Abstract
The oxidation of polyamines induced by antitumour polyamine analogues has been associated with tumour response to specific agents. The human spermine oxidase, SMO(PAOh1), is one enzyme that may play a direct role in the cellular response to the antitumour polyamine analogues. In the present study, the induction of SMO(PAOh1) enzyme activity by CPENSpm [N1-ethyl-N11-(cyclopropyl)methyl-4,8,diazaundecane] is demonstrated to be a result of newly synthesized mRNA and protein. Inhibition of new RNA synthesis by actinomycin D inhibits both the appearance of SMO(PAOh1) mRNA and enzyme activity. Similarly, inhibition of newly synthesized protein with cycloheximide prevents analogue-induced enzyme activity. Half-life determinations indicate that stabilization of SMO(PAOh1) protein does not play a significant role in analogue-induced activity. However, half-life experiments using actinomycin D indicate that CPENSpm treatment not only increases mRNA expression, but also leads to a significant increase in mRNA half-life (17.1 and 8.8 h for CPENSpm-treated cells and control respectively). Using reporter constructs encompassing the SMO(PAOh1) promoter region, a 30–90% increase in transcription is observed after exposure to CPENSpm. The present results are consistent with the hypothesis that analogue-induced expression of SMO(PAOh1) is a result of increased transcription and stabilization of SMO(PAOh1) mRNA, leading to increased protein production and enzyme activity. These data indicate that the major level of control of SMO(PAOh1) expression in response to polyamine analogues exposure is at the level of mRNA.
Keywords: N1-acetylpolyamine oxidase (PAO), hydrogen peroxide (H2O2), polyamine, reactive oxygen species, spermidine/spermine N1-acetyltransferase (SSAT), spermine oxidase [SMO(PAOh1)]
Abbreviations: CPENSpm, N1-ethyl-N11-(cyclopropyl)methyl-4,8,diazaundecane; PAO, N1-acetylpolyamine oxidase; SMO(PAOh1), human spermine oxidase; SSAT, spermidine/spermine N1-acetyltransferase
INTRODUCTION
The role of polyamine catabolism in determining the response of tumour cells to specific antitumour polyamine analogues has come under intense scrutiny since the finding that rapid and profound up-regulation of SSAT (spermidine/spermine N1-acetyltransferase) was associated with a cytotoxic response [1–8]. Interest was increased further with the discovery that a previously unrecognized mammalian spermine oxidase, SMO(PAOh1), is also inducible by many of the same polyamine analogues as SSAT [9–12]. These recent results demonstrate that two catabolic pathways exist, each producing toxic ROS (reactive oxygen species). One pathway produces H2O2 and 3-acetamidopropanal through the two-step process regulated by SSAT and PAO (N1-acetylpolyamine oxidase) [13–16], and the other produces H2O2 and 3-aminopropanal through the direct oxidation of spermine by SMO(PAOh1) [15,17]. Therefore both pathways have the potential to directly affect the cellular response to agents that induce the polyamine catabolic pathway.
The regulation of the two-step SSAT/PAO catabolic pathway has been studied extensively. In most cases, PAO is expressed as a constitutive enzyme that is limited by the availability of its acetylated substrate. Even in cases where it has been reported that PAO levels are inducible, the enzyme is still dependent on the activity of SSAT to provide substrate [13,15]. In the case of SSAT, it has been demonstrated that the gene is regulated extensively at both the transcriptional and post-transcriptional levels [18–23]. The promoter region of SSAT has been well defined, and some of the factors that control its transcription in response to inducing agents have been described previously [24–26]. However, little is currently known about the regulation of the two oxidases, PAO and SMO(PAOh1). In the present paper, data are provided indicating that SMO(PAOh1) is first regulated at the level of new mRNA synthesis, and that newly synthesized protein is required for the observed increase in activity in response to exposure to polyamine analogues. Unlike SSAT that is regulated at multiple levels, the current data indicate that the induction of SMO(PAOh1) by polyamine analogues is primarily the result of increased mRNA production, thus underscoring the multiple levels of control at which polyamines and analogues can affect the expression of polyamine-responsive genes.
EXPERIMENTAL
Chemicals
α-32P]dCTP was purchased from Amersham Biosciences (Piscataway, NJ, U.S.A.). CPENSpm [N1-ethyl-N11-(cyclopropyl)-methyl-4,8,diazaundecane] was synthesized as reported previously [27]. The luciferase assay system was from Promega (Madison, WI, U.S.A.). The Gal-XE chemiluminescent reporter gene assay system was purchased from ICN Pharmaceuticals (Cosa Mesa, CA, U.S.A.). HotStartTaq DNA polymerase was from Qiagen (Valencia, CA, U.S.A.). The TA cloning kit, Gene-Racer kit, LIPOFECTAMINE and TRIzol® total RNA reagent were from Invitrogen (Carlsbad, CA, U.S.A.). Actinomycin D, cycloheximide and spermine were from Sigma–Aldrich (St. Louis, MO, U.S.A.). Restriction and modifying enzymes were obtained from New England Biolabs (Beverly, MA, U.S.A.), Invitrogen and Sigma–Aldrich. The primers used were synthesized by Invitrogen. DNA sequencing was performed using a PerkinElmer ABI automated DNA sequencer. Other chemicals were from Bio-Rad (Hercules, CA, U.S.A.) and J. T. Baker (Phillipsburg, NJ, U.S.A.).
Cell Culture
The human non-small-cell lung carcinoma line A549 was maintained in culture as reported previously [28] in RPMI 1640 medium, supplemented with 9% (v/v) iron-supplemented calf serum, 100 units/ml penicillin and 100 units/ml streptomycin.
Northern blot analysis
Total cellular RNA was extracted from A549 cells with TRIzol® RNA reagent according to the provided protocol. RNA samples, 15 μg for each lane, were separated by 1.5% agarose/formaldehyde gel electrophoresis, transferred on to Zetaprobe membranes (Bio-Rad) and UV-cross-linked. The membrane was hybridized to a random-primer-labelled probe specific for the SMO(PAOh1) cDNA, and probed by 18 S ribosomal cDNA as a loading control. Results were quantified by PhosphorImage analysis performed on a Molecular Dynamics PhosphorImager (Sunnyvale, CA, U.S.A.) using ImageQuant software.
Determination of SMO(PAOh1) enzyme activity
SMO(PAOh1) activity in the cell lysate was determined by measuring the production of H2O2 upon the oxidation of spermine by SMO(PAOh1) as reported previously [17]. Briefly, enzyme activity was assayed in 83 mM glycine buffer, pH 8.0, 5 nmol of luminol, 20 μg/ml of horseradish peroxidase, 0.2 mM 2-bromoethylamine (catalase inhibitor), 15 μM deprenyl (copper-containing amine oxidase inhibitor), 0.15 μM clorgyline (mitochondrial oxidase inhibitor) and 250 μM spermine as the substrate. Where indicated, 250 μM N1-acetylspermine was used in place of spermine to determine oxidase activity attributable to PAO. All reagents, with the exception of substrate, were combined in a volume of 250 μl and incubated for 2 min at 37 °C, transferred to the luminometer where spermine was added, and the resulting chemiluminescence was integrated over 40 s.
Inhibition of newly synthesized SMO(PAOh1) protein and mRNA
A549 cells were exposed to 10 μM CPENSpm for the indicated times in the presence or absence of either 5 μg/ml actinomycin D, to inhibit RNA synthesis, or 100 μg/ml of cycloheximide, to inhibit protein synthesis. After treatment, total cellular RNA and protein were analysed for the expression of SMO(PAOh1) mRNA and oxidase activity.
Determination of SMO(PAOh1) mRNA and protein half-lives
For the determination of SMO(PAOh1) mRNA half-life, A549 cells were treated either with 5 μg/ml actinomycin D alone or a combination of 10 μM CPENSpm and 5 μg/ml actinomycin D for up to 18 h. At the indicated times, total cellular RNA was prepared and analysed by Northern Blot analysis. For determination of SMO(PAOh1) activity half-life, A549 cells were treated identically with those for mRNA half-life, with the exception that 100 μg/ml cycloheximide was used in place of actinomycin D. At the indicated times, samples were harvested for SMO(PAOh1) activity analysis. For both mRNA and oxidase activity, least-squares best-fit linear-regression analysis was used to determine half-life estimates (Prism software, GraphPad, San Diego, CA, U.S.A.).
Construction of luciferase reporter constructs
A genomic DNA plasmid library was constructed from A549 genomic DNA in the pBuescript SK(−) plasmid and screened with a cDNA probe homologous to exon 1 of the SMO(PAOh1) gene. From this library, a clone containing −4479 bp 5′ to the transcriptional start site was identified. From this clone, representative serial deletion constructs corresponding to −1117, −610, −126 and −55 bp were generated by restriction enzyme digestion, and subcloned into the pGL-2 basic vector.
Transient transfection assays
For transient transfection, 5×105 A549 cells were cultured in a 35-mm-diameter culture dish in RPMI 1640 medium for 24 h. LIPOFECTAMINE-mediated co-transfections were performed with 2 μg of SMO(PAOh1) promoter-containing reporter construct DNA and 0.2 μg of pSV-β-gal (Promega), according to the supplied protocol. After 5 h of incubation, the medium was replaced by serum-supplemented RPMI 1640 medium. Where indicated, 10 μM CPENSpm was added to the medium at 24 h post-transfection. Cell lysates were prepared 48 h post-transfection for luciferase activity analysis. Luciferase activity was normalized to β-galactosidase activity to account for variation in transfectional efficiency.
RESULTS
CPENSpm-induced expression of SMO(PAOh1) requires new protein and RNA synthesis
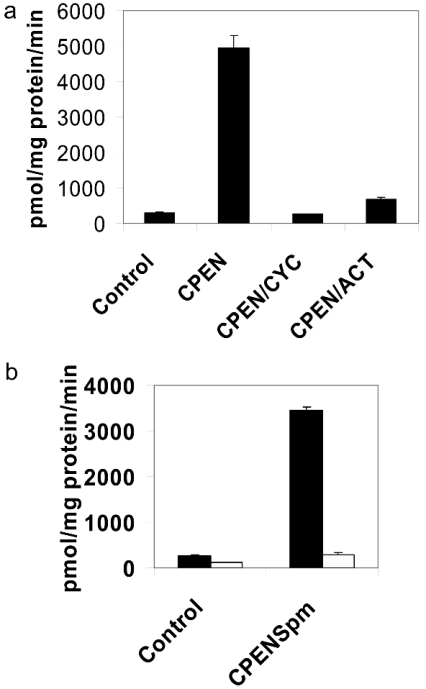
The induction of SMO(PAOh1) expression by antitumour polyamine analogues has been reported in multiple tumour cell lines, and is implicated in determining tumour cell sensitivity to specific antitumour polyamine analogues [9,11,12]. To evaluate the molecular mechanism of polyamine-analogue-induced expression of SMO(PAOh1), we first studied the requirement of newly synthesized mRNA and protein for the observed increase in SMO(PAOh1) expression in response to analogue exposure (Figure 1a). Exposing A549 cells to 10 μM CPENSpm resulted in an increase in SMO(PAOh1) activity >16-fold by 24 h. However, since it has been reported that the classical N1-acetylpolyamine oxidase is both inducible and capable of oxidizing spermine [13,15,16,29–31], it was important to demonstrate that the observed increase in oxidase activity is in fact due to SMO(PAOh1). After exposing A549 cells to 10 μM CPENSpm, cell extracts were prepared for analysis using either spermine or N1-acetylspermine as the substrate (Figure 1b). When spermine was used as the substrate with extracts from CPENSpm-treated cells, the oxidase activity measured was substantially greater than when N1-acetylspermine was used as the substrate (3448 compared with 296 pmol/mg of protein per min), indicating that the overwhelming majority of oxidase activity measured in the CPENSpm-treated cells is attributable to SMO(PAOh1) and not PAO.
Figure 1. Newly synthesized mRNA and protein are required for CPENSpm-induced SMO(PAOh1) activity in A549 human adenocarcinoma cells.
(a) The combination of either the RNA synthesis inhibitor, actinomycin D (ACT, 5 μg/ml), or the protein synthesis inhibitor, cycloheximide (CYC, 100 μg/ml), significantly reduces the CPENSpm (10 μM)-induced increase in SMO(PAOh1) activity after 24 h of co-treatment. Results are means±S.E.M. of four separate experiments performed in triplicate. (b) The oxidase activity induced by CPENSpm is predominately SMO(PAOh1) and not PAO. A549 cells were treated with 10 μM CPENSpm for 24 h, and oxidase activity of treated cell extracts was measured using 250 μM of either spermine (closed bars) or N1-acetylspermine (open bars). Results are means±S.D. from a representative experiment performed in triplicate.
To determine if newly synthesized SMO(PAOh1) protein was required for the observed increase of enzyme activity in analogue-exposed cells, cycloheximide was used to inhibit new protein synthesis in CPENSpm-treated cells. When cells were treated simultaneously with cycloheximide, the CPENSpm-dependent induction of SMO(PAOh1) was prevented (Figure 1a).
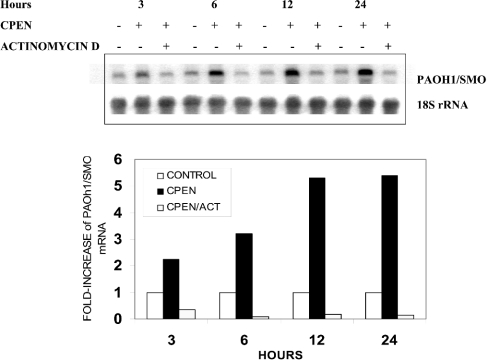
Similar to the results with cycloheximide, the increase in SMO(PAOh1) activity was almost entirely prevented when the cells were co-treated with actinomycin D. That the reduced activity is a result of inhibition of SMO(PAOh1) mRNA synthesis by actinomycin D is clearly demonstrated by the nearly complete inhibition of induced SMO(PAOh1) mRNA when A549 cells are co-treated with CPENSpm and actinomycin D (Figure 2). This treatment blocked the 5-fold induction of steady-state SMO(PAOh1) mRNA observed when cells were treated with CPENSpm alone. These results are consistent with the hypothesis that increased SMO(PAOh1) activity in response to analogue exposure is dependent on newly synthesized mRNA.
Figure 2. Simultaneous treatment of A549 cells with actinomycin D completely prevents the CPENSpm-dependent induction of SMO(PAOh1) mRNA.
A549 cells were treated for the indicated times with 10 μM CPENSpm (CPEN) in the presence or absence of 5 μg/ml actinomycin D (ACT). The results are from a representative experiment. The fold increase in SMO(PAOh1) mRNA is normalized to the 18 S rRNA loading control.
Consequently, the results of experiments with both actinomycin D and cycloheximide indicate that analogue-induced SMO(PAOh1) activity is dependent on both new mRNA and protein synthesis.
Increases in SMO(PAOh1) activity is not a result of post-translational stabilization of oxidase protein
Stabilization of SSAT protein in cells exposed to polyamine analogues is one of the major levels of control of SSAT in responsive cells [19,21]. To determine if SMO(PAOh1) was similarly regulated, half-life experiments were performed to determine whether exposure of A549 cells to CPENSpm altered the stability of SMO(PAOh1) protein. Cells were exposed to 100 μg/ml cycloheximide alone, or in combination with 10 μM CPENSpm, and oxidase activity was monitored for 24 h. In both cases, the half-life of SMO(PAOh1) activity was determined to be approx. 13.5 h, indicating that stabilization of protein does not play a major role in the up-regulation of SMO(PAOh1) activity in analogue-treated cells.
Exposure of A549 cells to CPENSpm increases the half-life of SMO(PAOh1) mRNA
In addition to increasing protein half-life, analogue exposure has been demonstrated to increase the half-life of SSAT mRNA, leading to post-transcriptional increases in SSAT mRNA [18]. This increase in mRNA half-life plays a significant role in the superinduction of SSAT expression in analogue-responsive cells. To determine whether a similar increase in SMO(PAOh1) mRNA stability is observed in response to CPENSpm exposure, the potential for analogue-induced changes in mRNA half-life was determined.
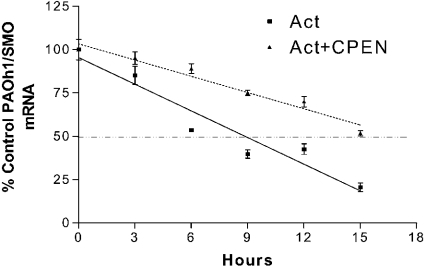
Treatment with 10 μM CPENSpm resulted in an increase of SMO(PAOh1) mRNA half-life from 8.8 h to 17.1 h (Figure 3). This near doubling in half-life suggests that stabilization of SMO(PAOh1) mRNA plays a significant role in CPENSpm-induced SMO(PAOh1) activity.
Figure 3. CPENSpm treatment increases the half-life of SMO(PAOh1) mRNA.
A549 cells were treated with 5 μg/ml actinomycin D (Act) alone or in combination with 10 μM CPENSpm (CPEN) for the indicated times. Results are means±S.D. from a representative experiment where Northern blot samples were run in triplicate, and specific SMO(PAOh1) mRNA expression was normalized to an 18 S rRNA loading. The mRNA half-life was calculated by linear regression analysis to be 8.8 and 17.1 h for actinomycin D alone and actinomycin D plus CPENSpm respectively. Note that the broken line parallel to the axis represents 50% decay.
CPENSpm exposure increases transcription of SMO(PAOh1) promoter constructs
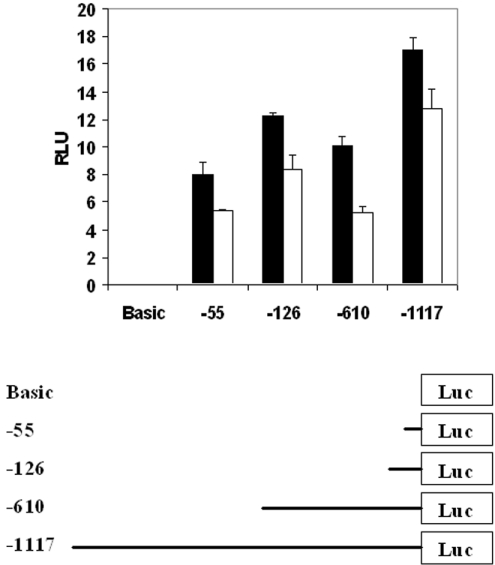
SMO(PAOh1) mRNA increases 2-fold within 3 h, and up to 5-fold within 12 h; increases that are blocked by actinomycin D inhibition of RNA synthesis. The increase in SMO(PAOh1) mRNA half-life may not fully account for the timing or magnitude of the observed increases in mRNA levels. Consequently, increased transcription of the SMO(PAOh1) is implicated in the CPENSpm treatment-dependent increase in steady-state mRNA. To test this possibility, luciferase reporter constructs containing regions of the SMO(PAOh1) promoter were transfected into A549 cells to analyse their response to CPENSpm treatment. The results indicate clearly (Figure 4) that the constructs ranging from −55 to −1117 bp relative to the transcription start site respond with modest, but significant, increases in expression in response to CPENSpm exposure. These increases, ranging from ~33 to 92%, when combined with the increase of mRNA half-life, are sufficient to account for the observed increases in steady-state mRNA. Since the analogue-induced expression of the reporter construct occurs with each of the constructs −1117 bp and shorter, these results also indicate that the response of the SMO(PAOh1) gene to analogue exposure may be under the complex control of multiple transcription elements, rather than a single responsive element.
Figure 4. CPENSpm exposure increases transcription of SMO(PAOh1) promoter constructs in A549 cells.
Serial-deleted SMO(PAOh1) promoter plasmids (2 μg for each) were co-transfected with pSV-β-gal (0.2 μg) into A549 cells. The cell lysate was prepared 48 h after transfection (control, open bars) and 24 h after exposure to 10 μM CPENSpm (closed bars), where indicated. The relative light units (RLU) are normalized to β-galactosidase activity and are represented as the means±S.D. for three replicates. Results are representative of three separate trials. Luc, luciferase.
DISCUSSION
The oxidation of polyamines has been implicated in the cytotoxic activity of several antitumour polyamine analogues [2,12,32]. Additionally, the role of polyamine oxidation in the maintenance of polyamine homoeostasis has been well documented [13]. However, with our discovery of a new and inducible oxidase in the polyamine catabolic pathway [9], the potential for greater complexity in both drug response and polyamine homoeostasis has become apparent. Therefore we have sought to elucidate the mechanisms by which the expression of SMO(PAOh1) is regulated.
SMO(PAOh1) activity is induced rapidly in multiple cell types, with initial studies indicating that the increase in activity was a result of new mRNA and protein synthesis, rather than due to activation of pre-existing protein [9,11,12]. The results of the protein and RNA synthesis experiments presented here confirm that the increase in oxidase activity in A549 cells exposed to the polyamine analogue CPENSpm is indeed a result of newly synthesized mRNA and protein.
In light of the complex regulation of SSAT in response to polyamines and polyamine analogues, the potential that SMO(PAOh1) was similarly regulated at the transcriptional and post-transcriptional level was also investigated. The activity half-life studies indicate clearly that analogue exposure does not alter the stability of the measured oxidase activity, confirming that the increase in activity is a result of newly synthesized protein. However, it is equally clear from the mRNA half-life studies that CPENSpm treatment of A549 cells produces a significant increase in mRNA half-life. It is important to note that the increase in mRNA half-life after analogue exposure may be the major regulatory mechanism responsible for the increase in SMO(PAOh1) mRNA observed here. Specifically, the regression analysis used to determine the half-life of the SMO(PAOh1) mRNA predicts that, after 18 h, >40-fold more SMO(PAOh1) mRNA exists in the CPENSpm/actinomycin D-treated cells than in the actinomycin D-treated cells. Consequently, only a small increase in transcription in the analogue-treated cells would be sufficient to produce the observed 5-fold increase in steady-state mRNA with analogue treatment. These results are similar to the increase in SSAT mRNA half-life in analogue-treated cells, as reported by Fogel-Petrovic et al. [18], and indicate a common mechanism responsible for analogue-induced up-regulation of these two important polyamine catabolic enzymes.
Although the increase in mRNA half-life may account for most of the differences observed between analogue-treated and untreated cells, it may not be entirely sufficient to account for the 5-fold increase in steady-state SMO(PAOh1) mRNA observed after 24 h of treatment. The use of reporter construct analyses indicates that a relatively large area spanning from −55 bp to −1117 bp relative to the transcriptional start site is moderately responsive to analogue exposure in A549 cells, producing an increase in transcription ranging from 30 to >90%. These results suggest that the transcriptional regulation of SMO(PAOh1) in response to analogue exposure is a complex process, potentially under the control of multiple cis-elements in the regulatory region of the gene. This is in contrast with the description of the regulation described for human SSAT, where the PRE (polyamineresponse element) has been demonstrated to be the critical cis-element that regulates increased transcription in response to increased polyamines and exposure to polyamine analogues [24,25,33]. Although the increase in analogue-induced transcription is modest in A549 cells, combined with the increase in mRNA half-life, it is clearly sufficient to account for the observed 5-fold increase in SMO(PAOh1) mRNA.
It should be noted that there is at least one more level at which SMO(PAOh1) can be regulated. We and others have demonstrated that both the human and mouse genes for spermine oxidase code for multiple splice variants [11,34–36]. However, there are no data to indicate that the transcription of the variants is individually modulated. Our preliminary studies in human breast cancer lines indicate that the expression of each of the major variants is increased by the same magnitude in response to analogue exposure [37]. Cervelli et al. [36] have provided evidence that the four major mouse splice variants have different cellular localization and they suggest that the inactive splice variants may play non-catalytic regulatory roles.
More work will be required to define fully the regulatory elements that are critical for the transcriptional regulation of SMO(PAOh1). Similarly, the mechanisms that are responsible for the stabilization of the mRNA in the presence of CPENSpm remain to be described. Although the molecular mechanisms regulating SMO(PAOh1) expression remain to be fully elucidated, the present results indicate clearly that the observed increase in SMO(PAOh1) activity in response to the antitumour polyamine analogue CPENSpm is the result of increased transcription, increased mRNA stability and new protein synthesis. Once the specific mechanisms underlying this regulation are understood fully, it may become possible to design agents that are more effective in increasing SMO(PAOh1) and thereby more effective in targeting tumour cells.
Acknowledgments
This research was supported by National Institutes of Health grants CA51085, CA85509, CA88843 and CA98454. We thank Dr Alison V. Fraser, Dr Yongchun Wang and Dr Naveen Babbar for their helpful discussions.
References
- 1.Casero R. A., Jr, Celano P., Ervin S. J., Porter C. W., Bergeron R. J., Libby P. R. Differential induction of spermidine/spermine N1-acetyltransferase in human lung cancer cells by the bis(ethyl)polyamine analogues. Cancer Res. 1989;49:3829–3833. [PubMed] [Google Scholar]
- 2.Ha H. C., Woster P. M., Yager J. D., Casero R. A., Jr The role of polyamine catabolism in polyamine analogue-induced programmed cell death. Proc. Natl. Acad. Sci. U.S.A. 1997;94:11557–11562. doi: 10.1073/pnas.94.21.11557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen Y., Kramer D. L., Diegelman P., Vujcic S., Porter C. W. Apoptotic signaling in polyamine analogue-treated SK-MEL-28 human melanoma cells. Cancer Res. 2001;61:6437–6444. [PubMed] [Google Scholar]
- 4.Vujcic S., Halmekyto M., Diegelman P., Gan G., Kramer D. L., Janne J., Porter C. W. Effects of conditional overexpression of spermidine/spermine N1-acetyltransferase on polyamine pool dynamics, cell growth, and sensitivity to polyamine analogs. J. Biol. Chem. 2000;275:38319–38328. doi: 10.1074/jbc.M003270200. [DOI] [PubMed] [Google Scholar]
- 5.McCloskey D. E., Pegg A. E. Altered spermidine/spermine N1-acetyltransferase activity as a mechanism of cellular resistance to bis(ethyl)polyamine analogues. J. Biol. Chem. 2000;275:28708–28714. doi: 10.1074/jbc.M004120200. [DOI] [PubMed] [Google Scholar]
- 6.Murray-Stewart T., Applegren N. B., Devereux W., Hacker A., Smith R., Wang Y., Casero R. A., Jr Spermidine/spermine N1-acetyltransferase (SSAT) activity in human small-cell lung carcinoma cells following transfection with a genomic SSAT construct. Biochem. J. 2003;373:629–634. doi: 10.1042/BJ20021895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wallace H. M., Fraser A. V. Polyamine analogues as anticancer drugs. Biochem. Soc. Trans. 2003;31:393–396. doi: 10.1042/bst0310393. [DOI] [PubMed] [Google Scholar]
- 8.Wallace H. M., Fraser A. V., Hughes A. A perspective of polyamine metabolism. Biochem. J. 2003;376:1–14. doi: 10.1042/BJ20031327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Y., Devereux W., Woster P. M., Stewart T. M., Hacker A., Casero R. A., Jr Cloning and characterization of a human polyamine oxidase that is inducible by polyamine analogue exposure. Cancer Res. 2001;61:5370–5373. [PubMed] [Google Scholar]
- 10.Cervelli M., Polticelli F., Federico R., Mariottini P. Heterologous expression and characterization of mouse spermine oxidase. J. Biol. Chem. 2003;278:5271–5276. doi: 10.1074/jbc.M207888200. [DOI] [PubMed] [Google Scholar]
- 11.Vujcic S., Diegelman P., Bacchi C. J., Kramer D. L., Porter C. W. Identification and characterization of a novel flavin-containing spermine oxidase of mammalian cell origin. Biochem. J. 2002;367:665–675. doi: 10.1042/BJ20020720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Devereux W., Wang Y., Stewart T. M., Hacker A., Smith R., Frydman B., Valasinas A. L., Reddy V. K., Marton L. J., Ward T. D., Woster P. M., Casero R. A. Induction of the PAOh1/SMO polyamine oxidase by polyamine analogues in human lung carcinoma cells. Cancer Chemother. Pharmacol. 2003;52:383–390. doi: 10.1007/s00280-003-0662-4. [DOI] [PubMed] [Google Scholar]
- 13.Seiler N. Polyamine oxidase, properties and functions. Prog. Brain Res. 1995;106:333–344. doi: 10.1016/s0079-6123(08)61229-7. [DOI] [PubMed] [Google Scholar]
- 14.Casero R. A., Jr, Pegg A. E. Spermidine/spermine N1-acetyltransferase – the turning point in polyamine metabolism. FASEB J. 1993;7:653–661. [PubMed] [Google Scholar]
- 15.Vujcic S., Liang P., Diegelman P., Kramer D. L., Porter C. W. Genomic identification and biochemical characterization of the mammalian polyamine oxidase involved in polyamine back-conversion. Biochem. J. 2003;370:19–28. doi: 10.1042/BJ20021779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu T., Yankovskaya V., McIntire W. S. Cloning, sequencing, and heterologous expression of the murine peroxisomal flavoprotein, N1-acetylated polyamine oxidase. J. Biol. Chem. 2003;278:20514–20525. doi: 10.1074/jbc.M302149200. [DOI] [PubMed] [Google Scholar]
- 17.Wang Y., Murray-Stewart T., Devereux W., Hacker A., Frydman B., Woster P. M., Casero R. A., Jr Properties of purified recombinant human polyamine oxidase, PAOh1/SMO. Biochem. Biophys. Res. Commun. 2003;304:605–611. doi: 10.1016/s0006-291x(03)00636-3. [DOI] [PubMed] [Google Scholar]
- 18.Fogel-Petrovic M., Shappell N. W., Bergeron R. J., Porter C. W. Polyamine and polyamine analog regulation of spermidine/spermine N1-acetyltransferase in MALME-3M human melanoma cells. J. Biol. Chem. 1993;268:19118–19125. [PubMed] [Google Scholar]
- 19.Fogel-Petrovic M., Vujcic S., Brown P. J., Haddox M. K., Porter C. W. Effects of polyamines, polyamine analogs, and inhibitors of protein synthesis on spermidine/spermine N1-acetyltransferase gene expression. Biochemistry. 1996;35:14436–14444. doi: 10.1021/bi9612273. [DOI] [PubMed] [Google Scholar]
- 20.Xiao L., Casero R. A., Jr Differential transcription of the human spermidine/spermine N1-acetyltransferase (SSAT) gene in human lung carcinoma cells. Biochem. J. 1996;313:691–696. doi: 10.1042/bj3130691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Coleman C. S., Huang H., Pegg A. E. Role of the carboxyl terminal MATEE sequence of spermidine/spermine N1-acetyltransferase in the activity and stabilization by the polyamine analog N1,N12-bis(ethyl)spermine. Biochemistry. 1995;34:13423–13430. doi: 10.1021/bi00041a020. [DOI] [PubMed] [Google Scholar]
- 22.Coleman C. S., Pegg A. E. Proteasomal degradation of spermidine/spermine N1-acetyltransferase requires the carboxyl-terminal glutamic acid residues. J. Biol. Chem. 1997;272:12164–12169. doi: 10.1074/jbc.272.18.12164. [DOI] [PubMed] [Google Scholar]
- 23.Coleman C. S., Huang H., Pegg A. E. Structure and critical residues at the active site of spermidine/spermine-N1-acetyltransferase. Biochem. J. 1996;316:697–701. doi: 10.1042/bj3160697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang Y., Xiao L., Thiagalingam A., Nelkin B. D., Casero R. A., Jr The identification of a cis-element and a trans-acting factor involved in the response to polyamines and polyamine analogues in the regulation of the human spermidine/spermine N1-acetyltransferase gene transcription. J. Biol. Chem. 1998;273:34623–34630. doi: 10.1074/jbc.273.51.34623. [DOI] [PubMed] [Google Scholar]
- 25.Wang Y., Devereux W., Stewart T. M., Casero R. A., Jr Cloning and characterization of human polyamine-modulated factor-1, a transcriptional cofactor that regulates the transcription of the spermidine/spermine N1-acetyltransferase gene. J. Biol. Chem. 1999;274:22095–22101. doi: 10.1074/jbc.274.31.22095. [DOI] [PubMed] [Google Scholar]
- 26.Babbar N., Ignatenko N. A., Casero R. A., Jr, Gerner E. W. Cyclooxygenase-independent induction of apoptosis by sulindac sulfone is mediated by polyamines in colon cancer. J. Biol. Chem. 2003;278:47762–47775. doi: 10.1074/jbc.M307265200. [DOI] [PubMed] [Google Scholar]
- 27.Saab N. H., West E. E., Bieszk N. C., Preuss C. V., Mank A. R., Casero R. A., Jr, Woster P. M. Synthesis and evaluation of unsymmetrically substituted polyamine analogues as modulators of human spermidine/spermine-N1-acetyltransferase (SSAT) and as potential antitumor agents. J. Med. Chem. 1993;36:2998–3004. doi: 10.1021/jm00072a020. [DOI] [PubMed] [Google Scholar]
- 28.Casero R. A., Jr, Mank A. R., Xiao L., Smith J., Bergeron R. J., Celano P. Steady-state messenger RNA and activity correlates with sensitivity to N1,N12-bis(ethyl)spermine in human cell lines representing the major forms of lung cancer. Cancer Res. 1992;52:5359–5363. [PubMed] [Google Scholar]
- 29.Holtta E. Oxidation of spermidine and spermine in rat liver: purification and properties of polyamine oxidase. Biochemistry. 1977;16:91–100. doi: 10.1021/bi00620a015. [DOI] [PubMed] [Google Scholar]
- 30.Holtta E. Polyamine oxidase (rat liver) Methods Enzymol. 1983;94:306–311. doi: 10.1016/s0076-6879(83)94054-5. [DOI] [PubMed] [Google Scholar]
- 31.Seiler N., Bolkenius F. N., Knodgen B., Mamont P. Polyamine oxidase in rat tissues. Biochim. Biophys. Acta. 1980;615:480–488. doi: 10.1016/0005-2744(80)90514-8. [DOI] [PubMed] [Google Scholar]
- 32.Chen Y., Kramer D. L., Diegelman P., Vujcic S., Porter C. W. Apoptotic signaling in polyamine analogue-treated SK-MEL-28 human melanoma cells. Cancer Res. 2001;61:6437–6444. [PubMed] [Google Scholar]
- 33.Wang Y., Devereux W., Stewart T. M., Casero R. A., Jr Characterization of the interaction between the transcription factors human polyamine modulated factor (PMF-1) and NF-E2-related factor 2 (Nrf-2) in the transcriptional regulation of the spermidine/spermine N1-acetyltransferase (SSAT) gene. Biochem. J. 2001;355:45–49. doi: 10.1042/0264-6021:3550045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang Y., Devereux W., Woster P. M., Casero R. A., Jr Cloning and characterization of the mouse polyamine-modulated factor-1 (mPMF-1) gene: an alternatively spliced homologue of the human transcription factor. Biochem. J. 2001;359:387–392. doi: 10.1042/0264-6021:3590387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Murray-Stewart T., Wang Y., Devereux W., Casero R. A., Jr Cloning and characterization of multiple human polyamine oxidase splice variants that code for isoenzymes with different biochemical characteristics. Biochem. J. 2002;368:673–677. doi: 10.1042/BJ20021587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cervelli M., Bellini A., Bianchi M., Marcocci L., Nocera S., Polticelli F., Federico R., Amendola R., Mariottini P. Mouse spermine oxidase gene splice variants: nuclear subcellular localization of a novel active isoform. Eur. J. Biochem. 2004;271:760–770. doi: 10.1111/j.1432-1033.2004.03979.x. [DOI] [PubMed] [Google Scholar]
- 37.Pledgie A. M., Wang Y., Huang Y., Hacker A., Woster P. M., Casero R. A., Davidson N. E. Differential induction of human spermine oxidase (PAOh1/SMO) mRNA and activity in human breast cancer cell lines. Proc. Am. Assoc. Cancer Res. 2004;45:5307. [Google Scholar]