Abstract
Although several reports showed the effect of compounds disrupting microtubules on NF-κB (nuclear factor κB) activation, nothing is known about agents perturbing actin dynamics. In the present study, we have shown that actin cytoskeleton disruption induced by actin-depolymerizing agents such as cytochalasin D and latrunculin B and actin-polymerizing compounds such as jasplakinolide induced NF-κB activation in myelomonocytic cells. The transduction pathway involved the IκB (inhibitory κB) kinase complex and a degradation of IκBα. We have shown that NF-κB activation in response to the perturbation of actin dynamics required reactive oxygen species, as demonstrated by the effect of antioxidants. Actin cytoskeleton disruption by cytochalasin D induced O2− release from human monocytes, through the activation of the NADPH oxidase, as confirmed by the phosphorylation and by the membrane translocation of p47phox. NF-κB activation after actin cytoskeleton disruption could be physiologically relevant during monocyte activation and/or recruitment into injured tissues, where cellular attachment, migration and phagocytosis result in cyclic shifts in cytoskeletal organization and disorganization.
Keywords: actin cytoskeleton, myelomonocytic cells, nuclear factor κB (NF-κB), reactive oxygen species (ROS)
Abbreviations: CytD, cytochalasin D; DFP, di-isopropyl fluorophosphate; DPI, diphenyleneiodonium chloride; DTT, dithiothreitol; EMSA, electrophoretic mobility-shift assay; FBS, fetal bovine serum; HRP, horseradish peroxidase; IκB, inhibitory κB; IKK, IκB kinase; IL-1β, interleukin-1β; JP, jasplakinolide; LatB, latrunculin B; LPS, lipopolysaccharide; LUC, luciferase; NAC, N-acetylcysteine; NF-κB, nuclear factor κB; PKC, protein kinase C; PX domain, phox homology domain; ROS, reactive oxygen species; SH3 domain, Src homology 3 domain; Tiron, 4,5-dihydroxy-1,3-benzenedisulphonic acid; TNF-α, tumour necrosis factor α
INTRODUCTION
The cytoskeleton is involved in many aspects of cellular function, such as cell movement [1], muscle contraction [2], phagocytosis [3] and mitosis [4]. Several studies suggested a link between cytoskeleton dynamics and alteration of gene expression through direct interactions with members of transduction pathways. Indeed, it has been demonstrated that cytoskeleton-disrupting factors can activate specific protein kinases [5,6] or transcription factors [7–10]. For example, a variety of agents that depolymerize microtubules were shown to be capable of activating the transcription factor NF-κB (nuclear factor κB) in many different cell types [11–14].
The transcription factor NF-κB regulates the expression of an exceptionally large number of genes, particularly those involved in immune and inflammatory responses, in the control of apoptosis and cell proliferation (for a review see [15]). Inappropriate regulation of NF-κB is directly involved in a wide range of human disorders, including a variety of cancers (reviewed in [16]), neurodegenerative diseases (reviewed in [17]), arthritis (reviewed in [18]), asthma (reviewed in [19]) and many other inflammatory diseases. NF-κB binds specific DNA sequences as dimers of the Rel/NF-κB family (reviewed in [15]). NF-κB complexes are sequestered in the cytoplasm of most resting cells by inhibitory proteins belonging to the IκB (inhibitory κB) family [15]. Upon stimulation with numerous pro-inflammatory agents such as cytokines [TNF-α (tumour necrosis factor α) and IL-1β (interleukin-1β)] or bacterial LPS (lipopolysaccharide), IκBα is phosphorylated, ubiquitinated, then degraded by the 26 S proteasome, allowing NF-κB to translocate to the nucleus and transactivate its target genes (reviewed in [20,21]). The canonical pathway induced by pro-inflammatory stimuli involves a specific IKK (IκB kinase), which phosphorylates IκBα on Ser-32 and Ser-36. IKK is a complex composed of two catalytic subunits, IKKα and IKKβ, and a regulatory subunit, IKKγ/NEMO [20,21]. Activation of IKK by all pro-inflammatory stimuli depends on phosphorylation of either the IKKβ or IKKα catalytic subunits at two conserved serine residues [20,21]. Exactly how any given signalling pathway targets the IKK complex is still a matter of debate. Such a phosphorylation may be achieved either through the action of an upstream kinase or through trans-autophosphorylation brought about by induced proximity of two catalytic subunits within the same complex.
Several lines of evidence indicate that the redox status of cells participates in modulating NF-κB activation. This is based on the following observations: (i) a broad range of antioxidants can block NF-κB activation [22], (ii) most, if not all, agents activating NF-κB are known to trigger the formation of ROS (reactive oxygen species) [23–25] and (iii) NF-κB activation can be induced by treatment with H2O2 [22,26].
Although several reports showed the effect of microtubule-disrupting compounds on NF-κB activation, only a little is known about agents perturbing actin dynamics. However, several stimuli known to activate NF-κB, such as the cytokines TNF-α [27] and IL-1β [28], PMA [29] and LPS [30], also induce actin cytoskeleton reorganization. Moreover, many physiological events such as phagocytosis [3], cellular adhesion [31] and chemotaxis [32] governed by actin-based cytoskeleton are often accompanied by NF-κB activation and the expression of pro-inflammatory genes. For these two reasons, we explored the potential role of the actin cytoskeleton in modulating the transduction pathway leading to NF-κB activation.
We showed a significant NF-κB activation in myelomonocytic cells in response to the perturbation of actin dynamics induced either by inhibitors of actin polymerization such as CytD (cytochalasin D) [33] and LatB (latrunculin B) [34] or by inducers of actin polymerization such as JP (jasplakinolide) [35]. The transduction pathway involved the activation of the IKK complex, followed by IκBα phosphorylation on Ser-32/Ser-36 and IκBα proteolysis. We also showed that actin cytoskeleton disruption by CytD induced O2− release from human monocytes through the NADPH oxidase. This oxygen species was required as a second messenger in the signalling pathway leading to NF-κB activation.
MATERIALS AND METHODS
Materials
CytD, Tiron (4,5-dihydroxy-1,3-benzenedisulphonic acid), DPI (diphenyleneiodonium chloride), isoluminol, LPS (serotype 0111/b4), PMA and the monoclonal anti-β-actin antibody were purchased from Sigma (St. Louis, MO, U.S.A.). JP, LatB and DFP (di-isopropyl fluorophosphate) were obtained from Calbiochem (La Jolla, CA, U.S.A.). Bay 11-7082 was purchased from Alexis Corporation (San Diego, CA, U.S.A.). The reporter construct (κB)5LUC (where LUC stands for luciferase) was purchased from Stratagene (La Jolla, CA, U.S.A.). Plasmid bearing the IκBα S32A (Ser32→Ala), S36A mutated gene under the control of the cytomegalovirus promoter was donated by A. Israel (Pasteur Institute, Paris, France). The IKKβ dominant-negative forms (K44M and S177/181A) were obtained from R. Gaynor (University of Texas Southwestern Medical Center). The polyclonal antibodies anti-p50, -p65, -cRel and -RelB were purchased from Euromedex (Souffelweyersheim, France) and Santa Cruz Biotechnology (Santa Cruz, CA, U.S.A.) respectively. Monoclonal antibody MAD10B that recognizes IκBα was donated by R. Hay (Department of Biology, University of St Andrews, St Andrews, Scotland, U.K.). The rabbit serum anti-p47phox was donated by Bernard M. Babior (The Scripps Research Institute, La Jolla, CA, U.S.A.). Human recombinant TNF-α, DNase I and HRP (horseradish peroxidase) were obtained from Roche Molecular Biochemicals (Mannheim, Germany).
Cell culture and transfection
Human promonocytes U937, promyelocytes HL-60, monocytes THP-1 and T lymphocytes CEM (A.T.C.C., Rockville, MD, U.S.A.) were cultivated in RPMI 1640 supplemented with 2 mM glutamine and 10% (v/v) FBS (fetal bovine serum). Mouse macrophages Mf4/4, a gift from R. Beyaert (University of Ghent, Belgium [36]), were cultivated in RPMI 1640 supplemented with 2 mM glutamine, 10% FBS and 2-mercaptoethanol (50 μM). The human cervix carcinoma cell line HeLa and the murine fibroblast cell line L929 (A.T.C.C.) were cultivated in modified Eagle's medium supplemented with 2 mM glutamine and 10% FBS.
The myelomonocytic cell lines U937, HL-60 and THP-1 were transfected by the DEAE–dextran method as described previously [37].
Preparation of human primary monocytes
Whole blood was centrifuged over a lymphoprep density solution. The platelets were removed by several washes through serum and the mononuclear cells were incubated on Petri dishes for 2 h in RPMI 1640 supplemented with 10% FBS. Non-adherent cells were eliminated after washing.
Gene reporter assays
The mononuclear cells were transfected with a reporter plasmid such as (κB)5LUC, and after 24 h, they were either treated or not with LPS, TNF-α, CytD, LatB or JP for 6 h. In experiments with antioxidants, cells were either preincubated or not with NAC (N-acetylcysteine), Tiron or DPI for 45 min before CytD or JP treatment. Then, cells were lysed for the determination of LUC activities in cellular extracts (luciferase reporter gene assay, Roche Molecular Biochemicals). The LUC activity of the samples was normalized with the protein concentration measured by the Bradford method (Bio-Rad, Hercules, CA, U.S.A.).
Nuclear and cytoplasmic protein extraction
The extraction method for HeLa and L929 cells was described previously [38]. The extraction procedures for the lymphocytic cell line CEM, myelomonocytic cell lines (U937, HL-60, THP-1, Mf4/4) and human monocytes were slightly different. Briefly, 5×106 cells were resuspended in 400 μl of cold hypo-osmotic buffer [10 mM Hepes/KOH, 2 mM MgCl2, 0.1 mM EDTA, 10 mM KCl, 1 mM DTT (dithiothreitol), 1 mM PMSF and protease inhibitors (Roche Molecular Biochemicals), pH 7.9] and left on ice for 10 min. Then, Nonidet P40 (0.007%) was added to the cell suspension that was immediately vortex-mixed and centrifuged at 15000 g for 30 s. The supernatant containing the cytoplasmic proteins could be stored at −80 °C. The pellets of nuclei were gently resuspended in 15 μl of cold saline buffer [50 mM Hepes/KOH, 50 mM KCl, 300 mM NaCl, 0.1 mM EDTA, 10% (v/v) glycerol, 1 mM DTT, 1 mM PMSF and protease inhibitors (Roche Molecular Biochemicals), pH 7.9] and left for 20 min on ice. After centrifugation at 15000 g for 15 min, at 4 °C, the supernatant containing nuclear proteins was stored at −80 °C. Protein concentrations were measured with the protein assay from Bio-Rad.
EMSA (electrophoretic mobility-shift assay)
In brief, 5 μg of nuclear proteins was incubated for 30 min at room temperature (25 °C) in a volume of 10 μl with 0.2 ng of 32P-labelled oligonucleotide probe in binding buffer [20 mM Hepes/KOH (pH 7.9), 75 mM NaCl, 1 mM EDTA, 5% glycerol, 0.5 mM MgCl2 and 1 mM DTT] containing 2 μg of BSA and 1.25 μg of poly(dI-dC)·(dI-dC) (Amersham Biosciences, Roosendael, The Netherlands). For competition experiments, the unlabelled probe was added in excess to the binding buffer. For supershift assays, specific antibodies against p50, p65, c-Rel and RelB were incubated with nuclear proteins in the binding buffer for 20 min on ice before the addition of the probe. DNA–protein complexes were then resolved by electrophoresis on a non-denaturing 6% (w/v) polyacrylamide gel for 2 h at 300 V in 0.25×TBE (2.5 mM Tris, 2.5 mM H3BO3 and 2 mM EDTA). The gels were then dried and autoradiographed on a Fuji X-ray film. The sequences of the oligonucleotide probes were as follows: wild-type κB probe (5′-GGTTACAAGGGACTTTCCGCTG-3′ and 5′-TTGGCAGCGGAAAGTCCCTTGT-3′), mutated κB probe (5′-GGTTACAACTCACTTTCCGCTG-3′ and 5′-TTGGCAGCGGGAAAGTGAGTTGT-3′), wild-type Sp1 probe (5′-GGTTATTCGATCGGGGCGGGGCGAGC-3′ and 5′-TTGGGCTCGCCCCGCCCCGATCGAAT-3′). The oligonucleotide probes were labelled by in- filling with the Klenow DNA polymerase (Roche Molecular Biochemicals).
Preparation of detergent-soluble and -insoluble cell extracts
Lysis buffer contains 50 mM Pipes/KOH (pH 6.9), 50 mM NaCl, 5 mM MgCl2, 5 mM EGTA, 5% glycerol, 0.1% Nonidet P40, 0.1% Triton X-100, 0.1% Tween 20, 0.1% (v/v) 2-mercaptoethanol, 1 mM ATP and protease inhibitors (Roche Molecular Biochemicals). Cells are lysed with approx. 50 vol. of prewarmed buffer. After incubation for 10 min at 37 °C, samples are centrifuged at 100000 g for 60 min at room temperature. Supernatants containing detergent-soluble cell proteins were harvested and put on ice. Pellets comprising detergent-insoluble proteins are resuspended in the same volume as supernatants with ice-cold lysis buffer.
Immunoblot
One volume of Laemmli buffer [60 mM Tris/HCl (pH 6.8), 2.5% (w/v) SDS, 10% glycerol, 5% 2-mercaptoethanol and 0.03% Bromophenol Blue] was added to protein samples that, after boiling for 5 min, were subjected to SDS/PAGE and electrotransferred on to PVDF membranes (Roche Molecular Biochemicals) or nitrocellulose membranes (in the case of the control of immunoprecipitation of p47phox). After probing with primary and secondary antibodies, membranes were finally analysed with ECL® system (Amersham Biosciences) or with alkaline-phosphatase system (Sigma).
Measure of O2− production by isoluminol-enhanced chemiluminescence
Human monocytes were scraped and resuspended in PBS supplemented with 7.5 mM glucose, 0.5 mM MgCl2 and 0.9 mM CaCl2. After the addition of isoluminol (50 μM) and HRP (4 units/ml), the cell suspension was divided in black 96-microplates (0.3×106 cells/250 μl/well). Cells were allowed to equilibrate for 15 min. Antioxidants were added for 30 min before stimulus (PMA/ionomycin, CytD or JP). Chemiluminescence was measured with the Wallac Victor 2 TM 1420 multilabel counter (PerkinElmer Life Sciences, Boston, MA, U.S.A.).
Preparation of membrane fractions
Membranes of human monocytes were prepared as described previously [39]. Cells were washed and scraped in PBS after PMA stimulation or treatment with CytD or JP. Approximately 3×106 monocytes were resuspended in 350 μl of relaxation buffer [100 mM KCl, 3 mM NaCl, 3.5 mM MgCl2, 10 mM Pipes, 0.5 mM PMSF, 1 mM EGTA and protease inhibitors (Roche Molecular Biochemicals)]. Cells were then disrupted by sonication on ice (3×10 s). Unbroken cells and nuclei were pelleted by centrifugation at 600 g for 10 min at 4 °C. The supernatant was then ultracentrifuged at 100000 g for 30 min at 4 °C. Then the pellet was washed with relaxation buffer, dissolved in Laemmli sample buffer and submitted to SDS/PAGE and immunoblot with anti-p47phox antibody.
Phosphorylation of p47phox in monocytes and immunoprecipitation
Human monocytes were washed and resuspended in Dulbecco's modified Eagle's medium without phosphate and serum. After the labelling with 32P (250 μCi/ml), monocytes (∼2×107 cells) were resuspended in loading buffer without phosphate [20 mM Hepes (pH 7.4), 140 mM NaCl, 5.7 mM KCl, 0.8 mM MgCl2, 1 mM CaCl2 and 0.025% BSA]. After various treatments, cells were scraped on ice in lysis buffer [20 mM Tris/HCl, pH 7.4, 150 mM NaCl, 0.5% Triton, 5 mM EGTA, 5 mM EDTA, 0.5 mM PMSF, 15 μg/ml leupeptin, 10 μg/ml pepstatin, 10 μg/ml aprotinin, 1 mM DFP, 8% (w/v) sucrose, 1 mg/ml DNase I, 25 mM NaF, 5 mM Na3VO4, 2 mM β-glycerophosphate and 0.5 mM p-nitrophenyl phosphate]. Cells were then disrupted by sonication on ice (2×10 s) and were ultracentrifuged at 100000 g for a period of 30 min at 4 °C. The supernatant containing total proteins was incubated in the lysis buffer with anti-p47phox antibody (1/100) and the immunocomplex was precipitated overnight using Protein G–Sepharose beads at 4 °C. Then, the beads were washed three times with the lysis buffer without DFP and DNase and resuspended in Laemmli sample buffer. The samples were submitted to SDS/PAGE and transferred on to a nitrocellulose membrane. The membrane was then autoradiographed and immunoblotted with anti-p47phox antibody.
RESULTS
Perturbation of actin dynamics induces NF-κB activation in myelomonocytic cells
To test whether cytoskeletal events can induce NF-κB activation, a naturally occurring actin-modulating substance, CytD, was used. CytD, by capping actin filaments and stimulating ATP hydrolysis on G-actin, inhibits actin polymerization [33]. Figure 1 shows the effect of CytD treatment on NF-κB activation determined by EMSA in various cell types. This actin-modulating treatment induced a very significant NF-κB nuclear translocation, when compared with classical inducers such as LPS and TNF-α, in myelomonocytic cell lines such as promonocytes U937, promyelocytes HL-60, mouse macrophages Mf4/4 and monocytes THP-1 (Figure 1A). On the other hand, no significant modification of DNA-binding activities was observed in nuclear extracts of several other cell lines, such as human epithelial cells (HeLa), murine fibroblasts (L929) and human T lymphocytes (CEM) (Figure 1A). NF-κB activation by CytD treatment was confirmed in freshly isolated human monocytes (Figure 1B). These results suggest that the responsiveness to the actin-disruption treatment is restricted to myelomonocytic cells.
Figure 1. CytD treatment induces NF-κB nuclear translocation in myelomonocytic cell lines and human monocytes.
(A) Effect of CytD on NF-κB activation in various cell lines. Various myelomonocytic (U937, HL-60, Mf4/4, THP-1) or non-myelomonocytic cell lines (HeLa, L-929, CEM) were either treated or not with TNF-α (100 units/ml, 30 min), LPS (10 μg/ml, 150 min) or CytD (150 min). The DNA-binding activities in nuclear extracts were analysed by EMSA with a consensus NF-κB probe. (B) Effect of CytD on NF-κB activation in primary human monocytes. Human monocytes were either treated or not with LPS (10 μg/ml) or CytD for 120 min. The DNA-binding activities in nuclear extracts were analysed by EMSA with a consensus NF-κB probe.
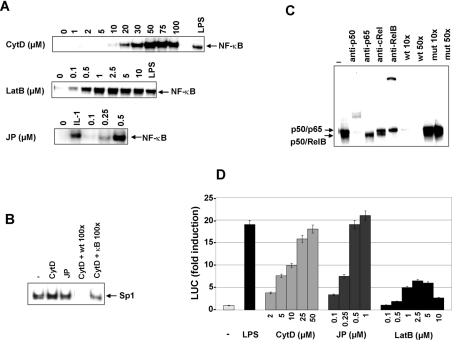
We used other modulators of actin assembly, namely LatB [34], a second actin polymerization inhibitor, and JP [35], known to induce actin polymerization. Similar to what was observed with CytD, LatB induced a significant NF-κB activation in promonocytes U937 (Figure 2A) as well as in promyelocytes HL-60 (results not shown). NF-κB activation by CytD and LatB was quantitatively important compared with LPS-treated promonocytes and was dependent on the drug concentration. NF-κB nuclear translocation started to appear with rather low doses of CytD (5 or 10 μM), but was optimal at 50 μM, depending on the cell type. Increasing CytD concentrations up to 100 μM did not lead to a further increase in NF-κB activation. Lower LatB concentrations allowed us to induce NF-κB translocation that appeared at 0.1 μM with a maximum at 1 μM (Figure 2A). A kinetic analysis of NF-κB activation after a Cyt D treatment of U937 cells was carried out, and a maximal activation was observed between 2 and 3 h (results not shown). Interestingly, JP also induced a strong and dose-dependent NF-κB activation in THP-1 monocytes (Figure 2A) as well as in U-937 and HL-60 cells (results not shown). The response to JP began at 0.1 μM and was maximal at 0.5 μM. Thus NF-κB activation was observed in response to both actin-polymerizing and -depolymerizing treatments. To prove the specificity of NF-κB activation in response to actin-cytoskeleton-disrupting drugs, we tested the effect of CytD and JP treatments on Sp1 activation. No change in the binding activities to the Sp1 probe could be observed in nuclear extracts of U937 cells after treatment with CytD or JP (Figure 2B).
Figure 2. Actin dynamics modulation by CytD, LatB and JP induces an activation of NF-κB in myelomonocytic cell lines.
(A) NF-κB nuclear translocation in response to actin cytoskeleton disruption by CytD, LatB and JP. Myelomonocytic cell lines were either treated or not with LPS (10 μg/ml), IL-1β (100 units/ml) or various concentrations of CytD (U937 cells), LatB (HL-60) or JP (THP-1) for 150 min. The DNA-binding activities in nuclear extracts were analysed by EMSA with a consensus binding site for NF-κB. (B) Nuclear proteins of CytD-treated cells (50 μM) or JP-treated cells (0.5 μM) were analysed by EMSA with a specific Sp1 probe. Competition experiments were performed with an excess of unlabelled oligonucleotide carrying the consensus site for Sp1 (CytD+wt, 100×) or NF-κB (CytD+κB, 100×). (C) Identification of NF-κB complexes. Supershift and competition assays were performed on nuclear extracts of U937 cells treated by CytD (50 μM) for 150 min. Nuclear proteins were incubated with antibodies specific for p50, p65, RelB and c-Rel or with an excess (10- or 50-fold) of unlabelled oligonucleotide wild-type (wt) or mutated (mut) before being analysed by EMSA with a consensus NF-κB binding site. (D) Actin cytoskeleton disruption by CytD, LatB and JP induces NF-κB-mediated transcription. HL-60 cells, 24 h post-transfection with a (κB)5LUC reporter plasmid, were either treated or not with LPS (10 μg/ml) or various concentrations of CytD, JP or LatB for 6 h before being harvested for LUC assays. Values are presented as means±S.D. (n=3).
The composition of NF-κB complex(es) induced in response to CytD and JP was determined by supershift assays with antibodies directed against the various members of the Rel family, and the band specificity was determined by competition experiments. Supershift assays allowed us to detect in the nucleus of CytD-treated cells two distinct complexes binding the κB probe (Figure 2C). These two bands were specific, as demonstrated by competition assays. Supershift experiments with antibodies against p50, p65, cRel and RelB demonstrated that the two complexes were the p50–RelB and p50–p65 heterodimers. The presence of the p52 subunit was not tested. These two NF-κB complexes were also induced after treatment with JP (results not shown).
We then investigated whether NF-κB was transcriptionally active by transient transfection assays with a reporter plasmid (κB)5LUC (Figure 2D). NF-κB-mediated transcription was significantly induced in CytD- and JP-treated HL-60 cells (about 20-fold). This induction was comparable with the one obtained for LPS and was also observed in two other cell lines, U937 and THP-1 (results not shown). The dose-dependent increase in NF-κB-mediated transcription by CytD and JP correlated with the results obtained by EMSA (Figure 2A). LatB was less efficient compared with the other two actin-modulating agents to induce NF-κB-mediated transcription, while this compound induced similar levels of NF-κB nuclear translocation (Figure 2A). The same actin-disrupting treatments did not lead to any significant increase in LUC activities in non-myelomonocytic cell lines such as HEK-293 cells (human embryonic kidney 293 cells) or HeLa cells (results not shown).
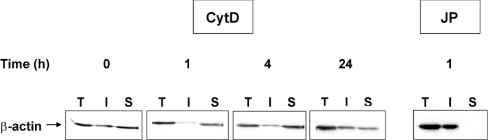
We wanted to confirm that CytD and JP had the expected effect on actin dynamics. This was demonstrated by examining the β-actin levels in Triton X-100-insoluble and -soluble fractions. Actin recovered from the Triton X-100-insoluble fraction corresponds to the cytoskeleton-associated actin, whereas the cellular actin found in the Triton X-100-soluble fraction is presumed to be either unpolymerized G-actin or polymerized F-actin, which is not functionally associated with the cytoskeleton. Western-blot analysis showed that β-actin levels in both fractions were similar in untreated U937 cells (Figure 3). As expected, CytD induced F-actin depolymerization, illustrated by the lack of β-actin in Triton X-100-insoluble fraction after 1 h treatment. However, F-actin depolymerization was transient, since β-actin could be detected again in Triton X-100-insoluble fraction after 4 h. After 24 h, β-actin distribution between both the fractions was comparable with that of the untreated cells. As expected, after 1 h treatment with JP, all of β-actin was present in the Triton X-100-insoluble fraction (Figure 3).
Figure 3. Actin depolymerization and polymerization after treatment by CytD and JP respectively.
Total (T), Triton X-100-insoluble (I) and Triton X-100-soluble (S) fractions were prepared from U937 cells treated for various times with CytD (50 μM) or JP (0.5 μM). These extracts were analysed by SDS/PAGE and immunoblotted with a monoclonal anti-β-actin antibody.
Taken together, these results suggest that there is no correlation between a specific state of actin polymerization and NF-κB activation. The signal seems to be a perturbation of actin dynamics, which is sensed by myelomonocytic cells and converted into NF-κB activation. It is important to notice that U937 cells were found to be unresponsive to antimicrotubule agents, such as paclitaxel and vinblastin (results not shown).
NF-κB activation by CytD and JP involves the IKK complex
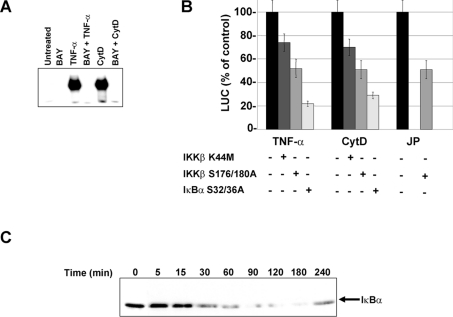
Most of the NF-κB activation pathways converge to the specific IKK complex, which is composed of two catalytic subunits, IKKα and IKKβ, and one regulatory subunit, IKKγ [20,21]. In response to all known pro-inflammatory stimuli, IKKβ phosphorylates the inhibitory protein IκBα on Ser-32 and Ser-36, allowing its degradation and NF-κB translocation to the nucleus [20,21]. The role of the IKK complex was investigated by using an inhibitor of IKK complex named Bay 11-7082 [40], or by transiently expressing a dominant-negative form of IKKβ. As shown in Figure 4(A), pretreatment of HL-60 cells with Bay completely abolished NF-κB activation in response to TNF-α and CytD. The expression of IKKβ K44M or S177/181A, both of which carry a point mutation either in the kinase domain or in the activation loop respectively, resulted in a decrease in NF-κB-mediated transcription in response to CytD (up to 75 and 50% of control respectively) (Figure 4B). In the case of JP treatment, the dominant-negative form IKKβ S177/181A also induced a significant decrease in NF-κB activation (up to 50%). As a control, we tested the effect of the same dominant-negative forms on TNF-α-mediated NF-κB activation and obtained the same inhibition percentage as with CytD and JP. A dominant-negative form of IκBα (IκBα S32A/S36A), which cannot be phosphorylated and therefore cannot be degraded, led to a significant inhibition in CytD- and TNF-α-mediated NF-κB activation (20 and 30% respectively) (Figure 4B). Taken together, these results indicate that the transduction pathway triggered by actin cytoskeleton disruption and leading to NF-κB activation requires the IKK complex and IκBα phosphorylation on Ser-32 and Ser-36. The next step, in the classical NF-κB activation pathway, involves IκBα ubiquitination followed by IκBα proteolysis. Figure 4(C) shows that CytD treatment led to the cytoplasmic IκBα degradation that began after 30 min and was maximal after 2 h. The time course of IκBα proteolysis was concomitant with the nuclear translocation of NF-κB (results not shown). Newly synthesized IκBα appeared after 4 h.
Figure 4. NF-κB activation pathway involves the IKK complex and IκBα proteolysis.
(A) Effect of Bay 11-7082, an inhibitor of IKK complex. HL-60 cells were either pretreated or not with Bay 11-7082 (10 μM) for 45 min, then treated with or without CytD (50 μM) for 150 min or with TNF-α (100 units/ml) for 30 min. The DNA-binding activities in nuclear extracts were analysed by EMSA with a consensus NF-κB binding site. (B) Effect of dominant-negative IKKβ and IκBα on CytD- and JP-induced NF-κB activation. U937 cells were co-transfected with the reporter plasmid (κB)5LUC and/or with the empty vector or the plasmids encoding dominant-negative IKKβ (K44M or S177A/S181A) or IκBα (S32A/S36A). The cells, 24 h post-transfection, were treated with TNF-α (250 units/ml), CytD (50 μM) or JP (0.5 μM) for 6 h before being harvested for LUC assays. Values are presented as the means±S.D. (n=3). (C) IκBα proteolysis. IκBα expression was analysed by immunoblot in cytoplasmic extracts of U937 cells treated with CytD (50 μM) for the times indicated.
NF-κB activation by CytD and JP involves ROS
Several reports stated that NF-κB induction after a number of stimuli depends on ROS production [22,26]. We therefore evaluated the role of ROS in NF-κB activation after actin cytoskeleton disruption by testing the effect of antioxidants and by measuring the ROS production.
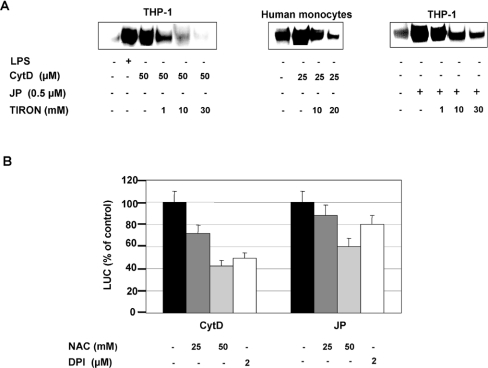
Pretreatment of THP-1 cells with Tiron, a superoxide (O2−) scavenger [41], inhibited NF-κB translocation in a dose-dependent manner after CytD or JP treatment (Figure 5A). The inhibition was particularly strong with CytD, since NF-κB translocation was completely abolished by Tiron at 30 mM (Figure 5A). The NF-κB activation pathway in response to CytD seems to depend equally on ROS in freshly isolated human monocytes, since NF-κB translocation is also decreased after the pretreatment with Tiron (Figure 5A). Preincubation of THP-1 cells with NAC resulted in a dose-dependent decrease in NF-κB-mediated transcription in response to both the actin-modulating treatments (Figure 5B). The effect of NAC was less marked when actin cytoskeleton disruption was induced by JP. DPI, an NADPH oxidase inhibitor [42], also induced a significant decrease in NF-κB activation in response to CytD (up to 50%) (Figure 5B). However, DPI had only a little effect on JP-induced NF-κB activation (Figure 5B).
Figure 5. Effect of antioxidants on NF-κB activation.
(A) Effect of Tiron on NF-κB nuclear translocation. THP-1 cells or human monocytes were either preincubated or not with Tiron for a period of 45 min and then treated either with or without LPS (10 μg/ml), Cyt D or JP for 120 min. The DNA-binding activities in nuclear extracts were analysed by EMSA with a consensus NF-κB probe. (B) Effect of NAC and DPI on NF-κB-mediated transcription. THP-1 cells, 24 h post-transfection with a (κB)5LUC reporter plasmid, were either preincubated or not with NAC or DPI for 45 min and then treated with Cyt D (50 μM) or JP (0.5 μM) for 6 h before being harvested for LUC assays. Values are presented as the means±S.D. (n=3).
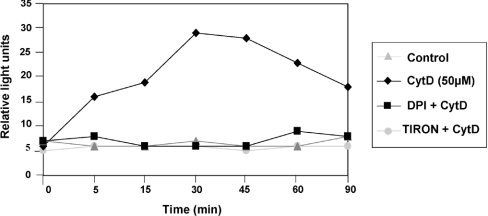
To measure the ROS production in response to the perturbation of actin dynamics, we used an isoluminol-amplified chemiluminescence assay on freshly isolated human monocytes [43]. This very sensitive technique is currently used for measuring O2− release from activated monocytes/macrophages or neutrophils. When we used PMA+ionomycin to stimulate the oxidative burst of monocytes, we observed a very strong O2− generation (results not shown). A significant and reproducible ROS generation, up to 5 times higher than in untreated cells, was observed after incubating human monocytes with CytD at 50 μM (Figure 6). The response began immediately after CytD addition, reached its maximum at 30 min and then decreased slowly. ROS production could be completely abolished after pretreatment of monocytes with DPI or Tiron. Lower CytD concentrations led to a more transient ROS production (results not shown). These results suggested that actin cytoskeleton disruption by CytD induced an extracellular generation of ROS whose major species is O2−, probably produced by membrane NADPH oxidase. On the other hand, no ROS release could be detected after the treatment of human monocytes with JP (0.1–1 μM). However, results obtained with Tiron and NAC (Figure 5) suggested that NF-κB activation by JP was ROS-dependent. The very low level of inhibition exerted by DPI on NF-κB activation together with the lack of production of extracellular ROS could suggest that actin disruption by JP induces ROS production in the intracellular compartment, independently of membrane NADPH oxidase.
Figure 6. O2− production in response to CytD treatment in human monocytes.
O2− production was measured by isoluminol-amplified chemiluminescence and was given as relative light units. After the incubation with isoluminol and HRP, cells were either preincubated or not incubated for 30 min with Tiron (10 mM) or DPI (2 μM) and then treated with CytD (50 μM). Control corresponded to cells incubated with only isoluminol and HRP. Measurement of O2− production began immediately after the addition of CytD. Results were representative of three experiments.
Cytoskeleton disruption by CytD induces the NADPH oxidase activation in human monocytes
We wanted to confirm that O2− release detected by isoluminol-enhanced chemiluminescence in response to CytD treatment resulted from the activation of the NADPH oxidase. In phagocytes, the NADPH oxidase complex includes membrane-bound cytochrome b558 (composed of the subunits gp91phox and p22phox) and cytosolic proteins (p47phox, p67phox, Rac and p40phox) (as reviewed in [44]). During stimulation, cytosolic subunits translocate to the membrane to form a catalytically active oxidase producing large amounts of O2− by reducing oxygen at the expense of NADPH [44]. A method currently used to estimate the NADPH oxidase activation involves the detection of the subunit p47phox in membrane extracts after cell stimulation.
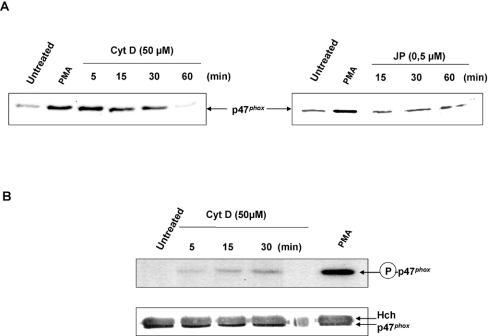
Human monocytes stimulated by PMA, CytD or JP for various times were harvested to prepare membrane fractions. Figure 7(A) shows a Western-blot analysis with anti-p47phox antibody. As expected, stimulation with PMA induced p47phox translocation. CytD treatment for 5 min resulted in a significant p47phox translocation, when compared with PMA. After 5 min, the level of p47phox in membranes slowly decreased to return to the basal level after 1 h. The time course of p47phox translocation correlated with the O2− release is shown in Figure 7. As expected, no p47phox membrane translocation could be observed after JP treatment (Figure 7A).
Figure 7. CytD treatment induces the phosphorylation and the translocation of p47phox.
(A) Human monocytes were either not activated or activated for 10 min with PMA (30 ng/ml) or treated for different times with CytD (50 μM) or JP (0.5 μM). The cells were then collected and fractionated as described under the Materials and methods section. The membrane fractions were separated by SDS/PAGE (10% gel). P47phox was detected by immunoblot with a rabbit serum against anti-human p47phox. (B) 32P-labelled human monocytes were either treated or not treated with PMA (30 ng/ml) or CytD (50 μM) for time periods of 5, 15 and 30 min. P47phox was then immunoprecipitated with the rabbit serum against anti-human p47phox, subjected to SDS/PAGE, blotted on to a nitrocellulose membrane and detected by autoradiography or immunoblot. Hch, heavy chain of the IgG antibody was used for immunoprecipitation. Results were representative of two experiments.
The membrane translocation of p47phox is promoted by its phosphorylation disrupting an intramolecular interaction to render its PX (phox homology) and SH3 (Src homology 3) domains in a state accessible to membrane phosphoinositides and p22phox respectively [45]. We wanted to confirm that the NADPH oxidase activation in response to cytoskeleton disruption also results from the p47phox phosphorylation. The phosphorylation of p47phox in human monocytes was analysed after 32P labelling, p47phox immunoprecipitation and SDS/PAGE. As expected, PMA stimulation triggered an important phosphorylation of p47phox (Figure 7B). Cytoskeleton disruption by CytD also induced the p47phox phosphorylation that began after 5 min and increased until 30 min. Western-blot results showed that the same amount of p47phox was loaded in each lane (Figure 7B).
These results demonstrated that cytoskeleton disruption by CytD induces kinase(s) activation, which allows the phosphorylation of p47phox, followed by its membrane translocation and the formation of a catalytically active NADPH oxidase.
DISCUSSION
Cytoskeleton is involved in many cellular functions, such as movement, muscle contraction, phagocytosis and mitosis (reviewed in [1–4]). Several studies suggest a link between the perturbation of cytoskeleton dynamics and an altered gene expression. For example, activation of the serum response factor, which regulates transcription of many serum-inducible and muscle-specific genes, and the nuclear factor of activated T cells is mediated by changes in actin dynamics [7–10].
Several works have described NF-κB activation in response to microtubule depolymerization [11–13]. Taxol, an agent that stabilizes microtubules, blocked NF-κB activation by phorbol ester [14]. NF-κB activation has already been described in several situations characterized by a reorganization of actin filaments, such as cellular adhesion on fibronectin [31], chemotaxis [32] or phagocytosis [3]. However, in each case, several pathways other than actin-filament reorganization may have contributed to the observed NF-κB activation, since these stimuli can initiate a variety of intracellular pathways.
This study demonstrates that disruption of the actin cytoskeleton induces a significant NF-κB activation in various myelomonocytic cell lines as well as in human monocytes. NF-κB activation was observed in response to both actin-polymerizing and -depolymerizing treatment by JP and CytD respectively. Consequently, there was no correlation between a specific state of CytD- or JP-modified actin polymerization and NF-κB activation. The signal seems to be triggered rather by changes in actin dynamics and/or by cytoskeleton disruption. Both actin-modulating treatments induced NF-κB activation through the canonical pathway involving the IKK complex that phosphorylates IκBα on Ser-32 and Ser-36, targeting it for ubiquitination and degradation by the 26 S proteasome.
By which mechanism does actin disruption lead to IKK complex activation? IKK complex stimulation depends on phosphorylation of either the IKKβ or IKKα catalytic subunits at two conserved serine residues [20,21]. How exactly any given signalling pathway targets the IKK complex is still a matter of debate. Such a phosphorylation may be achieved either through the action of an upstream kinase or through trans-autophosphorylation brought about by induced proximity of two catalytic subunits within the same complex. Actin cytoskeleton could sequester a mediator of a signalling pathway leading to the activation of the IKK complex. Disruption of dynamic cytoskeletal structures, such as membrane ruffles or lamellipodia, by CytD or JP would result in the release of this mediator, allowing either oligomerization or phosphorylation of the IKK complex by an upstream kinase. Such a model was already proposed to explain the activation of IL-1β expression in response to microtubule disruption in human monocytes [46]. It was then postulated that the microtubule network may act as a retention receptor for kinases or kinase activators. Since NF-κB activation in response to actin disruption seems to be restricted to myelomonocytic cells, such a mediator sensing the change of actin dynamics should be specific to this kind of cell. A candidate to play this role could be the protein Nod2, capable of inducing NF-κB activation and specifically expressed in myelomonocytic cells [47].
Several works demonstrated that the redox status of cells participates in the modulation of NF-κB activation [22–26]. By using antioxidants, we also demonstrated that the transduction pathway leading to NF-κB activation after actin cytoskeleton disruption involves ROS as second messengers. Since Tiron, a scavenger of O2−, was the most efficient antioxidant to inhibit the NF-κB activation, it seems that this oxygen species plays a central role in this pathway. Pretreatment with DPI, an NADPH oxidase inhibitor, significantly decreased CytD-induced NF-κB activation, but not JP-induced NF-κB activation. The Tiron- and DPI-inhibitable isoluminol oxidation after CytD addition to human monocytes allowed us to suggest that this treatment led to O2− release, probably through the NADPH oxidase. The involvement of the NADPH oxidase was confirmed by the phosphorylation and membrane translocation of p47phox in human monocytes treated with CytD. While JP treatment induced an ROS-dependent NF-κB activation, we could not detect either O2− release by isoluminol-dependent chemiluminescence or p47phox membrane translocation after the addition of this actin-modulating compound to human monocytes. A production of intracellular ROS in response to JP treatment could eventually be demonstrated by dichlorofluorescein diacetate fluorescence.
This study is the first to demonstrate that cytoskeleton disruption can lead to the activation of the NADPH oxidase of human monocytes. The currently proposed mechanism underlying the regulation of p47phox in the activation of the phagocyte NADPH oxidase is the following one [45]. Human p47phox comprises an N-terminal PX domain and two central SH3 domains. The C-terminal SH3 domain interacts intramolecularly with the PX domain. Stimulus-induced phosphorylation of p47phox in the C-terminal portion causes a conformational change, by which both PX and SH3 domains become accessible to their membranous targets, phosphoinositides and p22phox respectively. Co-operation of these two interactions, each being indispensable, enables p47phox to form a stable complex with cytochrome b558 (composed of the subunits gp91phox and p22phox), leading to activation of the phagocyte NADPH oxidase. In this work, we confirmed the activation of the NADPH oxidase in response to CytD by the detection of both p47phox phosphorylation and membrane translocation. However, the kinetics of phosphorylation did not exactly correspond to the time course of translocation. While the level of membrane p47phox was already optimal after 5 min, the level of phosphorylated p47phox reached its maximum only after 30 min. These results can be explained in different ways. First, the experimental conditions used to detect the p47phox phosphorylation or translocation are not exactly the same ones. Indeed, for 32P labelling, adherent monocytes are preincubated in a medium without phosphate and serum for 1 h before being treated by CytD in serum-free medium. In the protocol used to detect p47phox in membrane extracts, CytD treatments are performed in the presence of the serum. Secondly, the detection of phosphorylated p47phox after 32P labelling of monocytes does not allow us to distinguish between the different phosphorylated forms. Several serine residues of p47phox can be phosphorylated by different protein kinases, among which the PKC (protein kinase C) family plays a major role [48]. The identity of kinases that are involved in p47phox phosphorylation depends on the stimulus [PMA, FMLP (formyl-methionyl-leucyl-phenylalanine), zymosan etc.]. The phosphorylation of some serine residues induces stronger activation of the NADPH oxidase, while the phosphorylation of selective p47phox sites could have an inhibitory effect [49]. The p47phox sites, phosphorylated after 5 min of treatment with CytD, could induce an optimal p47phox translocation, while the p47phox hyperphosphorylation observed after 30 min could decrease the interaction between p47phox and the phosphoinositides or p22phox. Finally, the p47phox membrane translocation can be affected by events other than phosphorylation. For example, arachidonate released from the membrane by the phospholipase A2 is required for p47phox translocation, but not for phosphorylation in human monocytes stimulated by opsonized zymosan [50]. As shown by Shiose and Sumimoto [51], such a mechanism could synergize with phosphorylation of p47phox and could explain the important membrane translocation of p47phox detected in human monocytes treated by CytD for 5 min. These three arguments can also explain why the levels of membrane p47phox are similar after PMA or CytD treatment, while p47phox phosphorylation is much more important with PMA compared with CytD.
By which mechanism could cytoskeleton disruption induce p47phox phosphorylation and membrane translocation? Several kinases that phosphorylate p47phox and regulate oxidase activity have been identified, such as PKC isoforms [52–55], two mitogen-activated protein kinases, extracellular-signal-regulated kinase and p38 [56], as well as casein kinase-2 [57] and Akt [58]. The identity of kinases that are involved in p47phox phosphorylation depends on stimulus. Some reports described the activation of mitogen-activated protein kinases (p38 and extracellular-signal-regulated kinase) [59,60] and PKCs [61] in cell types other than monocytes after actin cytoskeleton disruption by CytD. The use of specific protein kinase inhibitors as well as the measurement of kinase activity should inform us about the role of these kinases in p47phox phosphorylation induced by CytD.
Interestingly, all of the O2−-generating activity of stimulated neutrophil membranes is found in the membrane cytoskeleton [62]. A very recent report demonstrated that the PX domain of p47phox targets cell membranes through moesin-mediated association with the actin cytoskeleton rather than through the interactions with phospholipids [63]. Since integrity of the membrane cytoskeleton is required for the activation of the NADPH oxidase, it can seem surprising that CytD treatment induces the activation of the NADPH oxidase. However, studies have shown that cytochalasins depolymerize some of the F-actin in neutrophils, but spare the cortical F-actin that lies directly beneath the plasma membrane [64].
Many physiological events such as phagocytosis [3], cellular adhesion [31] and chemotaxis [32] governed by actin-based cytoskeleton are often accompanied by NF-κB activation and the expression of pro-inflammatory genes. NF-κB activation after actin cytoskeleton disruption could be physiologically relevant during monocyte activation and/or recruitment into injured tissues, where cellular attachment, migration and phagocytosis result in cyclic shifts in cytoskeletal organization and disorganization.
Acknowledgments
This work was supported by grants from the National Fund for Scientific Research (FNRS, Brussels, Belgium) and by the Inter-university Attraction Poles (P5/12). G. K. is supported by a predoctoral fellowship from the Inter-university Attraction Poles (P5/12). S. L.-P. and J. P. are Research Associate and Research Director of the FNRS respectively.
References
- 1.Wittmann T., Waterman-Storer C. Cell motility: can Rho GTPases and microtubules point the way? J. Cell Sci. 2001;114:3795–3803. doi: 10.1242/jcs.114.21.3795. [DOI] [PubMed] [Google Scholar]
- 2.Clark K. A., McElhinny A. S., Beckerle M. C., Gregorio C. C. Striated muscle cytoarchitecture: an intricate web of form and function. Annu. Rev. Cell Dev. Biol. 2002;18:637–706. doi: 10.1146/annurev.cellbio.18.012502.105840. [DOI] [PubMed] [Google Scholar]
- 3.Friedland J. S., Constantin D., Shaw T. C., Stylianou E. Regulation of interleukin-8 gene expression after phagocytosis of zymosan by human monocytic cells. J. Leukoc. Biol. 2001;70:447–454. [PubMed] [Google Scholar]
- 4.Nanninga N. Cytokinesis in prokaryotes and eukaryotes: common principles and different solutions. Microbiol. Mol. Biol. Rev. 2001;65:319–333. doi: 10.1128/MMBR.65.2.319-333.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shtil A. A., Mandlekar S., Yu R., Walter R. J., Hagen K., Tan T.-H., Roninson I. B., Kong A.-N. Differential regulation of mitogen-activated protein kinases by microtubule-binding agents in human breast cancer cells. Oncogene. 1999;18:377–384. doi: 10.1038/sj.onc.1202305. [DOI] [PubMed] [Google Scholar]
- 6.Christerson L. B., Vanderbilt C. A., Cobb M. H. MEKK1 interacts with alpha-actinin and localizes to stress fibers and focal adhesions. Cell Motil. Cytoskeleton. 1999;43:186–198. doi: 10.1002/(SICI)1097-0169(1999)43:3<186::AID-CM2>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 7.Sotiropoulos A., Gineitis D., Copeland J., Treisman R. Signal-regulated activation of serum response factor is mediated by changes in actin dynamics. Cell (Cambridge, Mass.) 1999;98:159–169. doi: 10.1016/s0092-8674(00)81011-9. [DOI] [PubMed] [Google Scholar]
- 8.Mack C. P., Somlyo A. V., Hautmann M., Somlyo A. P., Owens G. K. Smooth muscle differentiation marker gene expression is regulated by RhoA-mediated actin polymerization. J. Biol. Chem. 2001;276:341–347. doi: 10.1074/jbc.M005505200. [DOI] [PubMed] [Google Scholar]
- 9.Holsinger L. J., Graef I. A., Swat W., Chi T., Bautista D. M., Davidson L., Lewis R. S., Alt F. W., Crabtree G. R. Defects in actin-cap formation in Vav-deficient mice implicate an actin requirement for lymphocyte signal transduction. Curr. Biol. 1998;8:563–572. doi: 10.1016/s0960-9822(98)70225-8. [DOI] [PubMed] [Google Scholar]
- 10.Rivas F. V., O'Keefe J. P., Alegre M.-L., Gajewski T. F. Actin cytoskeleton regulates calcium dynamics and NFAT nuclear duration. Mol. Cell. Biol. 2004;24:1628–1639. doi: 10.1128/MCB.24.4.1628-1639.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosette C., Karin M. Cytoskeletal control of gene expression: depolymerization of microtubules activates NF-kappa B. J. Cell Biol. 1995;128:1111–1119. doi: 10.1083/jcb.128.6.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bourgarel-Rey V., Vallee S., Rimet O., Champion D., Braguer D., Desobry A., Briand C., Barra Y. Involvement of nuclear factor kappaB in c-Myc induction by tubulin polymerization inhibitors. Mol. Pharmacol. 2001;59:1165–1170. doi: 10.1124/mol.59.5.1165. [DOI] [PubMed] [Google Scholar]
- 13.Algül H., Tando Y., Beil M., Weber C. K., Von Weyhern C., Schneider G., Adler G., Schmid R. M. Different modes of NF-kappaB/Rel activation in pancreatic lobules. Am. J. Physiol. Gastrointest. Liver Physiol. 2001;283:G270–G281. doi: 10.1152/ajpgi.00407.2001. [DOI] [PubMed] [Google Scholar]
- 14.Spencer W., Kwon H., Crépieux P., Leclerc N., Lin R., Hiscott J. Taxol selectively blocks microtubule dependent NF-kappaB activation by phorbol ester via inhibition of IkappaBalpha phosphorylation and degradation. Oncogene. 1999;18:495–505. doi: 10.1038/sj.onc.1202335. [DOI] [PubMed] [Google Scholar]
- 15.Ghosh S., May M. J., Kopp E. B. NF-kappa B and Rel proteins: evolutionarily conserved mediators of immune responses. Annu. Rev. Immunol. 1998;16:225–260. doi: 10.1146/annurev.immunol.16.1.225. [DOI] [PubMed] [Google Scholar]
- 16.Luque I., Gelinas C. Rel/NF-kappa B and I kappa B factors in oncogenesis. Semin. Cancer Biol. 1997;8:103–111. doi: 10.1006/scbi.1997.0061. [DOI] [PubMed] [Google Scholar]
- 17.Grilli M., Memo M. Nuclear factor-kappaB/Rel proteins: a point of convergence of signalling pathways relevant in neuronal function and dysfunction. Biochem. Pharmacol. 1999;57:1–7. doi: 10.1016/s0006-2952(98)00214-7. [DOI] [PubMed] [Google Scholar]
- 18.Foxwell B., Browne K., Bondeson J., Clarke C., de Martin R., Brennan F., Feldmann M. Efficient adenoviral infection with IkappaB alpha reveals that macrophage tumor necrosis factor alpha production in rheumatoid arthritis is NF-kappaB dependent. Proc. Natl. Acad. Sci. U.S.A. 1998;95:8211–8215. doi: 10.1073/pnas.95.14.8211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barnes P. J., Adcock I. M. Transcription factors and asthma. Eur. Respir. J. 1998;12:221–234. doi: 10.1183/09031936.98.12010221. [DOI] [PubMed] [Google Scholar]
- 20.Karin M., Ben-Neriah Y. Phosphorylation meets ubiquitination: the control of NF-[kappa]B activity. Annu. Rev. Immunol. 2000;18:621–663. doi: 10.1146/annurev.immunol.18.1.621. [DOI] [PubMed] [Google Scholar]
- 21.Ghosh S., Karin M. Missing pieces in the NF-kappaB puzzle. Cell (Cambridge, Mass.) 2002;109:S81–S96. doi: 10.1016/s0092-8674(02)00703-1. [DOI] [PubMed] [Google Scholar]
- 22.Schreck R., Rieber P., Baeuerle P. A. Reactive oxygen intermediates as apparently widely used messengers in the activation of the NF-kappaB transcription factor and HIV-1. EMBO J. 1991;10:2247–2258. doi: 10.1002/j.1460-2075.1991.tb07761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chandel N. S., Schumacker P. T., Arch R. H. Reactive oxygen species are downstream products of TRAF-mediated signal transduction. J. Biol. Chem. 2001;276:42728–42736. doi: 10.1074/jbc.M103074200. [DOI] [PubMed] [Google Scholar]
- 24.Bonizzi G., Piette J., Schoonbroodt S., Greimers R., Havard L., Merville M.-P., Bours V. Reactive oxygen intermediates-dependent NF-κB activation by interleukin-1β requires 5-lipoxygenase or NADPH oxidase activity. Mol. Cell. Biol. 1999;19:1950–1960. doi: 10.1128/mcb.19.3.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Legrand-Poels S., Maniglia S., Boelart J. R., Piette J. Activation of the transcription factor NF-κB in lipopolysaccharide-stimulated U937 cells. Biochem. Pharmacol. 1997;53:339–346. doi: 10.1016/s0006-2952(96)00715-0. [DOI] [PubMed] [Google Scholar]
- 26.Schoonbroodt S., Ferreira V., Best-Belpomme M., Boelaert J. R., Legrand-Poels S., Korner M., Piette J. Crucial role of the amino-terminal tyrosine residue 42 and the carboxyl-terminal PEST domain of I kappa B alpha in NF-kappa B activation by an oxidative stress. J. Immunol. 2000;164:4292–4300. doi: 10.4049/jimmunol.164.8.4292. [DOI] [PubMed] [Google Scholar]
- 27.Peppelenbosch M., Boone E., Jones G. E., van Deventer S. J. H., Haegemen G., Fiers W., Grooten J., Ridley A. J. Multiple signal transduction pathways regulate TNF-induced actin reorganization in macrophages: inhibition of Cdc42-mediated filopodium formation by TNF. J. Immunol. 1999;162:837–845. [PubMed] [Google Scholar]
- 28.Singh R., Wang B., Shirvaikar A., Khan S., Kamat S., Schelling J. R., Konieczkowski M., Sedor J. R. The IL-1 receptor and Rho directly associate to drive cell activation in inflammation. J. Clin. Invest. 1999;103:1561–1570. doi: 10.1172/JCI5754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Downey G. P., Chan C. K., Lea P., Takai A., Grinstein S. Phorbol ester-induced actin assembly in neutrophils: role of protein kinase C. J. Cell Biol. 1992;116:695–706. doi: 10.1083/jcb.116.3.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chakravortty D., Nanda Kumar K. S. Bacterial lipopolysaccharide induces cytoskeletal rearrangement in small intestinal lamina propria fibroblasts: actin assembly is essential for lipopolysaccharide signaling. Biochim. Biophys. Acta. 2000;1500:125–136. doi: 10.1016/s0925-4439(99)00098-8. [DOI] [PubMed] [Google Scholar]
- 31.Reyes-Reyes M., Mora N., Gonzalez G., Rosales C. β1 and β2 integrins activate different signalling pathways in monocytes. Biochem. J. 2002;363:273–280. doi: 10.1042/0264-6021:3630273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang S., Chen L.-Y., Zuraw B. L., Ye R. D., Pan Z. K. Chemoattractant-stimulated NF-kappaB activation is dependent on the low molecular weight GTPase RhoA. J. Biol. Chem. 2001;276:40977–40981. doi: 10.1074/jbc.M105242200. [DOI] [PubMed] [Google Scholar]
- 33.Sampath P., Pollard T. D. Effects of cytochalasin, phalloidin, and pH on the elongation of actin filaments. Biochemistry. 1991;30:1973–1980. doi: 10.1021/bi00221a034. [DOI] [PubMed] [Google Scholar]
- 34.Coue M., Brenner S. L., Spector I., Korn E. D. Inhibition of actin polymerization by latrunculin A. FEBS Lett. 1987;213:316–318. doi: 10.1016/0014-5793(87)81513-2. [DOI] [PubMed] [Google Scholar]
- 35.Bubb M. R., Senderowicz A. M., Sausville E. A., Duncan K. L., Korn E. D. Jasplakinolide, a cytotoxic natural product, induces actin polymerization and competitively inhibits the binding of phalloidin to F-actin. J. Biol. Chem. 1994;269:14869–14871. [PubMed] [Google Scholar]
- 36.Desmedt M., Rottiers P., Dooms H., Fiers W., Grooten J. Macrophages induce cellular immunity by activating Th1 cell responses and suppressing Th2 cell responses. J. Immunol. 1998;160:5300–5308. [PubMed] [Google Scholar]
- 37.Josse C., Boelaert J. R., Best-Belpomme M., Piette J. Importance of post-transcriptional regulation of chemokine genes by oxidative stress. Biochem. J. 2001;360:321–333. doi: 10.1042/0264-6021:3600321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Legrand-Poels S., Schoonbroodt S., Piette J. Regulation of interleukin-6 gene expression by pro-inflammatory cytokines in a colon cancer cell line. Biochem. J. 2000;349:765–773. doi: 10.1042/bj3490765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cachia O., Benna J. E., Pedruzzi E., Descomps B., Gougerot-Pocidalo M. A., Leger C. L. Alpha-tocopherol inhibits the respiratory burst in human monocytes. Attenuation of p47(phox) membrane translocation and phosphorylation. J. Biol. Chem. 1998;273:32801–32805. doi: 10.1074/jbc.273.49.32801. [DOI] [PubMed] [Google Scholar]
- 40.Pierce J. W., Schoenleber R., Jesmok G., Best J., Moore S. A., Collins T., Gerritsen M. E. Novel inhibitors of cytokine-induced IkappaBalpha phosphorylation and endothelial cell adhesion molecule expression show anti-inflammatory effects in vivo. J. Biol. Chem. 1997;272:21096–21103. doi: 10.1074/jbc.272.34.21096. [DOI] [PubMed] [Google Scholar]
- 41.Krishna C. M., Liebmann J. E., Kaufman D., DeGraff W., Hahn S. M., McMurry T., Mitchell J. B., Russo A. The catecholic metal sequestering agent 1,2-dihydroxybenzene-3,5-disulfonate confers protection against oxidative cell damage. Arch. Biochem. Biophys. 1992;294:98–106. doi: 10.1016/0003-9861(92)90142-j. [DOI] [PubMed] [Google Scholar]
- 42.O'Donnell B. V., Tew D. G., Jones O. T., England P. J. Studies on the inhibitory mechanism of iodonium compounds with special reference to neutrophil NADPH oxidase. Biochem. J. 1993;290:41–49. doi: 10.1042/bj2900041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lundqvist H., Dahlgren C. Isoluminol-enhanced chemiluminescence: a sensitive method to study the release of superoxide anion from human neutrophils. Free Radical Biol. Med. 1996;20:785–792. doi: 10.1016/0891-5849(95)02189-2. [DOI] [PubMed] [Google Scholar]
- 44.Takeya R., Sumimoto H. Molecular mechanism for activation of superoxide-producing NADPH oxidases. Mol. Cell. 2003;16:271–277. [PubMed] [Google Scholar]
- 45.Ago T., Kuribayashi F., Hiroaki H., Takeya R., Ito T., Kohda D., Sumimoto H. Phosphorylation of p47phox directs phox homology domain from SH3 domain toward phosphoinositides, leading to phagocyte NADPH oxidase activation. Proc. Natl. Acad. Sci. U.S.A. 2003;100:4474–4479. doi: 10.1073/pnas.0735712100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ritzenthaler J. D., Roman J. Interleukin-1beta gene transcription in U937 cells is modulated by type I collagen and cytoskeletal integrity via distinct signaling pathways. J. Interferon Cytokine Res. 2001;21:105–116. doi: 10.1089/107999001750069971. [DOI] [PubMed] [Google Scholar]
- 47.Ogura Y., Inohara N., Benito A., Chen F. F., Yamaoka S., Nùñez G. Nod2, a Nod1/Apaf-1 family member that is restricted to monocytes and activates NF-kappaB. J. Biol. Chem. 2001;276:4812–4818. doi: 10.1074/jbc.M008072200. [DOI] [PubMed] [Google Scholar]
- 48.El Benna J., Faust L. P., Babior B. M. The phosphorylation of the respiratory burst oxidase component p47phox during neutrophil activation. Phosphorylation of sites recognized by protein kinase C and by proline-directed kinases. J. Biol. Chem. 1994;269:23431–23436. [PubMed] [Google Scholar]
- 49.Fontayne A., Dang P. M., Gougerot-Pocidalo M. A., El-Benna J. Phosphorylation of p47phox sites by PKC alpha, beta II, delta, and zeta: effect on binding to p22phox and on NADPH oxidase activation. Biochemistry. 2002;41:7743–7750. doi: 10.1021/bi011953s. [DOI] [PubMed] [Google Scholar]
- 50.Zhao X., Bey E. A., Wientjes F. B., Cathcart M. K. Cytosolic phospholipase A2 (cPLA2) regulation of human monocyte NADPH oxidase activity. cPLA2 affects translocation but not phosphorylation of p67(phox) and p47(phox) J. Biol. Chem. 2002;277:25385–25392. doi: 10.1074/jbc.M203630200. [DOI] [PubMed] [Google Scholar]
- 51.Shiose A., Sumimoto H. Arachidonic acid and phosphorylation synergistically induce a conformational change of p47phox to activate the phagocyte NADPH oxidase. J. Biol. Chem. 2000;275:13793–13801. doi: 10.1074/jbc.275.18.13793. [DOI] [PubMed] [Google Scholar]
- 52.Dewas C., Fay M., Gougerot-Pocidalo M. A., El-Benna J. The mitogen-activated protein kinase extracellular signal-regulated kinase 1/2 pathway is involved in formyl-methionyl-leucyl-phenylalanine-induced p47phox phosphorylation in human neutrophils. J. Immunol. 2000;165:5238–5244. doi: 10.4049/jimmunol.165.9.5238. [DOI] [PubMed] [Google Scholar]
- 53.Dang P. M., Fontayne A., Hakim J., El Benna J., Perianin A. Protein kinase C zeta phosphorylates a subset of selective sites of the NADPH oxidase component p47phox and participates in formyl peptide-mediated neutrophil respiratory burst. J. Immunol. 2001;166:1206–1213. doi: 10.4049/jimmunol.166.2.1206. [DOI] [PubMed] [Google Scholar]
- 54.El Benna J., Faust R. P., Johnson J. L., Babior B. M. Phosphorylation of the respiratory burst oxidase subunit p47phox as determined by two-dimensional phosphopeptide mapping. Phosphorylation by protein kinase C, protein kinase A, and a mitogen-activated protein kinase. J. Biol. Chem. 1996;271:6374–6378. doi: 10.1074/jbc.271.11.6374. [DOI] [PubMed] [Google Scholar]
- 55.Inanami O., Johnson J. L., McAdara J. K., Benna J. E., Faust L. R., Newburger P. E., Babior B. M. Activation of the leukocyte NADPH oxidase by phorbol ester requires the phosphorylation of p47PHOX on serine 303 or 304. J. Biol. Chem. 1998;273:9539–9543. doi: 10.1074/jbc.273.16.9539. [DOI] [PubMed] [Google Scholar]
- 56.El Benna J., Han J., Park J. W., Schmid E., Ulevitch R. J., Babior B. M. Activation of p38 in stimulated human neutrophils: phosphorylation of the oxidase component p47phox by p38 and ERK but not by JNK. Arch. Biochem. Biophys. 1996;334:395–400. doi: 10.1006/abbi.1996.0470. [DOI] [PubMed] [Google Scholar]
- 57.Park H. S., Lee S. M., Lee J. H., Kim Y. S., Bae Y. S., Park J. W. Phosphorylation of the leucocyte NADPH oxidase subunit p47(phox) by casein kinase 2: conformation-dependent phosphorylation and modulation of oxidase activity. Biochem. J. 2001;358:783–790. doi: 10.1042/0264-6021:3580783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hoyal C. R., Gutierrez A., Young B. M., Catz S. D., Lin J. H., Tsichlis P. N., Babior B. M. Modulation of p47PHOX activity by site-specific phosphorylation: Akt-dependent activation of the NADPH oxidase. Proc. Natl. Acad. Sci. U.S.A. 2003;100:5130–5135. doi: 10.1073/pnas.1031526100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nemeth Z. H., Deitch E. A., Davidson M. T., Szabo C., Vizi E. S., Hasko G. Disruption of the actin cytoskeleton results in nuclear factor-kappaB activation and inflammatory mediator production in cultured human intestinal epithelial cells. J. Cell. Physiol. 2004;200:71–81. doi: 10.1002/jcp.10477. [DOI] [PubMed] [Google Scholar]
- 60.Samarakoon R., Higgins P. J. MEK/ERK pathway mediates cell-shape-dependent plasminogen activator inhibitor type 1 gene expression upon drug-induced disruption of the microfilament and microtubule networks. J. Cell Sci. 2002;115:3093–3103. doi: 10.1242/jcs.115.15.3093. [DOI] [PubMed] [Google Scholar]
- 61.Lim Y. B., Kang S. S., Park T. K., Lee Y. S., Chun J. S., Sonn J. K. Disruption of actin cytoskeleton induces chondrogenesis of mesenchymal cells by activating protein kinase C-alpha signalisation. Biochem. Biophys. Res. Commun. 2000;273:609–613. doi: 10.1006/bbrc.2000.2987. [DOI] [PubMed] [Google Scholar]
- 62.El Benna J., Ruedi J. M., Babior B. M. Cytosolic guanine nucleotide-binding protein Rac2 operates in vivo as a component of the neutrophil respiratory burst oxidase. Transfer of Rac2 and the cytosolic oxidase components p47phox and p67phox to the submembranous actin cytoskeleton during oxidase activation. J. Biol. Chem. 1994;269:6729–6734. [PubMed] [Google Scholar]
- 63.Zhan Y., He D., Newburger P. E., Zhou G. W. p47(phox) PX domain of NADPH oxidase targets cell membrane via moesin-mediated association with the actin cytoskeleton. J. Cell. Biochem. 2004;92:795–809. doi: 10.1002/jcb.20084. [DOI] [PubMed] [Google Scholar]
- 64.Cassimeris L., McNeill H., Zigmond S. H. Chemoattractant-stimulated polymorphonuclear leukocytes contain two populations of actin filaments that differ in their spatial distributions and relative stabilities. J. Cell Biol. 1990;110:1067–1075. doi: 10.1083/jcb.110.4.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]