Abstract
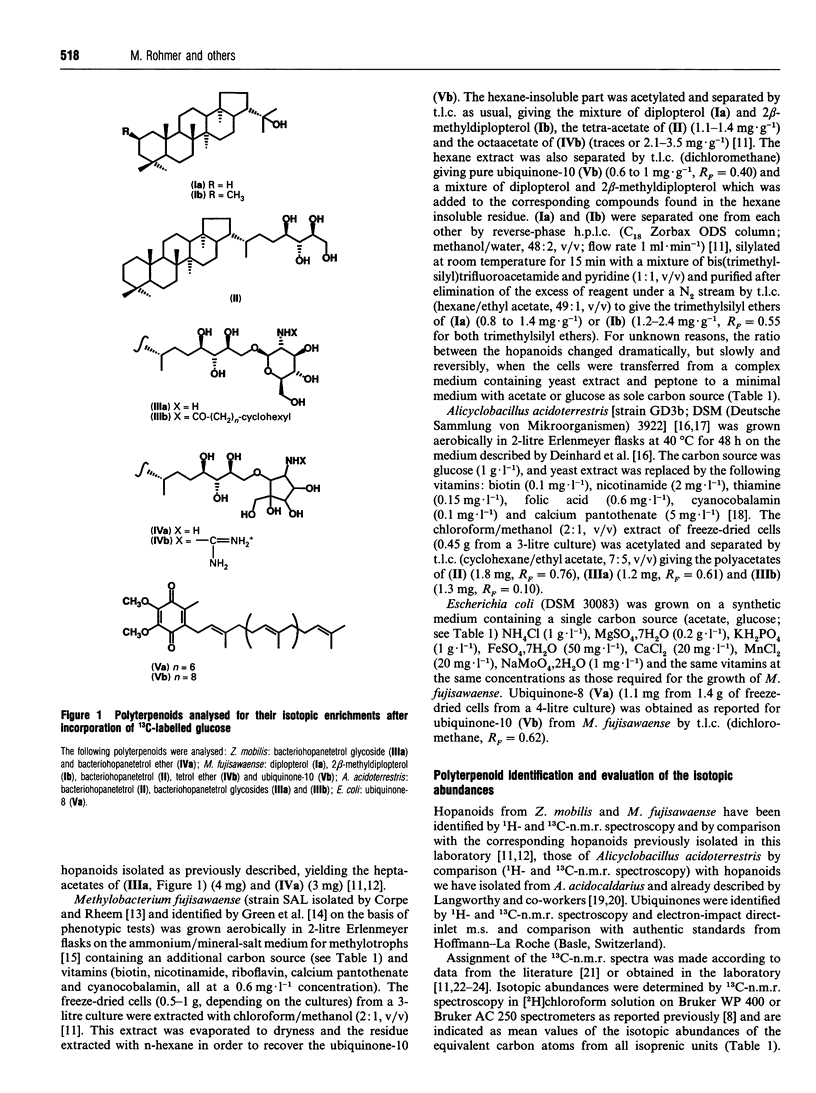
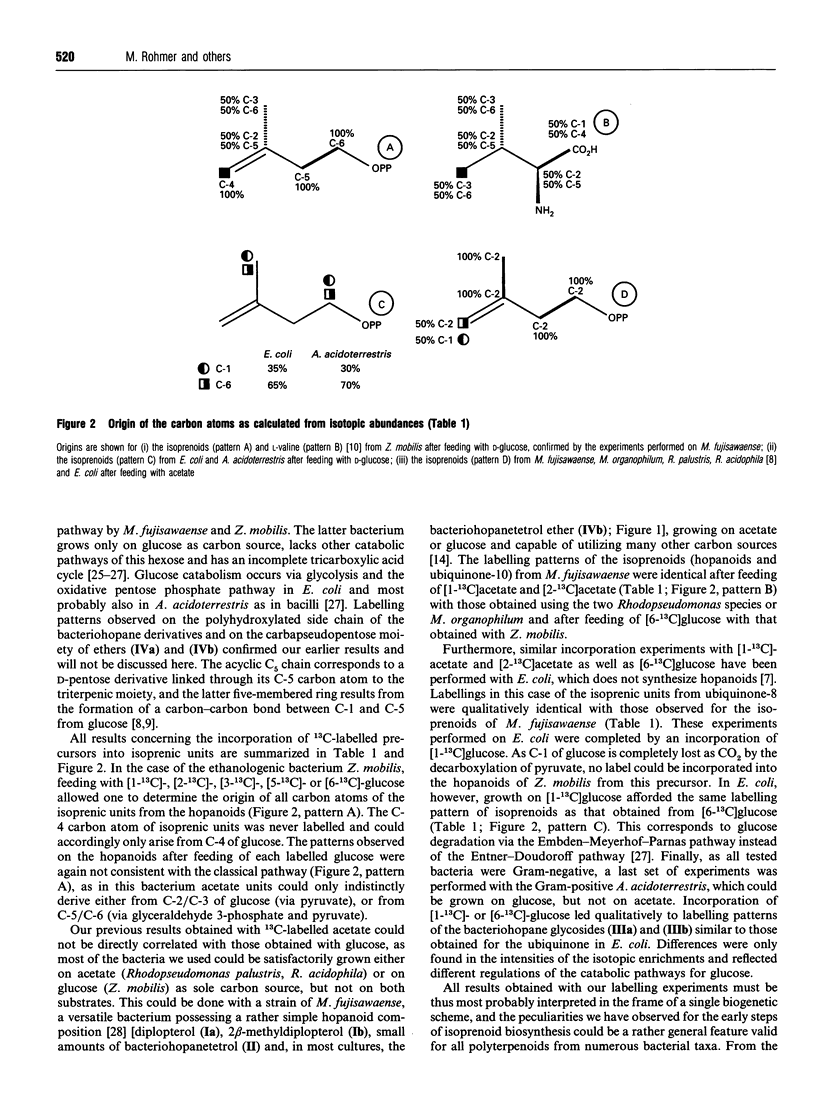
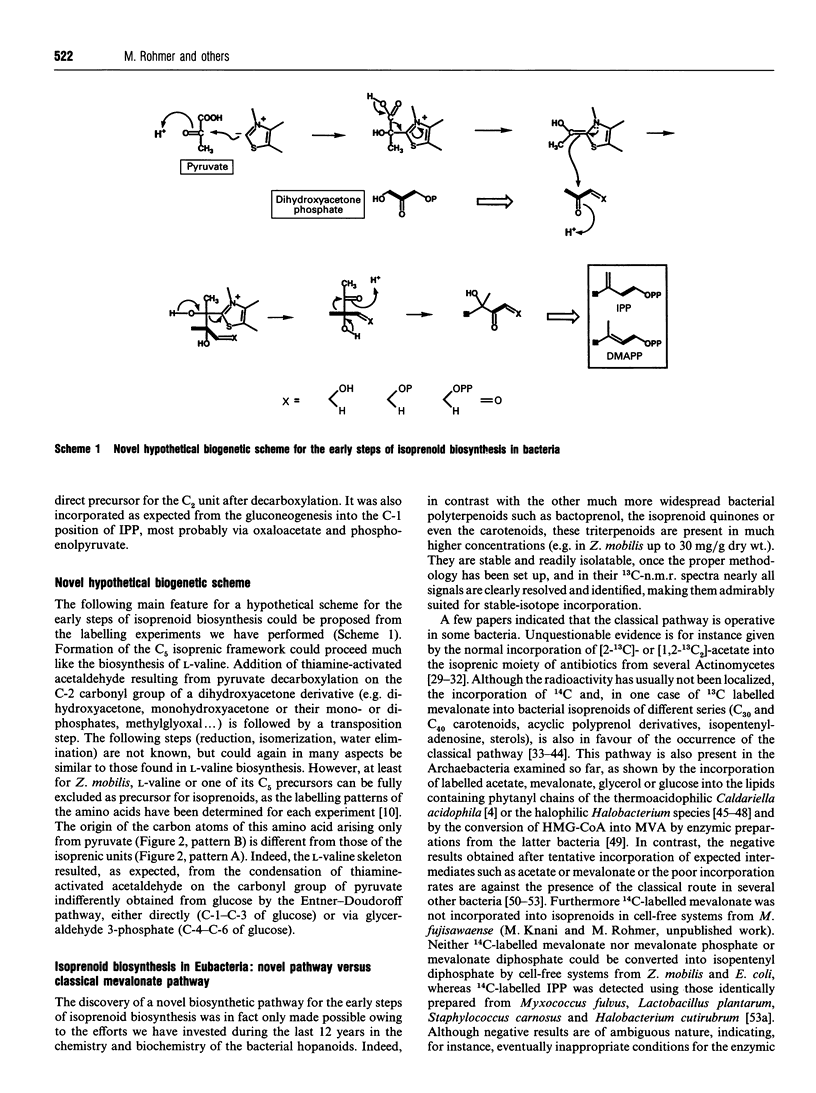
Incorporation of 13C-labelled glucose, acetate, pyruvate or erythrose allowed the determination of the origin of the carbon atoms of triterpenoids of the hopane series and/or of the ubiquinones from several bacteria (Zymomonas mobilis, Methylobacterium fujisawaense, Escherichia coli and Alicyclobacillus acidoterrestris) confirmed our earlier results obtained by incorporation of 13C-labelled acetate into the hopanoids of other bacteria and led to the identification of a novel biosynthetic route for the early steps of isoprenoid biosynthesis. The C5 framework of isoprenic units results most probably (i) from the condensation of a C2 unit derived from pyruvate decarboxylation (e.g. thiamine-activated acetaldehyde) on the C-2 carbonyl group of a triose phosphate derivative issued probably from dihydroxyacetone phosphate and not from pyruvate and (ii) from a transposition step. Although this hypothetical biosynthetic pathway resembles that of L-valine biosynthesis, this amino acid or its C5 precursors could be excluded as intermediates in the formation of isoprenic units.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen C. M., Keenan M. V., Sack J. Lactobacillus plantarum undecaprenyl pyrophosphate synthetase: purification and reaction requirements. Arch Biochem Biophys. 1976 Jul;175(1):236–248. doi: 10.1016/0003-9861(76)90504-x. [DOI] [PubMed] [Google Scholar]
- Baba T., Allen C. M. Prenyl transferases from Micrococcus luteus: characterization of undecaprenyl pyrophosphate synthetase. Arch Biochem Biophys. 1980 Apr 1;200(2):474–484. doi: 10.1016/0003-9861(80)90379-3. [DOI] [PubMed] [Google Scholar]
- Beach M. J., Rodwell V. W. Cloning, sequencing, and overexpression of mvaA, which encodes Pseudomonas mevalonii 3-hydroxy-3-methylglutaryl coenzyme A reductase. J Bacteriol. 1989 Jun;171(6):2994–3001. doi: 10.1128/jb.171.6.2994-3001.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisseret P., Zundel M., Rohmer M. Prokaryotic triterpenoids. 2. 2 beta-Methylhopanoids from Methylobacterium organophilum and Nostoc muscorum, a new series of prokaryotic triterpenoids. Eur J Biochem. 1985 Jul 1;150(1):29–34. doi: 10.1111/j.1432-1033.1985.tb08982.x. [DOI] [PubMed] [Google Scholar]
- Bloch K. Sterol molecule: structure, biosynthesis, and function. Steroids. 1992 Aug;57(8):378–383. doi: 10.1016/0039-128x(92)90081-j. [DOI] [PubMed] [Google Scholar]
- Cabrera J. A., Bolds J., Shields P. E., Havel C. M., Watson J. A. Isoprenoid synthesis in Halobacterium halobium. Modulation of 3-hydroxy-3-methylglutaryl coenzyme a concentration in response to mevalonate availability. J Biol Chem. 1986 Mar 15;261(8):3578–3583. [PubMed] [Google Scholar]
- Christenson J. G., Gross S. K., Robbins P. W. Enzymatic synthesis of the antigen carrier lipid. J Biol Chem. 1969 Oct 25;244(20):5436–5439. [PubMed] [Google Scholar]
- Conway T. The Entner-Doudoroff pathway: history, physiology and molecular biology. FEMS Microbiol Rev. 1992 Sep;9(1):1–27. doi: 10.1111/j.1574-6968.1992.tb05822.x. [DOI] [PubMed] [Google Scholar]
- Durr I. F., Habbal M. Z. The biosynthesis of C 55 polyprenols by a cell-free preparation of Lactobacillus plantarum. Biochem J. 1972 Apr;127(2):345–349. doi: 10.1042/bj1270345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekiel I., Sprott G. D., Smith I. C. Mevalonic acid is partially synthesized from amino acids in Halobacterium cutirubrum: a 13C nuclear magnetic resonance study. J Bacteriol. 1986 May;166(2):559–564. doi: 10.1128/jb.166.2.559-564.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flesch G., Rohmer M. Prokaryotic hopanoids: the biosynthesis of the bacteriohopane skeleton. Formation of isoprenic units from two distinct acetate pools and a novel type of carbon/carbon linkage between a triterpene and D-ribose. Eur J Biochem. 1988 Aug 1;175(2):405–411. doi: 10.1111/j.1432-1033.1988.tb14210.x. [DOI] [PubMed] [Google Scholar]
- Flesch G., Rohmer M. Prokaryotic triterpenoids. A novel hopanoid from the ethanol-producing bacterium Zymomonas mobilis. Biochem J. 1989 Sep 1;262(2):673–675. doi: 10.1042/bj2620673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii H., Koyama T., Ogura K. Hexaprenyl pyrophosphate synthetase from Micrococcus luteus B-P 26. Separation of two essential components. J Biol Chem. 1982 Dec 25;257(24):14610–14612. [PubMed] [Google Scholar]
- Fujisaki S., Nishino T., Izui K., Katsuki H. Specific 14C-labeling of isoprenoids of intact E. coli cells. Biochem Int. 1984 Jun;8(6):779–785. [PubMed] [Google Scholar]
- Fujisaki S., Nishino T., Katsuki H. Biosynthesis of isoprenoids in intact cells of Escherichia coli. J Biochem. 1986 Apr;99(4):1137–1146. doi: 10.1093/oxfordjournals.jbchem.a135577. [DOI] [PubMed] [Google Scholar]
- GIBBS M., DEMOSS R. D. Anaerobic dissimilation of C14-labeled glucose and fructose by Pseudomonas lindneri. J Biol Chem. 1954 Apr;207(2):689–694. [PubMed] [Google Scholar]
- Gough D. P., Kirby A. L., Richards J. B., Hemming F. W. The characterization of undecaprenol of Lactobacillus plantarum. Biochem J. 1970 Jun;118(1):167–170. doi: 10.1042/bj1180167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gräfe U., Reinhardt G., Hänel F., Schade W., Gumpert J. Occurrence of squalene and dehydrosqualene in streptomycetes. J Basic Microbiol. 1985;25(8):503–507. doi: 10.1002/jobm.3620250809. [DOI] [PubMed] [Google Scholar]
- Horbach S., Sahm H., Welle R. Isoprenoid biosynthesis in bacteria: two different pathways? FEMS Microbiol Lett. 1993 Aug 1;111(2-3):135–140. doi: 10.1111/j.1574-6968.1993.tb06375.x. [DOI] [PubMed] [Google Scholar]
- Isshiki K., Tamamura T., Sawa T., Naganawa H., Takeuchi T., Umezawa H. Biosynthetic studies of terpentecin. J Antibiot (Tokyo) 1986 Nov;39(11):1634–1635. doi: 10.7164/antibiotics.39.1634. [DOI] [PubMed] [Google Scholar]
- KANDUTSCH A. A., PAULUS H., LEVIN E., BLOCH K. PURIFICATION OF GERANYLGERANYL PYROPHOSPHATE SYNTHETASE FROM MICROCOCCUS LYSODEIKTICUS. J Biol Chem. 1964 Aug;239:2507–2515. [PubMed] [Google Scholar]
- Kleinig H. On the utilization in vivo of lycopene and phytoene as precursors for the formation of carotenoid glucoside ester and on the regulation of carotenoid biosynthesis in Myxococcus fulvus. Eur J Biochem. 1975 Sep 1;57(1):301–308. doi: 10.1111/j.1432-1033.1975.tb02301.x. [DOI] [PubMed] [Google Scholar]
- Kushwaha S. C., Kates M., Porter J. W. Enzymatic synthesis of C40 carotenes by cell-free preparation from Halobacterium cutirubrum. Can J Biochem. 1976 Sep;54(9):816–823. doi: 10.1139/o76-117. [DOI] [PubMed] [Google Scholar]
- Langworthy T. A., Mayberry W. R. A 1,2,3,4-tetrahydroxy pentane-substituted pentacyclic triterpene from Bacillus acidocaldarius. Biochim Biophys Acta. 1976 Jun 22;431(3):570–577. doi: 10.1016/0005-2760(76)90221-6. [DOI] [PubMed] [Google Scholar]
- Langworthy T. A., Mayberry W. R., Smith P. F. A sulfonolipid and novel glucosamidyl glycolipids from the extreme thermoacidophile Bacillus acidocaldarius. Biochim Biophys Acta. 1976 Jun 22;431(3):550–569. doi: 10.1016/0005-2760(76)90220-4. [DOI] [PubMed] [Google Scholar]
- Moshier S. E., Chapman D. J. Biosynthetic studies on aromatic carotenoids. Biosynthesis of chlorobactene. Biochem J. 1973 Oct;136(2):395–404. doi: 10.1042/bj1360395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandian S., Saengchjan S., Raman T. S. An alternative pathway for the biosynthesis of isoprenoid compounds in bacteria. Biochem J. 1981 Jun 15;196(3):675–681. doi: 10.1042/bj1960675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterkofsky A. The incorporation of mevalonic acid into the N6-(delta 2-isopentenyl) adenosine of transfer ribonucleic acid in Lactobacillus acidophilus. Biochemistry. 1968 Jan;7(1):472–482. doi: 10.1021/bi00841a059. [DOI] [PubMed] [Google Scholar]
- RAMAN T. S., SHARMA B. V., JAYARAMAN J., RAMASARMA T. BIOSYNTHESIS OF COENZYME Q IN MICROORGANISMS. Arch Biochem Biophys. 1965 Apr;110:75–84. doi: 10.1016/0003-9861(65)90156-6. [DOI] [PubMed] [Google Scholar]
- Raman T. S., Rudney H., Buzzelli N. K. The incorporation of p-hydroxybenzoate and isopentenyl pyrophosphate into polyprenylphenol precursors of ubiquinone by broken cell preparations of Rhodospirillum rubrum. Arch Biochem Biophys. 1969 Mar;130(1):164–174. doi: 10.1016/0003-9861(69)90022-8. [DOI] [PubMed] [Google Scholar]
- Renoux J. M., Rohmer M. Prokaryotic triterpenoids. New bacteriohopanetetrol cyclitol ethers from the methylotrophic bacterium Methylobacterium organophilum. Eur J Biochem. 1985 Sep 2;151(2):405–410. doi: 10.1111/j.1432-1033.1985.tb09116.x. [DOI] [PubMed] [Google Scholar]
- Sagami H., Ogura K., Seto S. Solanesyl pyrophosphate synthetase from Micrococcus lysodeikticus. Biochemistry. 1977 Oct 18;16(21):4616–4622. doi: 10.1021/bi00640a014. [DOI] [PubMed] [Google Scholar]
- Shiomi K., Iinuma H., Naganawa H., Isshiki K., Takeuchi T., Umezawa H. Biosynthesis of napyradiomycins. J Antibiot (Tokyo) 1987 Dec;40(12):1740–1745. doi: 10.7164/antibiotics.40.1740. [DOI] [PubMed] [Google Scholar]
- Swings J., De Ley J. The biology of Zymomonas. Bacteriol Rev. 1977 Mar;41(1):1–46. doi: 10.1128/br.41.1.1-46.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi I., Ogura K. Prenyltransferases of Bacillus subtilis: undecaprenyl pyrophosphate synthetase and geranylgeranyl pyrophosphate synthetase. J Biochem. 1982 Nov;92(5):1527–1537. doi: 10.1093/oxfordjournals.jbchem.a134077. [DOI] [PubMed] [Google Scholar]
- Taylor R. F., Davies B. H. A cell-free system for Streptococcus faecium for studies on the biosynthesis of triterpenoid carotenoids. Can J Biochem. 1982 Jun;60(6):675–683. doi: 10.1139/o82-083. [DOI] [PubMed] [Google Scholar]
- Thorne K. J. Identification of prenol intermediates of wall biosynthesis in growing cells of Lactobacillus plantarum. J Bacteriol. 1973 Oct;116(1):235–244. doi: 10.1128/jb.116.1.235-244.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne K. J. Incorporation of radioactive mevalonate into C50 and C55 phenols by Streptococcus mutans. Biochem J. 1973 Nov;135(3):567–568. doi: 10.1042/bj1350567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne K. J., Kodicek E. The structure of bactoprenol, a lipid formed by lactobacilli from mevalonic acid. Biochem J. 1966 Apr;99(1):123–127. doi: 10.1042/bj0990123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisotzkey J. D., Jurtshuk P., Jr, Fox G. E., Deinhard G., Poralla K. Comparative sequence analyses on the 16S rRNA (rDNA) of Bacillus acidocaldarius, Bacillus acidoterrestris, and Bacillus cycloheptanicus and proposal for creation of a new genus, Alicyclobacillus gen. nov. Int J Syst Bacteriol. 1992 Apr;42(2):263–269. doi: 10.1099/00207713-42-2-263. [DOI] [PubMed] [Google Scholar]
- Zhou D., White R. H. Early steps of isoprenoid biosynthesis in Escherichia coli. Biochem J. 1991 Feb 1;273(Pt 3):627–634. doi: 10.1042/bj2730627. [DOI] [PMC free article] [PubMed] [Google Scholar]


