Abstract
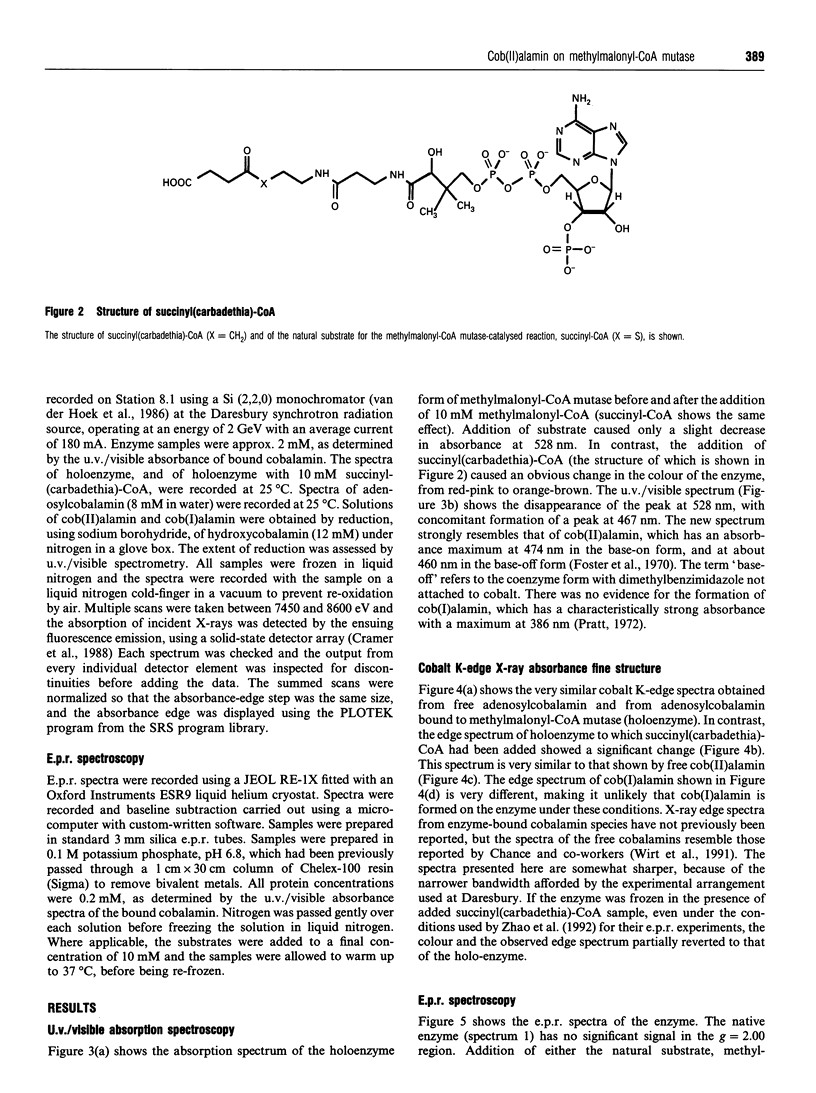
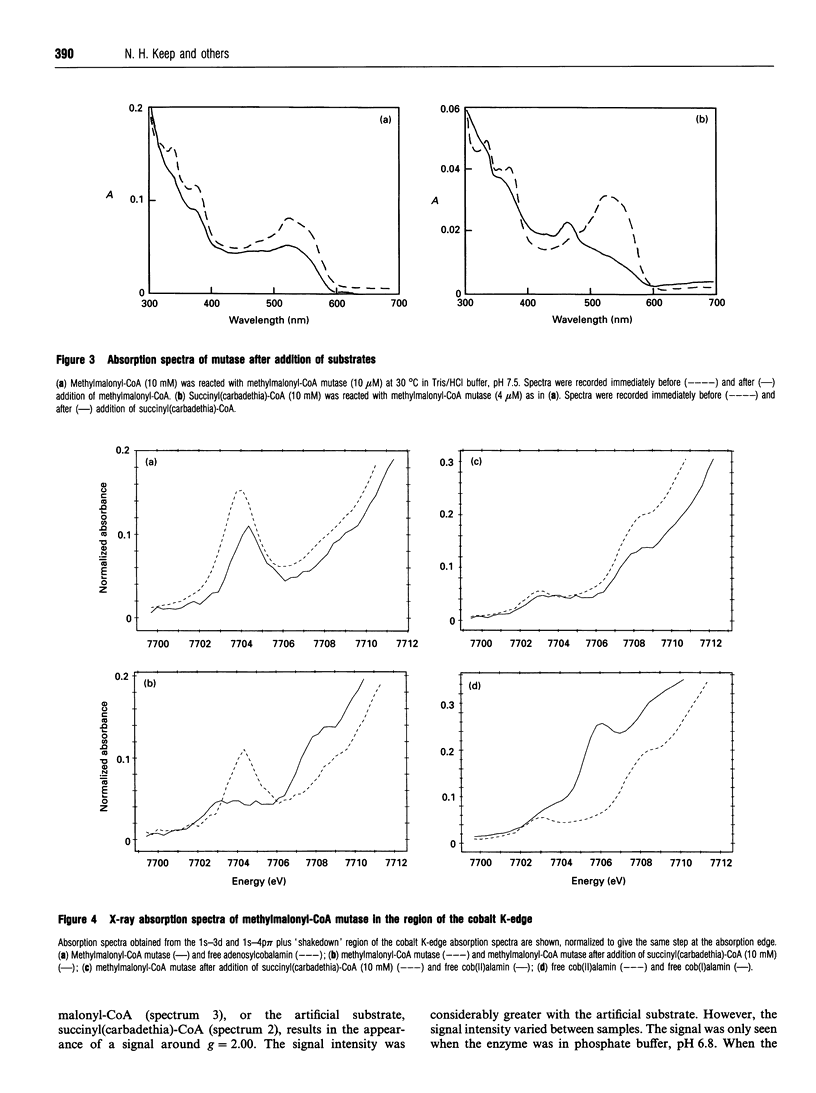
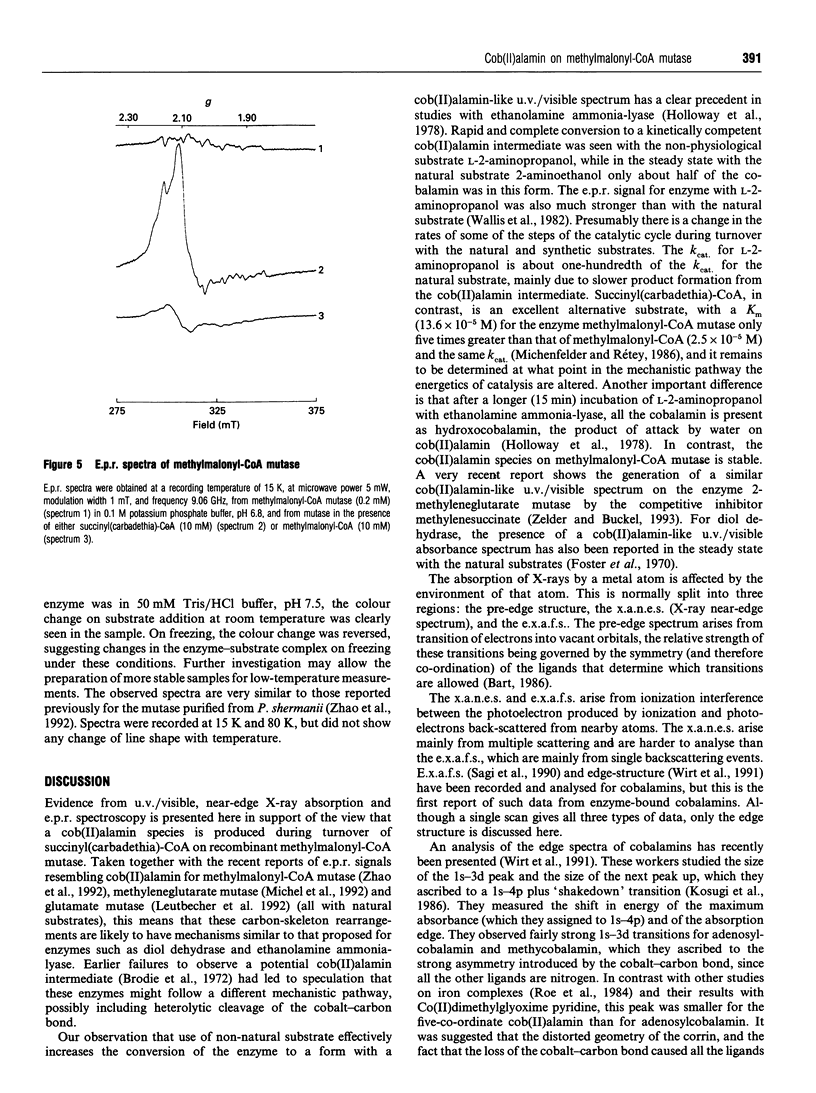
Succinyl(carbadethia)-coenzyme A, a synthetic substrate for adenosylcobalamin-dependent methylmalonyl-CoA mutase, has been prepared by a simplified procedure. When recombinant mutase was mixed with the synthetic substrate, the u.v./visible absorption spectrum of the bound cofactor changed rapidly to resemble that of cob(II)alamin, with an absorption maximum at 467 nm. Addition of the natural substrates, in contrast, produced only minor changes in the u.v./visible spectrum. The recent report of the generation of a complex e.p.r. spectrum on addition of substrate to the holo-methylmalonyl-CoA mutase was confirmed with the recombinant enzyme. The signals observed were stronger when the succinyl(carbadethia) analogue was used. Cobalt K-edge X-ray absorption spectroscopy confirmed that the addition of this analogue to holoenzyme leads to the generation of a cob(II)alamin-like species. These results strongly support the generation of cob(II)alamin during the 1,2-skeletal rearrangement catalysed by methylmalonyl-CoA mutase, as required if this enzyme follows the reaction pathway involving radical intermediates previously proposed for other adenosylcobalamin-dependent enzymes.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babior B. M., Moss T. H., Orme-Johnson W. H., Beinert H. The mechanism of action of ethanolamine ammonia-lyase, a B-12-dependent enzyme. The participation of paramagnetic species in the catalytic deamination of 2-aminopropanol. J Biol Chem. 1974 Jul 25;249(14):4537–4544. [PubMed] [Google Scholar]
- Buettner G. R., Coffman R. E. EPR determination of the Co(II)-free radical magnetic geometry of the "doublet" species arising in a coenzyme B-12-enzyme reaction. Biochim Biophys Acta. 1977 Feb 9;480(2):495–505. doi: 10.1016/0005-2744(77)90042-0. [DOI] [PubMed] [Google Scholar]
- Cockle S. A., Hill H. A., Williams R. J. The detection of intermediates during the conversion of propane-1,2-diol to propionaldehyde by glyceroldehydrase, a coenzyme B 12 dependent reaction. J Am Chem Soc. 1972 Jan 12;94(1):275–277. doi: 10.1021/ja00756a050. [DOI] [PubMed] [Google Scholar]
- Finlay T. H., Valinsky J., Mildvan A. S., Abeles R. H. Electron spin resonance studies with dioldehydrase. Evidence for radical intermediates in reactions catalyzed by coenzyme B 12. J Biol Chem. 1973 Feb 25;248(4):1285–1290. [PubMed] [Google Scholar]
- Foster M. A., Hill H. A., Williams R. J. Cobalt as a functional group. Biochem Soc Symp. 1970;31:187–202. [PubMed] [Google Scholar]
- Halpern J. Mechanisms of coenzyme B12-dependent rearrangements. Science. 1985 Feb 22;227(4689):869–875. doi: 10.1126/science.2857503. [DOI] [PubMed] [Google Scholar]
- Hollaway M. R., White H. A., Joblin K. N., Johnson A. W., Lappert M. F., Wallis O. C. A spectrophotometric rapid kinetic study of reactions catalysed by coenzyme-B12-dependent ethanolamine ammonia-lyase. Eur J Biochem. 1978 Jan 2;82(1):143–154. doi: 10.1111/j.1432-1033.1978.tb12005.x. [DOI] [PubMed] [Google Scholar]
- Leadlay P. F. Purification and characterization of methylmalonyl-CoA epimerase from Propionibacterium shermanii. Biochem J. 1981 Aug 1;197(2):413–419. doi: 10.1042/bj1970413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leutbecher U., Albracht S. P., Buckel W. Identification of a paramagnetic species as an early intermediate in the coenzyme B12-dependent glutamate mutase reaction. A cob(II)amide? FEBS Lett. 1992 Jul 28;307(2):144–146. doi: 10.1016/0014-5793(92)80754-5. [DOI] [PubMed] [Google Scholar]
- Marsh N., Leadlay P. F., Evans P. R. Crystallization and preliminary diffraction data for adenosylcobalamin-dependent methylmalonyl-CoA mutase from Propionibacterium shermanii. J Mol Biol. 1988 Mar 20;200(2):421–422. doi: 10.1016/0022-2836(88)90252-5. [DOI] [PubMed] [Google Scholar]
- McKie N., Keep N. H., Patchett M. L., Leadlay P. F. Adenosylcobalamin-dependent methylmalonyl-CoA mutase from Propionibacterium shermanii. Active holoenzyme produced from Escherichia coli. Biochem J. 1990 Jul 15;269(2):293–298. doi: 10.1042/bj2690293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel C., Albracht S. P., Buckel W. Adenosylcobalamin and cob(II)alamin as prosthetic groups of 2-methyleneglutarate mutase from Clostridium barkeri. Eur J Biochem. 1992 Apr 15;205(2):767–773. doi: 10.1111/j.1432-1033.1992.tb16841.x. [DOI] [PubMed] [Google Scholar]
- Schepler K. L., Dunham W. R., Sands R. H., Fee J. A., Abeles R. H. A physical explanation of the EPR spectrum observed during catalysis by enzymes utilizing coenzyme B12. Biochim Biophys Acta. 1975 Aug 26;397(2):510–518. doi: 10.1016/0005-2744(75)90141-2. [DOI] [PubMed] [Google Scholar]
- Wallis O. C., Bray R. C., Gutteridge S., Hollaway M. R. The extents of formation of cobalt(II)-radical intermediates in the reactions with different substrates catalysed by the adenosylcobalamin-dependent enzyme ethanolamine ammonia-lyase. Eur J Biochem. 1982 Jul;125(2):299–303. doi: 10.1111/j.1432-1033.1982.tb06682.x. [DOI] [PubMed] [Google Scholar]
- Wood H. G., Chiao J. P., Poto E. M. A new large form of transcarboxylase with six outer subunits and twelve biotinyl carboxyl carrier subunits. J Biol Chem. 1977 Feb 25;252(4):1490–1499. [PubMed] [Google Scholar]
- Zagalak B., Rétey J. Studies on methylmalonyl-CoA mutase from Propionibacterium shermanii. Eur J Biochem. 1974 May 15;44(2):529–535. doi: 10.1111/j.1432-1033.1974.tb03512.x. [DOI] [PubMed] [Google Scholar]
- Zelder O., Buckel W. On the role of two different cobalt(II) species in coenzyme B12-dependent 2-methyleneglutarate mutase from Clostridium barkeri. Biol Chem Hoppe Seyler. 1993 Jan;374(1):85–90. doi: 10.1515/bchm3.1993.374.1-6.85. [DOI] [PubMed] [Google Scholar]


