rho−) and MY001 (mam3Δ).
Mam3p is a vacuolar membrane protein
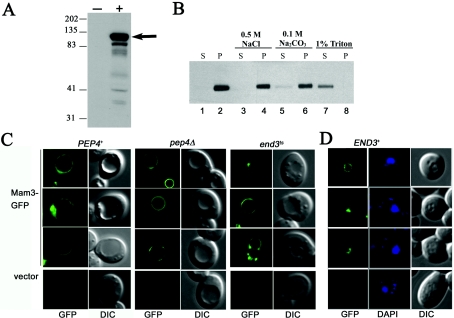
To address the intracellular localization of Mam3p, a Mam3–GFP fusion was created in which one copy of GFP was fused to the soluble C-terminus of Mam3p expressed on a CEN plasmid. This Mam3–GFP chimera was capable of complementing the manganese-resistance phenotype of a mam3Δ mutant (results not shown). Production of Mam3–GFP was analysed by immunoblotting using an anti-GFP antibody. A distinct band of ∼110 kDa was observed in cells expressing Mam3–GFP from the CEN vector, but not in control cells (Figure 4A). The size of this band was equivalent to the predicted molecular mass of the Mam3–GFP fusion protein (Mam3p, 78 kDa; GFP, 27 kDa).
Figure 4. Mam3p is a permanent member of the vacuolar membrane.
(A) Lysate protein (40 μg) prepared from strain BY4741 transformed with vector pRS415 (−) or pAP003 (Mam3–GFP; +) were analysed by Western blotting with a rabbit anti-GFP antibody. The arrow marks the position of the Mam3–GFP fusion protein. Protein molecular-mass markers (in kDa) run in parallel are indicated. (B) Extracts prepared from BY4741 transformed with pAP003 (Mam3–GFP) were treated with 0.5 M NaCl, 0.1 M Na2CO3 or 1% Triton X-100 respectively, followed by centrifugation at 100000 g, as described in the Experimental section. Supernatant and pellet fractions (20 μl) were analysed by Western blotting using an anti-GFP antibody. P, pellet; S, supernatant. (C) Strain PEP4+ (YR98), pep4Δ (XL126) and end3ts (SM2186) transformed with Mam3–GFP or vector were grown in SD medium to a D600 of 1.0, examined as live cells by fluorescence microscopy (×1000 magnification) and viewed by Normarski DIC (differential interference contrast) microscopy. (D) Strains END3+ (SM2188) transformed with Mam3–GFP, were fixed with formaldehyde, stained with DAPI and examined by fluorescence microscopy (×1000 magnification) and viewed by DIC. The images in green correspond to GFP fluorescence, grey are viewed DIC and blue correspond to cells stained with DAPI for DNA.
Since Mam3p is predicted to contain at least three transmembrane domains (Figure 1B), we addressed whether it is an integral membrane protein. After centrifugation of cell lysates at 100000 g, Mam3–GFP fractionated to the pellet containing cellular membranes (Figure 4B, lane 2). Treatment with salts that are known to release peripheral membrane proteins [36] had no effect on this fractionation pattern of Mam3–GFP (Figure 4B, lanes 4 and 6). By comparison, detergent treatments that are known to solubilize integral membrane proteins [36] resulted in release of Mam3–GFP from the membrane fraction (Figure 4B, lane 7). These observations strongly indicate that Mam3p is an integral membrane protein.
To determine the intracellular localization of Mam3p, live cells expressing Mam3–GFP were subjected to fluorescence microscopy. Figure 4(C) (‘GFP’) shows that Mam3–GFP localizes on the periphery/membrane of the vacuole, but not the vacuolar lumen. The vacuole can be visualized as indentions under Nomarski optics (Figure 4C; ‘DIC’). The localization of Mam3p is consistent with a high throughput analysis of yeast protein localization that was published while the present study was in progress [37].
Since Mam3p is localized at the vacuole, we addressed whether the bulk of Mam3–GFP is actually targeted to the vacuolar lumen for degradation. Mam3–GFP was expressed in pep4Δ mutant, in which vacuolar proteases are inactive [38]. An intense lumen staining was not observed (Figure 4C, middle panel). Therefore, Mam3p does not appear to be degraded in the vacuole lumen.
Although there was no obvious cell surface staining of Mam3p, a transient localization was still possible. In yeast, certain plasma membrane proteins, such as Ste6p, are rapidly internalized and recycled through endocytosis [27]. To test the possibility that Mam3p transiently localizes in the plasma membrane, we expressed Mam3–GFP in end3ts mutants, in which endocytosis is blocked at the non-permissive temperature (37 °C). At the non-permissive temperature, end3ts cells expressing Mam3–GFP exhibited no rim staining predicted for a plasma membrane protein (Figure 4C, right-hand panel), whereas the control Ste6–GFP moved to the cell surface under these conditions (results not shown). Thus Mam3p does not reside at the cell surface.
In studies published elsewhere, human ACDPs were reported to be localized in the nucleus [24], although mouse Acdp1 was localized to the plasma membrane [39]. To verify that Mam3p does not localize to the nucleus, cells expressing Mam3–GFP were fixed and permeabilized to enhance staining of the nuclei. As seen in Figure 4(D), there was no overlapping staining of Mam3–GFP with the round nuclei marked by staining with DAPI. Overall, these studies demonstrate that Mam3–GFP is an integral membrane protein of the vacuole.
Metal ion accumulation in mam3Δ mutants
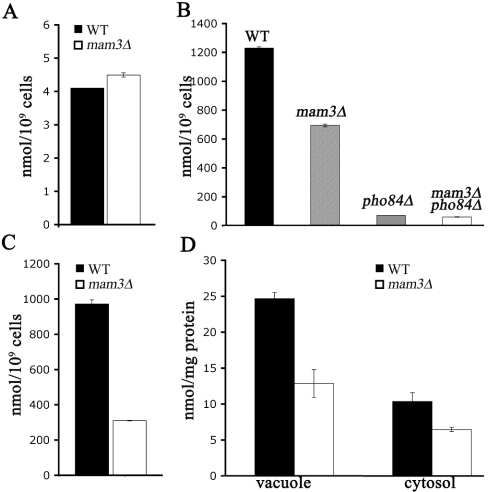
In an attempt to address the mechanism of metal resistance associated with mam3Δ, we analysed metal ion accumulation by atomic absorption spectrophotometry. Under standard growth conditions, we failed to detect any significant changes in total cell accumulation of manganese (Figure 5A), or of cobalt (results not shown). The only effect seen with mam3Δ mutations was a somewhat lowered manganese accumulation under metal toxicity conditions. In the experiment of Figure 5(C), cells were cultured for 16 h in the presence of 500 μM MnCl2, a condition in which growth of wild-type cells was inhibited by approx. 50%. A 2-fold reduction in manganese accumulation was seen in mam3Δ mutants. When cells were treated with manganese for a short period (1.5 h), the effect of mam3Δ mutations seemed somewhat more pronounced (Figure 5B), yet even this decrease in manganese accumulation may not account for the strong resistance to manganese toxicity seen in mam3Δ mutants (see below).
Figure 5. Manganese accumulation in mam3Δ mutant cells.
The indicated strains were grown to mid-log phase in YPD medium without (A) or with (B) supplementation with 500 μM MnCl2. (C, D) Cells were grown as in (A), then supplemented with 500 μM MnCl2 for 1.5 h. (D) Vacuole and crude cytosol fractions were isolated by Ficoll gradient centrifugation. Manganese content of whole cells, isolated vacuole and crude cytosol were monitored by atomic absorption spectroscopy. The results are expressed as the means±S.D. for at least two independent experiments. Strains used: wild-type BY4741 (WT), MY001 (mam3Δ), 6524 (pho84Δ) and MY003 (mam3Δ pho84Δ).
Since Mam3p is vacuolar, and as the vacuole is a known site for metal ion storage and detoxification [18–23,40,41], we tested whether metal accumulation in vacuoles was specifically affected. Vacuoles were isolated from mam3Δ and isogenic wild-type cells by Ficoll gradient centrifugation [18] and analysed for metal contents. Under normal growth conditions, there were no noticeable differences in vacuolar manganese of mam3Δ versus wild-type strains (results not shown). The same was observed for other metals tested, including iron and zinc, as well as copper and sodium (results not shown). Under manganese toxicity conditions, where mam3Δ mutants accumulate lower levels of whole-cell manganese (as in Figures 5B and 5C), the vacuoles likewise contained lower levels of manganese (Figure 5D). However, this same decrease was seen in the cytosol as well (Figure 5D). Therefore, Mam3p does not specifically control vacuolar compartmentalization of metals and may work indirectly to influence manganese toxicity.
Manganese detoxification in mam3Δ mutants is distinct from the well-established Pho84p, Pmr1p and Smf2p pathways
We tested whether Mam3p operates through known pathways for manganese homoeostasis to effect manganese damage. Under manganese toxicity conditions, yeast cells take up manganese by way of the cell surface phosphate and manganese transporter Pho84p [10]. Toxic manganese can be eliminated from the cell by pumping the metal into the secretory pathway via Pmr1p, a Golgi-localized transporter for manganese and calcium [6,14,42]. The high-affinity manganese transport pathway involving Smf1p and Smf2p does not normally contribute to manganese toxicity; however, if these proteins are over-expressed, metal toxicity can ensue [43]. Hence it was possible that Mam3p affects manganese toxicity by having an impact on the metal transport activities of Smf1p/Smf2p, Pmr1p or Pho84p.
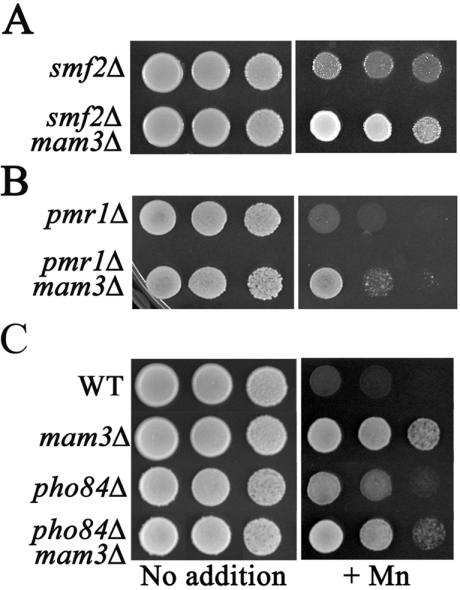
To address this, we tested whether mam3Δ mutations can still confer manganese resistance on yeast strains containing either smf2Δ, pmr1Δ or pho84Δ mutations. As seen in Figure 6, mam3Δ mutations were able to increase manganese resistance in an smf2Δ mutant (Figure 6A) and also helped reverse the very strong manganese sensitivity of a pmr1Δ mutation (Figure 6B). Furthermore, mam3Δ mutations still improved manganese resistance in strains lacking Pho84p (compare the +Mn growth of pho84Δ versus mam3Δ pho84Δ strains, Figure 6C). In agreement with the idea that Mam3p does not control Pho84p, we observed no effect of mam3 mutations on the expression of the PHO84 gene (results not shown) nor on the phosphate transport role of Pho84p, as monitored by polyphosphate accumulation (results not shown). Overall, these genetic studies demonstrate that Mam3p does not operate through Smf2p, Pmr1p or Pho84p to affect manganese toxicity.
Figure 6. Manganese detoxification in mam3Δ mutant is distinct from the well-established Smf2p, Pmr1p and Pho84p pathways.
The indicated strains of yeast were tested for growth as described in Figure 2 on YPD supplemented where indicated with 5.0 mM (A, C) or 100 μM (B) MnCl2 (+Mn). Cells were allowed to grow at 30 °C for 2 days. Strains used: 1878 (smf2Δ); MY002 (smf2Δ mam3Δ); 4534 (pmr1Δ); MY006 (mam3Δ pmr1Δ); wild-type BY4741 (WT); MY001 (mam3Δ); 6524 (pho84Δ); MY003 (mam3Δ pho84Δ).
In the course of these studies, we noted a discordance between manganese resistance and total cellular levels of manganese. For example, when pho84Δ and mam3Δ single mutants were compared, mam3Δ mutants were considerably more manganese resistant (Figure 6C), however, pho84Δ mutants showed much lower cellular accumulation of manganese (Figure 5B). Furthermore, even though mam3Δ pho84Δ mutants were more resistant to manganese toxicity than single pho84Δ mutants (Figure 6C), these double mutants were not further reduced in manganese accumulation (compare pho84Δ with mam3Δ pho84Δ in Figure 5B). The lack of correlation between metal accumulation and metal toxicity indicates that the somewhat lowered manganese accumulation in mam3 mutants (Figures 5B and 5C) is not the underlying basis for metal resistance. In this regard, it is noteworthy that mam3Δ mutants only exhibit reduced manganese accumulation when the wild-type control strain is confronted with manganese toxicity. Hence, the effects seen may represent differences in vigor of the wild-type versus mam3Δ strains under these conditions.
Collectively, these studies demonstrate that S. cerevisiae Mam3p can contribute to manganese toxicity through a pathway that does not involve vacuolar sequestration of metals, Golgi pumping of manganese via Pmr1p or regulated manganese transport by Pho84p and Smf2p. Although the precise mechanism is still elusive, these studies establish a role for a eukaryotic ACDP protein in metal homoeostasis.
DISCUSSION
The goal of this study was to identify factors that contribute to manganese toxicity in a eukaryotic cell. Through a genetic screen, S. cerevisiae mam3 was identified as a mutant conferring strong resistance to manganese toxicity. Mutations in MAM3 also increased cells' tolerance to cobalt and zinc. S. cerevisiae MAM3 is a member of the ACD family of proteins. In humans, ACDP1 has been linked to UFS, a rare genetic disorder affecting facial expression and urinary tract function. The underlying etiology for UFS is completely unknown, largely due to a lack of understanding of ACDP function. An extensive analysis of yeast MAM3 might therefore provide insight as to the role of eukaryotic ACDP proteins.
Mutants of MAM3 (mitochondria aberrant morphology) were first identified on the basis of defects in mitochondrial morphology and respiration [34]. However, such mitochondrial anomalies were not observed in our mam3Δ mutants. The difference may reflect strain backgrounds or possible occurrence of a petite mutation in the previously described mam3Δ mutant [34]. In any case, the metal resistance of mam3 cells cannot be readily attributed to the mitochondria.
S. cerevisiae Mam3p is a vacuolar membrane protein. Murine ACDP1 has also been localized to a membrane, in this case the plasma membrane [39]. Although human ACDPs were first reported to be nuclear [24], it was subsequently suggested that this finding reflected the non-specific nature of the antibody employed [39]. Hence both human and murine ACDP1 are likely to be membrane-associated, even though these particular ACDP family members lack the N-terminal hydrophobic domain of mammalian ACDP2–ACDP4 and of S. cerevisiae Mam3p. Perhaps all ACDPs function at the site of a membrane, as either membrane-associated or as integral membrane proteins.
The yeast vacuole is an important site for the storage and detoxification of essential metals, such as manganese [18,19,40], iron [18,41], zinc [20], cobalt [21] and copper [22,23]. The vacuole also plays a role in detoxifying non-essential toxic metals, such as cadmium [44,45]. Although the spectrum of metals affected by a mam3Δ mutation show some overlap with the aforementioned ions (for example, manganese, cobalt and zinc), not all the vacuolar metals are affected in mam3Δ mutants (for example, copper). In addition, mam3Δ mutants exhibited no noticeable change in vacuolar morphology, vacuolar acidification (results not shown) or vacuolar partitioning of manganese, or of other ions including zinc, iron and sodium (results not shown). It is therefore unlikely that Mam3p has an impact globally on vacuolar function.
One possibility is that Mam3p may be a transporter for the vacuole, but since we detected no change in vacuolar partitioning of metals, Mam3p may instead act on non-metal substrates, such as metal-liganding molecules. Although GSH seems a likely candidate, because GSH–metal conjugates can be transported into the vacuole [44,45], we noted no obvious changes in vacuolar GSH content of mam3Δ mutants (results not shown). Yet it is possible that Mam3p might transport metal binding substrates other than GSH. Mam3p might also facilitate transport of molecules that indirectly affect metals. For example, mitochondrial Yhm1p in yeast has a dramatic impact on iron homoeostasis [46], but is a mitochondrial transporter for GTP/GDP [47]. A similar scenario may be true for Mam3p.
We tested whether MAM3 operates through known metal metabolism pathways to cause manganese toxicity. Our results demonstrate that mutations in MAM3 lead to manganese resistance through mechanisms that do not involve Golgi sequestration of manganese via Pmr1p [11,13] or the uptake of toxic manganese via Pho84p [10]. We have likewise excluded a requirement for the high-affinity manganese transport system involving Smf2p [6]. Hence, the role of Mam3p in manganese metabolism appears unique to previously reported pathways, although the exact mechanism is still unknown. In any case, these studies have exposed a role for a eukaryotic ACDP in metal metabolism.
As further evidence for the role of ACD-containing proteins in metal homoeostasis, a bacterial ACDP-like polypeptide, known as CorC from Salmonella typhimurium [24], has been implicated in magnesium and cobalt homoeostasis [48,49]. Mutations in corC are associated with cobalt resistance [49], however, there is no evidence to date that CorC itself is a metal transporter. Hence, both S. cerevisiae MAM3 and bacteria corC influence metal ion homoeostasis through mechanisms that may not involve direct membrane transport of the ions. We hypothesize that human ACDP1 may function in a similar way in metal metabolism.
Acknowledgments
We thank E. Luk and C. Outten for helpful discussions. This work is supported by the Johns Hopkins University National Institute of Environmental Health Sciences (NIEHS) Center and by the National Institutes of Health grant ES 08996 awarded to V.C.C. M.Y. is supported by NIEHS training grant ES 07141.
References
- 1.Pal P. K., Samii A., Calne D. B. Manganese neurotoxicity: a review of clinical features, imaging and pathology. Neurotoxicology. 1999;20:227–238. [PubMed] [Google Scholar]
- 2.Kaiser J. Manganese: a high-octane dispute. Science. 2003;300:926–928. doi: 10.1126/science.300.5621.926. [DOI] [PubMed] [Google Scholar]
- 3.Luk E., Jensen L. T., Culotta V. C. The many highways for intracellular trafficking of metals. J. Biol. Inorg. Chem. 2003;8:803–809. doi: 10.1007/s00775-003-0482-3. [DOI] [PubMed] [Google Scholar]
- 4.Supek F., Supekova L., Nelson H., Nelson N. A yeast manganese transporter related to the macrophage protein involved in conferring resistance to mycobacteria. Proc. Natl. Acad. Sci. U.S.A. 1996;93:5105–5110. doi: 10.1073/pnas.93.10.5105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu X. F., Culotta V. C. Post-translation control of Nramp metal transport in yeast. Role of metal ions and the BSD2 gene. J. Biol. Chem. 1999;274:4863–4868. doi: 10.1074/jbc.274.8.4863. [DOI] [PubMed] [Google Scholar]
- 6.Luk E. E., Culotta V. C. Manganese superoxide dismutase in Saccharomyces cerevisiae acquires its metal co-factor through a pathway involving the Nramp metal transporter, Smf2p. J. Biol. Chem. 2001;276:47556–47562. doi: 10.1074/jbc.M108923200. [DOI] [PubMed] [Google Scholar]
- 7.Liu X. F., Culotta V. C. Mutational analysis of Saccharomyces cerevisiae Smf1p, a member of the Nramp family of metal transporters. J. Mol. Biol. 1999;289:885–891. doi: 10.1006/jmbi.1999.2815. [DOI] [PubMed] [Google Scholar]
- 8.Hettema E. H., Valdez-Taubas J., Pelham H. R. Bsd2 binds the ubiquitin ligase Rsp5 and mediates the ubiquitination of transmembrane proteins. EMBO J. 2004;23:1279–1288. doi: 10.1038/sj.emboj.7600137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Portnoy M. E., Liu X. F., Culotta V. C. Saccharomyces cerevisiae expresses three functionally distinct homologues of the nramp family of metal transporters. Mol. Cell. Biol. 2000;20:7893–7902. doi: 10.1128/mcb.20.21.7893-7902.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jensen L. T., Ajua-Alemanji M., Culotta V. C. The Saccharomyces cerevisiae high affinity phosphate transporter encoded by PHO84 also functions in manganese homeostasis. J. Biol. Chem. 2003;278:42036–42040. doi: 10.1074/jbc.M307413200. [DOI] [PubMed] [Google Scholar]
- 11.Antebi A., Fink G. R. The yeast Ca2+-ATPase homologue, PMR1, is required for normal Golgi function and localizes in a novel Golgi-like distribution. Mol. Biol. Cell. 1992;3:633–654. doi: 10.1091/mbc.3.6.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Durr G., Strayle J., Plemper R., Elbs S., Klee S. K., Catty P., Wolf D. H., Rudolph H. K. The medial-Golgi ion pump Pmr1 supplies the yeast secretory pathway with Ca2+ and Mn2+ required for glycosylation, sorting, and endoplasmic reticulum-associated protein degradation. Mol. Biol. Cell. 1998;9:1149–1162. doi: 10.1091/mbc.9.5.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rudolph H. K., Antebi A., Fink G. R., Buckley C. M., Dorman T. E., LeVitre J., Davidow L. S., Mao J. I., Moir D. T. The yeast secretory pathway is perturbed by mutations in PMR1, a member of a Ca2+ ATPase family. Cell. 1989;58:133–145. doi: 10.1016/0092-8674(89)90410-8. [DOI] [PubMed] [Google Scholar]
- 14.Mandal D., Woolf T. B., Rao R. Manganese selectivity of pmr1, the yeast secretory pathway ion pump, is defined by residue Gln783 in transmembrane segment 6. Residue Asp778 is essential for cation transport. J. Biol. Chem. 2000;275:23933–23938. doi: 10.1074/jbc.M002619200. [DOI] [PubMed] [Google Scholar]
- 15.Lapinskas P. J., Cunningham K. W., Liu X. F., Fink G. R., Culotta V. C. Mutations in PMR1 suppress oxidative damage in yeast cells lacking superoxide dismutase. Mol. Cell. Biol. 1995;15:1382–1388. doi: 10.1128/mcb.15.3.1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sudbrak R., Brown J., Dobson-Stone C., Carter S., Ramser J., White J., Healy E., Dissanayake M., Larregue M., Perrussel M., et al. Hailey–Hailey disease is caused by mutations in ATP2C1 encoding a novel Ca2+ pump. Hum. Mol. Genet. 2000;9:1131–1140. doi: 10.1093/hmg/9.7.1131. [DOI] [PubMed] [Google Scholar]
- 17.Ton V. K., Mandal D., Vahadji C., Rao R. Functional expression in yeast of the human secretory pathway Ca2+, Mn2+-ATPase defective in Hailey–Hailey disease. J. Biol. Chem. 2002;277:6422–6427. doi: 10.1074/jbc.M110612200. [DOI] [PubMed] [Google Scholar]
- 18.Li L., Chen O. S., McVey Ward D., Kaplan J. CCC1 is a transporter that mediates vacuolar iron storage in yeast. J. Biol. Chem. 2001;276:29515–29519. doi: 10.1074/jbc.M103944200. [DOI] [PubMed] [Google Scholar]
- 19.Ramsay L. M., Gadd G. M. Mutants of Saccharomyces cerevisiae defective in vacuolar function confirm a role for the vacuole in toxic metal ion detoxification. FEMS Microbiol. Lett. 1997;152:293–298. doi: 10.1111/j.1574-6968.1997.tb10442.x. [DOI] [PubMed] [Google Scholar]
- 20.MacDiarmid C. W., Milanick M. A., Eide D. J. Induction of the ZRC1 metal tolerance gene in zinc-limited yeast confers resistance to zinc shock. J. Biol. Chem. 2003;278:15065–15072. doi: 10.1074/jbc.M300568200. [DOI] [PubMed] [Google Scholar]
- 21.Conklin D. S., Culbertson M. R., Kung C. Interactions between gene products involved in divalent cation transport in Saccharomyces cerevisiae. Mol. Gen. Genet. 1994;244:303–311. doi: 10.1007/BF00285458. [DOI] [PubMed] [Google Scholar]
- 22.Szczypka M. S., Zhu Z., Silar P., Thiele D. J. Saccharomyces cerevisiae mutants altered in vacuole function are defective in copper detoxification and iron-responsive gene transcription. Yeast. 1997;13:1423–1435. doi: 10.1002/(SICI)1097-0061(199712)13:15<1423::AID-YEA190>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 23.Eide D. J., Bridgham J. T., Zhao Z., Mattoon J. R. The vacuolar H+-ATPase of Saccharomyces cerevisiae is required for efficient copper detoxification, mitochondrial function, and iron metabolism. Mol. Gen. Genet. 1993;241:447–456. doi: 10.1007/BF00284699. [DOI] [PubMed] [Google Scholar]
- 24.Wang C. Y., Shi J. D., Yang P., Kumar P. G., Li Q. Z., Run Q. G., Su Y. C., Scott H. S., Kao K. J., She J. X. Molecular cloning and characterization of a novel gene family of four ancient conserved domain proteins (ACDP) Gene. 2003;306:37–44. doi: 10.1016/s0378-1119(02)01210-6. [DOI] [PubMed] [Google Scholar]
- 25.Wang C. Y., Davoodi-Semiromi A., Shi J. D., Yang P., Huang Y. Q., Agundez J. A., Moran J. M., Ochoa B., Hawkins-Lee B., She J. X. High resolution mapping and mutation analyses of candidate genes in the urofacial syndrome (UFS) critical region. Am. J. Med. Genet. 2003;119A:9–14. doi: 10.1002/ajmg.a.20042. [DOI] [PubMed] [Google Scholar]
- 26.Wang C. Y., Shi J. D., Huang Y. Q., Cruz P. E., Ochoa B., Hawkins-Lee B., Davoodi-Semiromi A., She J. X. Construction of a physical and transcript map for a 1-Mb genomic region containing the urofacial (Ochoa) syndrome gene on 10q23-q24 and localization of the disease gene within two overlapping BAC clones (<360 kb) Genomics. 1999;60:12–19. doi: 10.1006/geno.1999.5908. [DOI] [PubMed] [Google Scholar]
- 27.Paddon C., Loayza D., Vangelista L., Solari R., Michaelis S. Analysis of the localization of STE6/CFTR chimeras in a Saccharomyces cerevisiae model for the cystic fibrosis defect CFTRΔF508. Mol. Microbiol. 1996;19:1007–1017. doi: 10.1046/j.1365-2958.1996.444973.x. [DOI] [PubMed] [Google Scholar]
- 28.Gietz R. D., Schiestl R. H. Applications of high efficiency lithium acetate transformation of intact yeast cells using single-stranded nucleic acids as carrier. Yeast. 1991;7:253–263. doi: 10.1002/yea.320070307. [DOI] [PubMed] [Google Scholar]
- 29.Hobbs A. E., Srinivasan M., McCaffery J. M., Jensen R. E. Mmm1p, a mitochondrial outer membrane protein, is connected to mitochondrial DNA (mtDNA) nucleoids and required for mtDNA stability. J. Cell Biol. 2001;152:401–410. doi: 10.1083/jcb.152.2.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sesaki H., Jensen R. E. Division versus fusion: Dnm1p and Fzo1p antagonistically regulate mitochondrial shape. J. Cell Biol. 1999;147:699–706. doi: 10.1083/jcb.147.4.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eason R. G., Pourmand N., Tongprasit W., Herman Z. S., Anthony K., Jejelowo O., Davis R. W., Stolc V. Characterization of synthetic DNA bar codes in Saccharomyces cerevisiae gene-deletion strains. Proc. Natl. Acad. Sci. U.S.A. 2004;101:11046–11051. doi: 10.1073/pnas.0403672101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin S. J., Culotta V. C. Suppression of oxidative damage by Saccharomyces cerevisiae ATX2, which encodes a manganese-trafficking protein that localizes to Golgi-like vesicles. Mol. Cell. Biol. 1996;16:6303–6312. doi: 10.1128/mcb.16.11.6303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ochoa B. Can a congenital dysfunctional bladder be diagnosed from a smile? The Ochoa syndrome updated. Pediatr. Nephrol. 2004;19:6–12. doi: 10.1007/s00467-003-1291-1. [DOI] [PubMed] [Google Scholar]
- 34.Entian K. D., Schuster T., Hegemann J. H., Becher D., Feldmann H., Guldener U., Gotz R., Hansen M., Hollenberg C. P., Jansen G., et al. Functional analysis of 150 deletion mutants in Saccharomyces cerevisiae by a systematic approach. Mol. Gen. Genet. 1999;262:683–702. doi: 10.1007/pl00013817. [DOI] [PubMed] [Google Scholar]
- 35.Jensen R. E., Hobbs A. E., Cerveny K. L., Sesaki H. Yeast mitochondrial dynamics: fusion, division, segregation, and shape. Microsc. Res. Tech. 2000;51:573–583. doi: 10.1002/1097-0029(20001215)51:6<573::AID-JEMT7>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 36.Feldheim D., Rothblatt J., Schekman R. Topology and functional domains of Sec63p, an endoplasmic reticulum membrane protein required for secretory protein translocation. Mol. Cell. Biol. 1992;12:3288–3296. doi: 10.1128/mcb.12.7.3288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huh W. K., Falvo J. V., Gerke L. C., Carroll A. S., Howson R. W., Weissman J. S., O'shea E. K. Global analysis of protein localization in budding yeast. Nature (London) 2003;425:686–691. doi: 10.1038/nature02026. [DOI] [PubMed] [Google Scholar]
- 38.Jones E. W. Three proteolytic systems in the yeast Saccharomyces cerevisiae. J. Biol. Chem. 1991;266:7963–7966. [PubMed] [Google Scholar]
- 39.Wang C. Y., Yang P., Shi J. D., Purohit S., Guo D., An H., Gu J. G., Ling J., Dong Z., She J. X. Molecular cloning and characterization of the mouse Acdp gene family. BMC Genomics. 2004;5:7. doi: 10.1186/1471-2164-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Paidhungat M., Garrett S. Cdc1 and the vacuole coordinately regulate Mn2+ homeostasis in the yeast Saccharomyces cerevisiae. Genetics. 1998;148:1787–1798. doi: 10.1093/genetics/148.4.1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Davis-Kaplan S. R., Ward D. M., Shiflett S. L., Kaplan J. Genome-wide analysis of iron-dependent growth reveals a novel yeast gene required for vacuolar acidification. J. Biol. Chem. 2004;279:4322–4329. doi: 10.1074/jbc.M310680200. [DOI] [PubMed] [Google Scholar]
- 42.Sorin A., Rosas G., Rao R. PMR1, a Ca2+-ATPase in yeast Golgi, has properties distinct from sarco/endoplasmic reticulum and plasma membrane calcium pumps. J. Biol. Chem. 1997;272:9895–9901. doi: 10.1074/jbc.272.15.9895. [DOI] [PubMed] [Google Scholar]
- 43.Liu X. F., Supek F., Nelson N., Culotta V. C. Negative control of heavy metal uptake by the Saccharomyces cerevisiae BSD2 gene. J. Biol. Chem. 1997;272:11763–11769. doi: 10.1074/jbc.272.18.11763. [DOI] [PubMed] [Google Scholar]
- 44.Li Z. S., Lu Y. P., Zhen R. G., Szczypka M., Thiele D. J., Rea P. A. A new pathway for vacuolar cadmium sequestration in Saccharomyces cerevisiae: YCF1-catalyzed transport of bis(glutathionato)cadmium. Proc. Natl. Acad. Sci. U.S.A. 1997;94:42–47. doi: 10.1073/pnas.94.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li Z. S., Szczypka M., Lu Y. P., Thiele D. J., Rea P. A. The yeast cadmium factor protein (YCF1) is a vacuolar glutathione S-conjugate pump. J. Biol. Chem. 1996;271:6509–6517. doi: 10.1074/jbc.271.11.6509. [DOI] [PubMed] [Google Scholar]
- 46.Lesuisse E., Lyver E. R., Knight S. A., Dancis A. Role of YHM1, encoding a mitochondrial carrier protein, in iron distribution of yeast. Biochem. J. 2004;378:599–607. doi: 10.1042/BJ20031387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vozza A., Blanco E., Palmieri L., Palmieri F. Identification of the mitochondrial GTP/GDP transporter in Saccharomyces cerevisiae. J. Biol. Chem. 2004;279:20850–20857. doi: 10.1074/jbc.M313610200. [DOI] [PubMed] [Google Scholar]
- 48.Smith R. L., Banks J. L., Snavely M. D., Maguire M. E. Sequence and topology of the CorA magnesium transport systems of Salmonella typhimurium and Escherichia coli. Identification of a new class of transport protein. J. Biol. Chem. 1993;268:14071–14080. [PubMed] [Google Scholar]
- 49.Gibson M. M., Bagga D. A., Miller C. G., Maguire M. E. Magnesium transport in Salmonella typhimurium: the influence of new mutations conferring Co2+ resistance on the CorA Mg2+ transport system. Mol. Microbiol. 1991;5:2753–2762. doi: 10.1111/j.1365-2958.1991.tb01984.x. [DOI] [PubMed] [Google Scholar]