Abstract
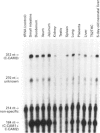
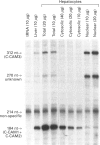
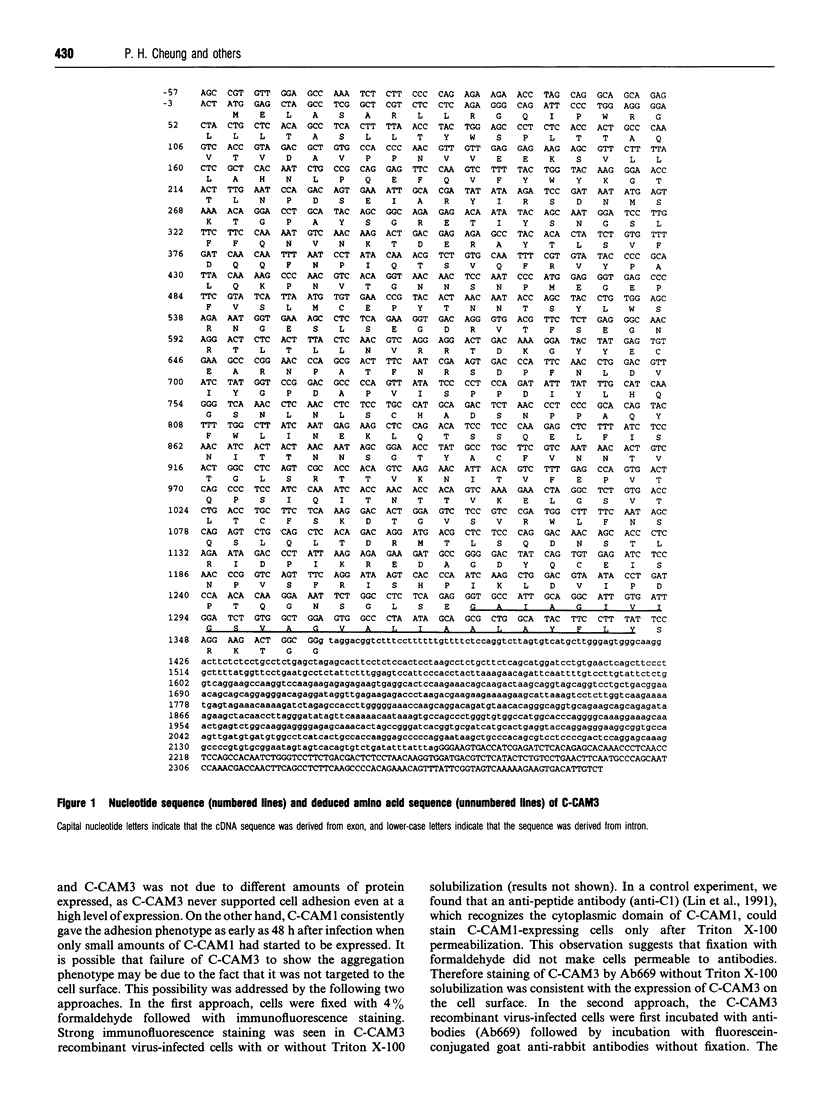
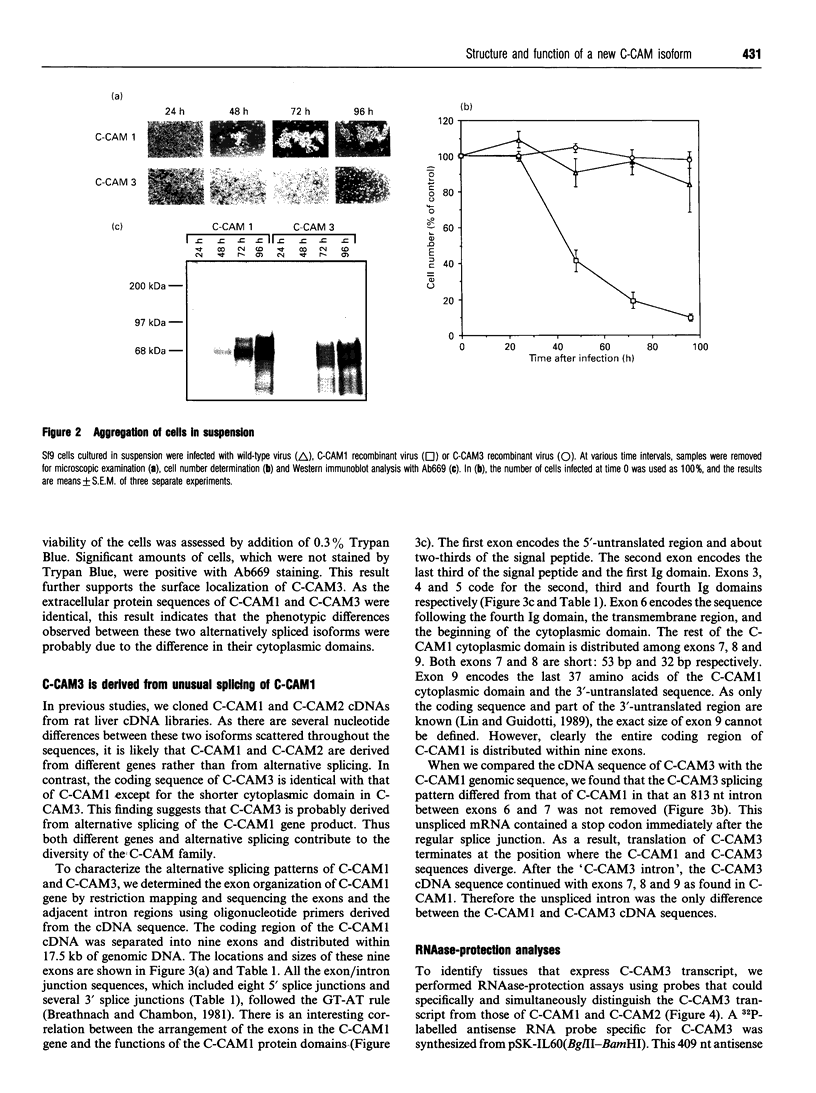
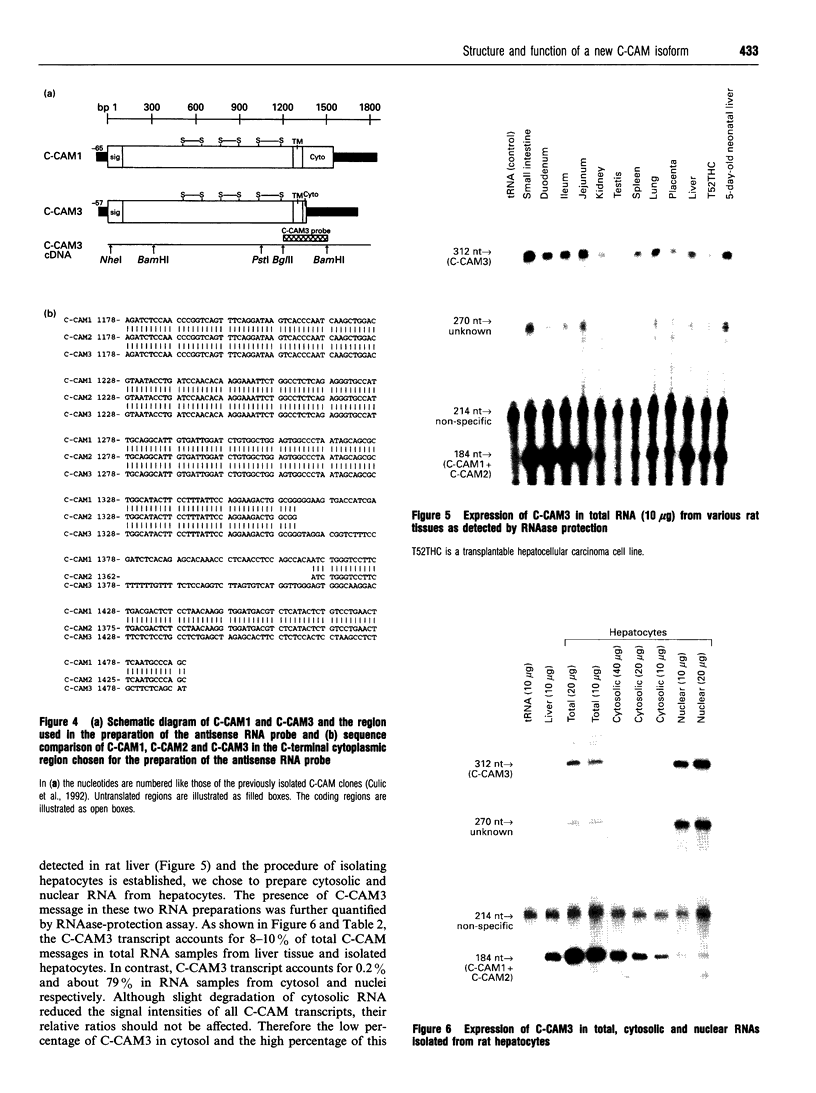
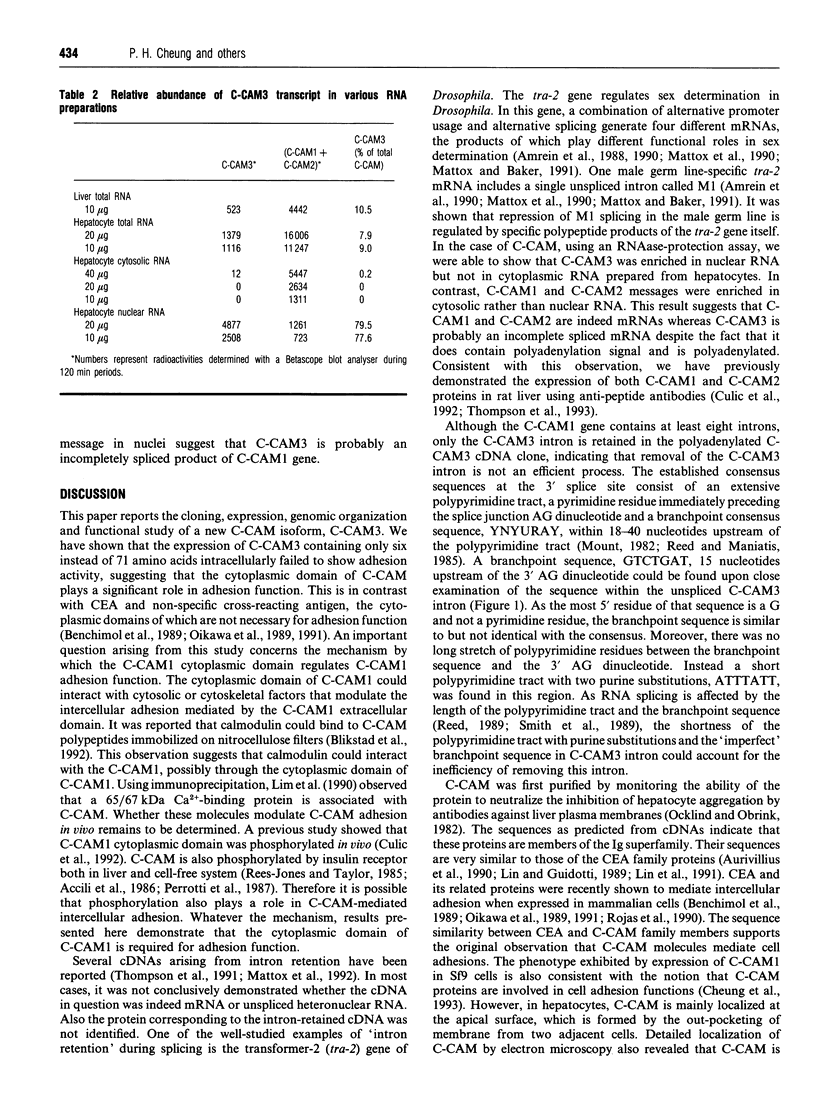
Cell-CAM105 (also named C-CAM) is a cell surface glycoprotein involved in intercellular adhesion of rat hepatocytes. It has four extracellular immunoglobulin (Ig) domains, a transmembrane domain and a cytoplasmic domain and therefore is a member of the Ig supergene family. We have characterized multiple cDNAs of the C-CAM genes in rat intestine. Sequence analyses showed that rat intestine contained not only the previously reported L-form and S-form C-CAMs (renamed C-CAM1 and C-CAM2 respectively) but also a new isoform, C-CAM3. The C-CAM3 transcript codes for a polypeptide with a truncated C-terminus that lacks 65 amino acids from the previously reported C-CAM1 cytoplasmic domain. Unlike C-CAM1, C-CAM3 did not mediate cell adhesion when expressed in insect cells using the baculoviral expression system. Thus the extra 65 amino acids in the cytoplasmic domain of C-CAM1 are important for adhesion phenotype when expressed in insect cells. Although C-CAM1 and C-CAM2 are encoded by different genes, sequence analysis suggests that C-CAM3 is probably derived from alternative splicing of the C-CAM1 gene. To examine this possibility, we have determined the exon organization of the C-CAM1 gene. C-CAM3 differed from C-CAM1 by the presence of a single unspliced intron which contained a stop codon immediately after the regular splice junction. As a result, translation of C-CAM3 terminates at the point where C-CAM1 and C-CAM3 sequences diverge. To investigate the expression of C-CAM1, C-CAM2 and C-CAM3 in different tissues, we used an RNAase-protection assay to simultaneously assess the levels of expression of these transcripts. Using total RNA prepared from various tissues, we showed that expression of C-CAM3 was tissue-specific, and the C-CAM3 transcript accounted for about 25% of the transcripts derived from the C-CAM1 gene. However, further analysis revealed that C-CAM3 transcript was not present in cytosolic RNA, rather it was enriched in nuclear RNA prepared from hepatocytes. Although C-CAM3 cDNA contains the polyadenylation signal and is polyadenylated, these results indicate that C-CAM3 is probably an incomplete spliced product of C-CAM1 gene.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Accili D., Perrotti N., Rees-Jones R., Taylor S. I. Tissue distribution and subcellular localization of an endogenous substrate (pp 120) for the insulin receptor-associated tyrosine kinase. Endocrinology. 1986 Sep;119(3):1274–1280. doi: 10.1210/endo-119-3-1274. [DOI] [PubMed] [Google Scholar]
- Amrein H., Gorman M., Nöthiger R. The sex-determining gene tra-2 of Drosophila encodes a putative RNA binding protein. Cell. 1988 Dec 23;55(6):1025–1035. doi: 10.1016/0092-8674(88)90247-4. [DOI] [PubMed] [Google Scholar]
- Amrein H., Maniatis T., Nöthiger R. Alternatively spliced transcripts of the sex-determining gene tra-2 of Drosophila encode functional proteins of different size. EMBO J. 1990 Nov;9(11):3619–3629. doi: 10.1002/j.1460-2075.1990.tb07573.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aurivillius M., Hansen O. C., Lazrek M. B., Bock E., Obrink B. The cell adhesion molecule Cell-CAM 105 is an ecto-ATPase and a member of the immunoglobulin superfamily. FEBS Lett. 1990 May 21;264(2):267–269. doi: 10.1016/0014-5793(90)80264-j. [DOI] [PubMed] [Google Scholar]
- Benchimol S., Fuks A., Jothy S., Beauchemin N., Shirota K., Stanners C. P. Carcinoembryonic antigen, a human tumor marker, functions as an intercellular adhesion molecule. Cell. 1989 Apr 21;57(2):327–334. doi: 10.1016/0092-8674(89)90970-7. [DOI] [PubMed] [Google Scholar]
- Blikstad I., Wikström T., Aurivillius M., Obrink B. C-CAM (Cell-CAM 105) is a calmodulin binding protein. FEBS Lett. 1992 May 4;302(1):26–30. doi: 10.1016/0014-5793(92)80276-m. [DOI] [PubMed] [Google Scholar]
- Boyer B., Thiery J. P. Epithelial cell adhesion mechanisms. J Membr Biol. 1989 Dec;112(2):97–108. doi: 10.1007/BF01871271. [DOI] [PubMed] [Google Scholar]
- Breathnach R., Chambon P. Organization and expression of eucaryotic split genes coding for proteins. Annu Rev Biochem. 1981;50:349–383. doi: 10.1146/annurev.bi.50.070181.002025. [DOI] [PubMed] [Google Scholar]
- Cheung P. H., Thompson N. L., Earley K., Culic O., Hixson D., Lin S. H. Cell-CAM105 isoforms with different adhesion functions are coexpressed in adult rat tissues and during liver development. J Biol Chem. 1993 Mar 25;268(9):6139–6146. [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Culic O., Huang Q. H., Flanagan D., Hixson D., Lin S. H. Molecular cloning and expression of a new rat liver cell-CAM105 isoform. Differential phosphorylation of isoforms. Biochem J. 1992 Jul 1;285(Pt 1):47–53. doi: 10.1042/bj2850047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman G. M., Crossin K. L. Cell adhesion molecules: implications for a molecular histology. Annu Rev Biochem. 1991;60:155–190. doi: 10.1146/annurev.bi.60.070191.001103. [DOI] [PubMed] [Google Scholar]
- Hansson M., Blikstad I., Obrink B. Cell-surface location and molecular properties of cell-CAM 105 in intestinal epithelial cells. Exp Cell Res. 1989 Mar;181(1):63–74. doi: 10.1016/0014-4827(89)90182-1. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lin S. H., Culic O., Flanagan D., Hixson D. C. Immunochemical characterization of two isoforms of rat liver ecto-ATPase that show an immunological and structural identity with a glycoprotein cell-adhesion molecule with Mr 105,000. Biochem J. 1991 Aug 15;278(Pt 1):155–161. doi: 10.1042/bj2780155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S. H., Guidotti G. Cloning and expression of a cDNA coding for a rat liver plasma membrane ecto-ATPase. The primary structure of the ecto-ATPase is similar to that of the human biliary glycoprotein I. J Biol Chem. 1989 Aug 25;264(24):14408–14414. [PubMed] [Google Scholar]
- Mattox W., Baker B. S. Autoregulation of the splicing of transcripts from the transformer-2 gene of Drosophila. Genes Dev. 1991 May;5(5):786–796. doi: 10.1101/gad.5.5.786. [DOI] [PubMed] [Google Scholar]
- Mattox W., Palmer M. J., Baker B. S. Alternative splicing of the sex determination gene transformer-2 is sex-specific in the germ line but not in the soma. Genes Dev. 1990 May;4(5):789–805. doi: 10.1101/gad.4.5.789. [DOI] [PubMed] [Google Scholar]
- Mattox W., Ryner L., Baker B. S. Autoregulation and multifunctionality among trans-acting factors that regulate alternative pre-mRNA processing. J Biol Chem. 1992 Sep 25;267(27):19023–19026. [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mount S. M. A catalogue of splice junction sequences. Nucleic Acids Res. 1982 Jan 22;10(2):459–472. doi: 10.1093/nar/10.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mowery J., Hixson D. C. Detection of cell-CAM 105 in the pericanalicular domain of the rat hepatocyte plasma membrane. Hepatology. 1991 Jan;13(1):47–56. [PubMed] [Google Scholar]
- Nicolson G. L. Metastatic tumor cell interactions with endothelium, basement membrane and tissue. Curr Opin Cell Biol. 1989 Oct;1(5):1009–1019. doi: 10.1016/0955-0674(89)90073-2. [DOI] [PubMed] [Google Scholar]
- Ocklind C., Obrink B. Intercellular adhesion of rat hepatocytes. Identification of a cell surface glycoprotein involved in the initial adhesion process. J Biol Chem. 1982 Jun 25;257(12):6788–6795. [PubMed] [Google Scholar]
- Odin P., Asplund M., Busch C., Obrink B. Immunohistochemical localization of cellCAM 105 in rat tissues: appearance in epithelia, platelets, and granulocytes. J Histochem Cytochem. 1988 Jul;36(7):729–739. doi: 10.1177/36.7.3290331. [DOI] [PubMed] [Google Scholar]
- Oikawa S., Inuzuka C., Kuroki M., Arakawa F., Matsuoka Y., Kosaki G., Nakazato H. A specific heterotypic cell adhesion activity between members of carcinoembryonic antigen family, W272 and NCA, is mediated by N-domains. J Biol Chem. 1991 May 5;266(13):7995–8001. [PubMed] [Google Scholar]
- Oikawa S., Inuzuka C., Kuroki M., Matsuoka Y., Kosaki G., Nakazato H. Cell adhesion activity of non-specific cross-reacting antigen (NCA) and carcinoembryonic antigen (CEA) expressed on CHO cell surface: homophilic and heterophilic adhesion. Biochem Biophys Res Commun. 1989 Oct 16;164(1):39–45. doi: 10.1016/0006-291x(89)91679-3. [DOI] [PubMed] [Google Scholar]
- Perrotti N., Accili D., Marcus-Samuels B., Rees-Jones R. W., Taylor S. I. Insulin stimulates phosphorylation of a 120-kDa glycoprotein substrate (pp120) for the receptor-associated protein kinase in intact H-35 hepatoma cells. Proc Natl Acad Sci U S A. 1987 May;84(10):3137–3140. doi: 10.1073/pnas.84.10.3137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed R., Maniatis T. Intron sequences involved in lariat formation during pre-mRNA splicing. Cell. 1985 May;41(1):95–105. doi: 10.1016/0092-8674(85)90064-9. [DOI] [PubMed] [Google Scholar]
- Reed R. The organization of 3' splice-site sequences in mammalian introns. Genes Dev. 1989 Dec;3(12B):2113–2123. doi: 10.1101/gad.3.12b.2113. [DOI] [PubMed] [Google Scholar]
- Rees-Jones R. W., Taylor S. I. An endogenous substrate for the insulin receptor-associated tyrosine kinase. J Biol Chem. 1985 Apr 10;260(7):4461–4467. [PubMed] [Google Scholar]
- Rojas M., Fuks A., Stanners C. P. Biliary glycoprotein, a member of the immunoglobulin supergene family, functions in vitro as a Ca2(+)-dependent intercellular adhesion molecule. Cell Growth Differ. 1990 Nov;1(11):527–533. [PubMed] [Google Scholar]
- Smith C. W., Porro E. B., Patton J. G., Nadal-Ginard B. Scanning from an independently specified branch point defines the 3' splice site of mammalian introns. Nature. 1989 Nov 16;342(6247):243–247. doi: 10.1038/342243a0. [DOI] [PubMed] [Google Scholar]
- Thompson J. A., Grunert F., Zimmermann W. Carcinoembryonic antigen gene family: molecular biology and clinical perspectives. J Clin Lab Anal. 1991;5(5):344–366. doi: 10.1002/jcla.1860050510. [DOI] [PubMed] [Google Scholar]
- Thompson N. L., Panzica M. A., Hull G., Lin S. H., Curran T. R., Gruppuso P. A., Baum O., Reutter W., Hixson D. C. Spatiotemporal expression of two cell-cell adhesion molecule 105 isoforms during liver development. Cell Growth Differ. 1993 Apr;4(4):257–268. [PubMed] [Google Scholar]
- Zinn K., DiMaio D., Maniatis T. Identification of two distinct regulatory regions adjacent to the human beta-interferon gene. Cell. 1983 Oct;34(3):865–879. doi: 10.1016/0092-8674(83)90544-5. [DOI] [PubMed] [Google Scholar]