Abstract
PPD (PAZ Piwi domain) proteins and the Dicer family have been the subjects of intense study over the last 6 years. These proteins have well-established roles in RNAi (RNA interference), a process that relies on siRNAs (small interfering RNAs) or miRNAs (microRNAs) to mediate specificity. The development of techniques for applying RNAi as a laboratory tool and a molecular therapeutic technique has rapidly outpaced our understanding of the biology of this process. However, over the last 2 years, great strides have been made towards elucidating how PPD proteins and Dicer regulate gene-silencing at the pre- and post-transcriptional levels. In addition, evidence is beginning to emerge that suggests that these proteins have additional siRNA-independent roles as cell-cycle regulators. In the present review, we summarize the well-known roles of these two classes of proteins in gene-silencing pathways, as well as explore the evidence for novel roles of PPD and Dicer proteins.
Keywords: Dicer, gene expression, gene silencing, PAZ Piwi domain protein (PPD protein), RNA-induced silencing complex (RISC), RNA interference (RNAi)
Abbreviations: ds, double-stranded; FXR, fragile X mental retardation protein; GFP, green fluorescent protein; miRNA, microRNA; miRNP, miRNA-containing ribonucleoprotein; MVH, mammalian Vasa homologue; PPD, PAZ Piwi domain; RISC, RNA-induced silencing complex; RITS, RNA-induced initiation of transcriptional gene silencing; RNAi, RNA interference; siRNA, small interfering RNA; ss, single-stranded; UTR, untranslated region; VIG, Vasa intronic gene protein
INTRODUCTION
RNAi (RNA interference) is a process utilized by eukaryotes to modulate gene expression at pre- and post-transcriptional levels [1]. In its original form, RNAi may have evolved as a genome immune system that acted to maintain the integrity of eukaryotic genomes. For example, in lower eukaryotes and plants, components of the RNAi apparatus are required for transposon silencing, resistance to viruses and maintenance of chromosome stability. Since the discovery 7 years ago that introduction of dsRNA (double-stranded RNA) into cells initiates sequence-specific gene silencing [2], the use of RNAi as a laboratory tool has revolutionized the study of eukaryotic gene function. Moreover, it holds great promise as a molecular therapeutic technique for the treatment of cancers and infectious diseases [3–5].
Specificity in RNAi-dependent gene silencing is provided by small (21–26 nt) guide RNAs. Two main types of small RNAs that differ in origin and function are known to play integral roles in RNAi: siRNAs (small interfering RNAs) and miRNAs (microRNAs). siRNAs are the cleavage products of longer dsRNAs that are generated by RNA-dependent RNA polymerases, or from bi-directional transcription of genes or transposable elements. The siRNAs usually guide the RNAi machinery to carry out mRNA degradation and/or chromatin modification. Endogenous transcripts that contain near-complementary inverted repeats fold back on themselves to form dsRNA hairpins, which, after a twostep maturation process involving two RNase III enzymes, give rise to miRNAs. miRNAs mediate translational repression, although, in some cases, they can also direct mRNA degradation. For example, in plants, miRNAs mainly function in the cleavage of cognate mRNAs, while, in animals, miRNAs predominantly inhibit translation by targeting partially complementary sequences in the 3′ UTR (untranslated region) of mRNAs [6].
Based on genetic and biochemical studies, the canonical RNAi pathway has been divided into two stages: initiation and effector. The initiation stage involves the generation of siRNAs [7–10] and miRNAs [11,12] from long dsRNA or hairpin RNA precursors respectively. Cleavage of precursors into si/miRNAs is mediated by the type III RNase Dicer [13]. The effector stage requires the transfer of the si/miRNAs into ribonucleoprotein complexes known as RISCs (RNA-induced silencing complexes) [14]. Functional RISCs contain only single-stranded siRNAs or miRNAs [15]. Gene silencing by RISC is accomplished via homology-dependent mRNA degradation [16–18], translational repression [19] or transcriptional gene silencing [20–22]. PPD (PAZ Piwi domain) proteins form the core of RISCs [23–26], which are then guided by the si/miRNAs to specific mRNA targets or genomic loci. A third core component of the RNAi pathway, RNA-dependent RNA polymerase, is utilized by a number of lower eukaryotes, including fission yeast and nematodes [20,22,27]. Its purported function in gene-silencing pathways is to amplify siRNAs and possibly miRNAs in order to increase the speed and/or the magnitude of the RNAi response after detection of the dsRNA trigger. Interestingly, this enzyme is not required for RNAi in mammals or Drosophila melanogaster.
CORE COMPONENTS OF THE RNAi APPARATUS
PPD proteins
PPD proteins form a highly conserved superfamily of proteins that are found in diverse organisms ranging from archaea to humans. By definition, members of this family contain two signature domains [28], a centrally located PAZ domain and a C-terminally located Piwi domain (Figure 1). The 100-amino-acid PAZ domain binds the 2 nt 3′-overhang of the siRNA duplex, and facilitates anchoring of this guide RNA into the effector complex [29]. The Piwi domain, comprising approx. 300 amino acids, has been shown to mediate the interaction of PPD proteins with Dicer [30,31]. Recent structural and bioinformatic analyses suggest that Piwi domains share similarity with endonucleases, an observation that led to the discovery that PPD proteins are directly involved in cleavage of targeted mRNAs during the effector stage of RNAi [32–35]. The mechanisms by which PPD proteins mediate translational suppression and chromatin silencing are not known at this time.
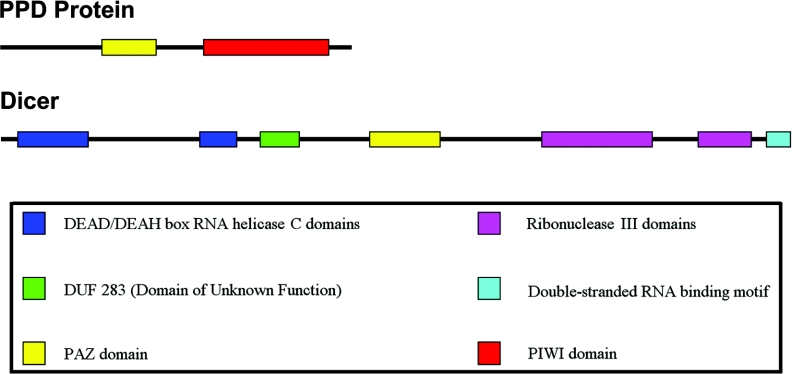
Figure 1. PPD protein and Dicer domain structures.
PPD proteins are classically defined by the presence of PAZ and Piwi domains. The PAZ domain binds to siRNAs, whereas the Piwi domain serves as the binding site for Dicer. The Piwi domains of some PPD proteins possess endonuclease activity. The two ribonuclease III domains of Dicer dimerize to form the catalytic centre that is responsible for cleaving long dsRNA. Some Dicers, such as Schiz. pombe Dcr1, A. thaliana DCL4 and D. melanogaster Dcr1 do not have recognizable PAZ domains. One or more nuclear localization signal can be found in A. thaliana DCL1 and DCL4, D. melanogaster Dcr1, and mouse and human Dicers.
Functions of PPD proteins in siRNA and miRNA pathways
The genes encoding Arabidopsis thaliana AGO1 and ZWILLE were the first PPD family members to be characterized [36,37]. Before the discovery that PPD proteins are RNAi effectors, AGO1 and ZWILLE were shown to have overlapping functions in plant development [38]. Although it has yet to be proved that ZWILLE has RNAi-related functions, it is well-documented that AGO1 is integral to gene silencing [39–41].
The Caenorhabditis elegans genome encodes 24 PPD proteins, a striking number when compared with the fact that mice and humans each contain only eight PPD genes. It appears that, in nematodes at least, PPD proteins perform highly specialized functions. For example, the PPD proteins RDE-1 and PPW-1 are required for efficient siRNA-mediated mRNA cleavage [40,42,43], whereas ALG-1 and ALG-2 are not required for this process [19]. However, the last two PPD proteins function in maturation and translational inhibition activities of miRNAs that regulate developmental timing pathways [19]. In D. melanogaster, Argonaute2 (dAgo2) is required for the incorporation of siRNAs into RISC and subsequent targeting of cognate mRNAs for destruction [44]. Fly embryos that lack dAgo2 activity are defective for siRNA-targeted mRNA cleavage. In contrast, dAgo1 is required for miRNA biogenesis [44], but not siRNA-mediated RISC activities. In humans, four PPD proteins (Argonautes1–4) were shown to bind miRNAs, but only hAgo2 is associated with the catalytic activity required for mRNA cleavage [45]. These examples serve to reinforce the idea that in metazoans, PPD proteins exhibit isoform-specific functions in gene-silencing pathways. Since sequence conservation among family members is greatest in the C-termini, it is likely that their N-terminal domains determine the isoform-specific roles of PPD proteins.
In unicellular organisms, it seems that PPD proteins are multifunctional. The Schizosaccharomyces pombe PPD protein Ago1 is required for silencing of pericentric chromatin, and for accurate chromosome segregation during mitosis and meiosis [20,22,46–48]. Ago1 is also purported to function in concert with short heterochromatic RNAs, which are derived from repetitive sequences, to guide chromatin-silencing machinery to sites of heterochromatin formation [22,49]. It was later demonstrated that this protein also functions in the classic RNAi pathway that culminates in siRNA-directed cleavage of mRNAs [50].
PPD proteins and development
As mentioned above, genetic studies first pointed to the importance of PPD proteins in developmental pathways in plants and animals [36,37,51,52]. For instance, AGO1 and ZWILLE are required for leaf development in A. thaliana, whereas Piwi and dAgo1 are essential for normal development of gametes and nervous system tissues respectively in D. melanogaster [51,52]. PPD protein activity is also required for maintaining pools of stem cells in developing plants [37,38] and animals [51]. Conversely, loss of a given PPD protein activity can result in differentiation and ultimately depletion of stem cell populations, whereas overexpression of the same protein results in increased proliferation of these cell types [53].
In vertebrates, PPD proteins appear to be important for regulating multiple developmental pathways, and certain family members are highly expressed in undifferentiated cells and germline tissues. For example, levels of Hiwi mRNA expression are high in Cdc34+ haematopoietic progenitor bone marrow stem cells, but, as these cells differentiate, Hiwi expression levels decrease dramatically [54]. Similarly, Mili is preferentially expressed in germ cells, and is essential for spermatogenesis in mice. Interestingly, Mili−/− strains of mice resemble MVH−/− strains in that they are developmentally normal, but are sterile owing to arrest of spermatogenesis at the spermatocyte stage [55]. MVH is the mammalian homologue of Vasa, an ATP-dependent DEAD-box RNA helicase that was previously found to co-localize with the PPD protein Aub in germline-specific granules [56], and in a RISC-like complex [26]. Expression and localization of Vasa mRNA is an important determinant of germline fate commitment [57,58]. Accordingly, since Mili associates with MVH, it is likely that these two proteins function together as a complex that regulates cell differentiation. Finally, mouse Ago2 was demonstrated recently to play a critical role in embryogenesis. Targeted disruption of the ago2 gene results in embryonic lethality owing to multiple developmental abnormalities, the most severe of which are defects in neural crest tube closure and cardiac failure [33]. Taken together, these data suggest that it is not only the level of expression and/or activity of PPD family members, but also their intracellular localization, that dictates specific developmental processes.
Bioinformatic analyses have revealed that many of the miRNA targets in plants are transcription factors [59,60]. Accordingly, it seems likely that the majority of the developmental defects exhibited by PPD mutants result from aberrant expression of transcription factors that determine cell fate. However, it is important to consider the possibility that at least some of the developmental roles of PPD proteins are unrelated to gene silencing. In A. thaliana, for instance, some allelic mutants of ago1 are defective for RNAi, but are developmentally normal [61]. This study indicates that the gene-silencing functions of PPD proteins can be genetically uncoupled from their roles in development. D. melanogaster has also served as an excellent experimental system to study the roles of PPD proteins in signalling pathways that control cell fate and developmental patterning. For example, dAGO1 was isolated in a genetic screen for dominant activators of the Wingless signal transduction pathway [52]. Moreover, the phenotypes of piwi mutants can be rescued by overexpression of Hedgehog, a secreted signalling protein that is not implicated in RNAi [62–65]. Of course, these results do not discount the possibility that Wingless and Hedgehog function downstream of dAgo1 and Piwi respectively. In this case, overexpression of Hedgehog may simply bypass the need for Piwi at an upstream miRNA-dependent step.
RNase III enzyme family
Dicer was first identified as the ribonuclease that functions at the initiation stage of RNAi [13]. It is a member of the RNase III family, a group of enzymes that exhibit specificity for dsRNA [66]. The RNase III family is divided into three structural classes. Class I enzymes contain one catalytic endonuclease domain (RIII) and a dsRNA-binding domain, and are involved in numerous RNA-processing reactions, including maturation of rRNAs, tRNAs and mRNAs [67]. Enzymes such as Escherichia coli RNase III are part of the class I subgroup. The second class of enzymes is represented by the nuclear protein Drosha. Class II enzymes comprise two RIII domains, a dsRNA-binding domain, and long N-termini that are rich in proline, serine and arginine residues [68]. The latter regions are presumably involved in protein–protein interactions. Drosha homologues are encoded in the D. melanogaster, C. elegans, mouse and human genomes [69–71], and are the nucleases that are responsible for processing primary miRNAs into stem-loop precursor miRNAs in the nucleus [72].
Drosha exists in two multiprotein complexes. One is a large complex that displays weak primary miRNA-processing activity as well as non-specific RNase activity, contains DEAD-box RNA helicases, dsRNA-binding proteins, heterogeneous nuclear ribonucleoproteins and the Ewing's sarcoma family of proteins [73]. This complex may function in other Drosha-mediated RNA processing pathways, such as pre-ribosomal RNA processing [71]. The smaller complex, named the Microprocessor, displays high primary miRNA-processing activity and contains a dsRNA-binding protein, CG1800 in D. melanogaster or DGCR8 in C. elegans and mammals [73–75]. The function of DGCR8 may be to recognize and present the primary miRNA transcripts to Drosha. It interacts with the middle region and the two RNase III domains of Drosha, and is required for efficient primary miRNA processing [75]. Its function may be to help determine the specificity of Drosha cleavage [75]. The RNA cleavage products of Drosha are then exported to the cytoplasm, where Dicer processes them further to miRNAs. Nuclear export of the miRNA precursors is dependent upon Exportin 5 and RanGTP [76–78].
Dicer belongs to the third class of RNase III enzymes, which contain an N-terminal DEXH-box RNA helicase/ATPase domain, two RIII domains and a dsRNA-binding domain (Figure 1). Most, but not all, Dicer proteins also contain PAZ domains. Whereas C. elegans and D. melanogaster Dicer activities are stimulated by ATP [79–82], mammalian Dicer enzymes do not require ATP for activity, and are instead strongly stimulated by limited proteolysis in vitro [83]. Fascinatingly, some Dicer mRNAs are subject to negative-feedback regulation by miRNA-dependent mechanisms [84].
Based on the structural comparisons with Aquifex aeolicus RNase III enzyme and biochemical analyses of residues within the catalytic domains of E. coli RNase III and human Dicer [85], the current model predicts that Dicer molecules possess one RNA-processing centre. Within the processing centre, each RIII domain cleaves one strand of the dsRNA. Intramolecular dimerization of the two RNase III domains in co-operation with two RNA-binding domains, PAZ and dsRNA-binding domain, results in a processing centre that generates products with the characteristic two-nucleotide 3′ overhangs [85]. Cleavage of RNA substrates by Drosha is also thought to involve the formation of an intramolecular dimer [75]. The first RIII domain cuts the RNA strand bearing a 3′-hydroxy group, approx. 21 nucleotides from the end. In conjunction with the PAZ domain, it is probably responsible for determining the distance from the terminus of the RNA to the cleavage site [85]. By analogy with its role in PPD protein function, the PAZ domain of Dicer may also be involved in RNA substrate recognition [86–88]. If this is the case, it raises the question as to whether Dicer enzymes that lack PAZ domains function in a similar manner.
Functional diversification and characteristics of Dicer proteins
The number of Dicer genes in a given organism varies from one to four. For example, C. elegans, Schiz. pombe and vertebrates each appear to encode only one Dicer protein [19,22,79,80,89–91]. Dicer enzymes are essential for the normal development of zebrafish [89] and mice [79], as well as for the formation of heterochromatin in vertebrate cells [92]. Curiously, Neurospora crassa expresses two Dicer proteins that are functionally redundant [93], whereas the two Dicer paralogues in D. melanogaster (Dcr1 and Dcr2) have unique functions. The former enzyme, which is essential for miRNA processing and miRNA-directed translational repression [94], contains a PAZ domain, but lacks a functional helicase domain. Surprisingly, a complex that includes Dcr1 and Dcr2 is required for siRNA-directed mRNA cleavage. This points to a role for Dicer enzymes at the effector stage of RNAi [94]. The A. thaliana genome encodes four Dicer homologues, at least three of which have unique functions: DCL1 processes miRNA precursors [12,49,95], DCL2 is required for processing virus-specific dsRNA [96] and, finally, DCL3 processes endogenously generated siRNAs [96]. To date, the role of DCL4 in gene silencing has not been elucidated. Lastly, one or more predicted nuclear localization signals are present in DCL1 and DCL4 [95]. D. melanogaster Dcr1 and mouse and human Dicers also contain nuclear localization signals [95]; however, it is unlikely that these motifs are functional, since, to date, these proteins have not been detected in the nucleus. In contrast, the nuclear localization signal of A. thaliana DCL1 does indeed appear to function in mediating nuclear localization [97].
PROTEIN–PROTEIN INTERACTIONS IN RNAi PATHWAYS
Interactions between PPD proteins and Dicer
Shortly after PPD and Dicer proteins were determined to be central to RNAi, an obligatory interaction between these two groups of proteins was predicted to be important for the transfer of si/miRNAs from Dicer to RISC. Initially, heterotypic PAZ–PAZ interactions were thought to mediate PPD protein–Dicer binding [98]; however, the discovery of PAZ-less Dicer isoforms [95] cast doubt upon that hypothesis. Subsequently, two independent studies revealed that PPD protein–Dicer interactions are mediated through the Piwi domains of PPD proteins [30,31]. Moreover, PAZ domains are not required at all for stable PPD protein–Dicer complex formation. Instead, it appears to be the Piwi-box region of PPD proteins that binds to the first RIII domain of human Dicer [31]. Interestingly, the stability of mammalian PPD protein–Dicer complexes is dependent on the activity of Hsp90 (heat-shock protein 90), suggesting that assembly of RNAi components is regulated at multiple steps [31,99]. Finally, PPD proteins and Dicer were shown to be present in soluble and membrane-associated fractions [31], a fact that may indicate that RISCs are present at multiple intracellular locations.
Curiously, binding of PPD proteins to Dicer inhibits its RNase activity in vitro [31]. One of the possible consequences of this effect is to prevent Dicer from releasing si/miRNAs before they associate with other RISC components that mediate mRNA target cleavage or translational repression. This would be expected to maintain the specificity of a given RISC for a single mRNA species.
Interactions between Dicer and dsRNA-binding proteins
In addition to PPD proteins, several dsRNA-binding proteins (RDE-4, R2D2 and HYL1) are known to associate with Dicer [81,100–102]. These proteins may function as physical links between the initiation and effector stages of RNAi. For example, R2D2 was identified as a dsRNA-binding protein that co-fractionated with dsRNA-processing activity from D. melanogaster S2 cell extracts [81]. This protein forms a stable complex with Dcr2 and dsRNA. Maximum stimulation of dsRNA-initiated RISC activity was observed in the presence of Dcr2–R2D2 complexes. In contrast, Dcr2 does not bind detectable amounts of dsRNA in the absence of R2D2. Furthermore, RISC activity was abolished when the dsRNA-binding domains of R2D2 were ablated by site-directed mutagenesis. Similar results were obtained when pre-cleaved siRNAs were used to trigger RISC activity, indicating that R2D2 is needed for RISC to efficiently utilize siRNAs [81]. Finally, the Dcr2–R2D2 complex is required for efficient siRNA transfer and binding to dAgo2 in RISC [102a].
The R2D2 sequence is approx. 33% similar to that of the C. elegans dsRNA-binding protein RDE-4, which interacts with Dicer, PPD proteins and Dicer-related helicase [100]. RDE-4 was first identified as a protein necessary for the initiation step of RNAi in C. elegans [43,103], but its activity is not required for miRNA processing or development [43,100]. It binds to long dsRNA molecules, but not to ds or ss (single-stranded) siRNAs [100]. The PPD protein RDE-1, whose activity was previously shown to be required for the effector stage of RNAi [102], is necessary for the accumulation of long dsRNAs which are then bound by RDE-4 [100]. Thus the function of RDE-4 may be to recognize long dsRNAs, and, together with RDE-1, mediate their transfer to DCR1 for processing into siRNAs. The helicase activity of Dicer-related helicase is likely to be necessary to unwind dsRNA in order to facilitate the movement of the RDE-4–DCR1–RDE-1 complex along the molecule. Alternatively, it may be involved in the transfer of dsRNA from RDE-4 to DCR1 [100].
Recently, it was discovered that a nuclearly localized dsRNA-binding protein, HYL1, is required for miRNA accumulation and development in A. thaliana, but not for post-transcriptional gene silencing [101]. Interestingly, the developmental defects of hyl1 mutants overlap with those of hen1 and dcl1. HEN1 is a nuclear protein that functions in plant development, presumably through its roles in miRNA metabolism and siRNA-mediated post-transcriptional gene silencing [12,39]. hyl1-, hen1- and dcr1-null mutants also share common RNAi defects, specifically reduced miRNA accumulation and a concomitant reduction in targeted mRNA cleavage. In addition, the reported or predicted nuclear localizations of HYL1, HEN1 and DCR1 are consistent with the possibility that these proteins function together as a complex in the nucleus [101].
RISC
Protein components of RISC
The first published reports of RISC purification were difficult to reconcile, since preparations that displayed sequence-specific nuclease activity varied in size from 160 kDa [15] to 500 kDa [14,25,82]. One common thread between all of these studies was that the RISC-like complexes always purified with 21–25-nt-long RNAs. The earliest identified protein component of RISCs were PPD proteins, specifically dAgo2 [13,25,82] and hAgo1 and hAgo2 [15].
Additional RISC components are continually being identified, a situation that makes the precise determination of RISC composition difficult. Two putative RNA-binding proteins, the D. melanogaster homologue of the fragile X mental retardation protein, dFXR, and VIG (Vasa intronic gene protein) were recently identified as components of RISC [26]. Although the specific roles of these proteins in RISC function are not known, it is interesting to note that the dFXR-associated RISC also contains the ribosomal proteins L5 and L11, as well as 5 S RNA, dAgo2 and p68 RNA helicase [104]. Furthermore, dFXR was found to bind Dicer and p68 RNA helicase, the latter protein being important for RNAi in D. melanogaster [104]. Given that one of the functions of dFXR is to repress translation of specific mRNAs, it is reasonable to assume that dFXR-containing complexes function in siRNA- and miRNA-initiated pathways [104].
Characterization of an miRNP (miRNA-containing ribonucleoprotein) complex revealed that it contained components similar to those in siRNA-containing RISC [23,105]. The 15 S miRNP complex isolated from HeLa cells contains hAgo2, Gemin3, a DEAD-box helicase, Gemin4, and is associated with multiple miRNAs [23]. In a human retinoblastoma cell line, a let-7b-containing miRNP complex was shown to bind to the 3′ UTR of its lin-28 mRNA target in polyribosome-containing fractions. The levels of a reporter protein, but not its corresponding mRNA containing the lin28 3′ UTR, were significantly lower in the presence of let-7b-miRNP, suggesting that the miRNP functions through translational suppression [106]. In contrast, human let-7-programmed RISC was shown to be a multiple turnover enzyme complex, capable of catalysing the cleavage of more than ten target mRNA molecules [24]. These findings are not surprising, since miRNAs that are perfectly complementary to mRNAs can mediate targeted mRNA degradation similarly to siRNAs and vice versa [107,108]. In general, perfect complementarity between a miRNA and its cognate mRNA results in degradation of the target rather than translational repression, which occurs when base pairing is not perfectly matched [107].
Dynamics of RISC formation
Recently, the application of native gel electrophoresis to the study of RISC dynamics [109] has led to the identification of three stages of RISC formation defined by three distinct siRNA-containing complexes: R1, R2 and R3 (Figure 2). A role for Dicer in RISC function is indicated by the fact that Dcr2 is found in all of these RNP complexes. The R1 complex probably corresponds to the 360 kDa RISC-like structure described previously [82]. It consists of Dcr2, R2D2, and one or more as yet unidentified proteins. As mentioned above, both Dcr2 and R2D2 are required for association of siRNAs with RISC. The latter protein is known to contact siRNAs directly in the R1 complex. The function of R1 may be to process long dsRNA and initiate RISC assembly by serving as a precursor to R2 and R3 [109]. The high rate at which R2 is formed suggests that it may be derived from the binding of R1 to an as yet unidentified pre-assembled complex. The R2 complex is thought to function in siRNA duplex unwinding.
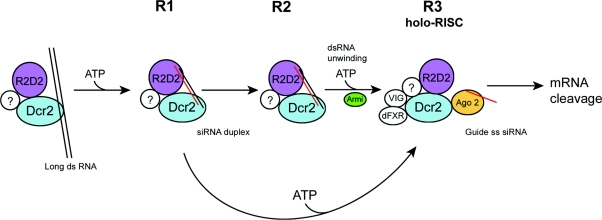
Figure 2. Model of RISC assembly in D. melanogaster.
The principal siRNA-generating enzyme complex in D. melanogaster is composed of Dcr2 and R2D2. Although R2D2 does not affect the ability of Dcr2 to cleave long dsRNA, it is important for binding to the siRNA product after cleavage. R2D2 binds to the 3′ end of the guide strand (red) of the siRNA duplex, while Dcr2 binds to the 3′ end of the passenger strand. R1 forms after Dcr2-mediated RNA cleavage of long dsRNAs. The R2 complex may be an intermediate that derives from R1 and ultimately matures into R3. Alternatively, it has been suggested that R3 may form directly from R1. The R3 complex is large (80 S) and associates with ribosomes (not shown). Ago2 displaces the Dcr2–R2D2 complex from the siRNA duplex by binding to the 3′ end of the guide siRNA strand. Formation of this complex is dependent upon ATP. The siRNAs in the R3 complex are single-stranded, having possibly been unwound by the helicase Armitage. R3 binds the cognate mRNA target, which is then cleaved by the Ago2 subunit. It is possible that there are other unidentified proteins (?) associated with each of the intermediate complexes.
Thermodynamic differences in the base-pairing of the two siRNA strands determine which siRNA strand is assembled into RISC: the one whose 5′ end is less tightly paired to its complement [110]. The Dcr2–R2D2 complex senses the thermodynamic stabilities of the ends of an siRNA duplex and selects which RNA strand becomes associated with Ago2. R2D2 binds to the 3′ end of the siRNA guide strand that enters RISC, as well as to the 5′ phosphate of the non-incorporated siRNA strand. Dcr2 binds to the opposite end of the siRNA duplex. As siRNA unwinding proceeds, the Dcr2–R2D2 complex is replaced with Ago2, which binds the 2 nt 3′ overhang of the guide strand. The unwinding of the siRNA is initiated by the Dcr2–R2D2 complex, but can proceed only in the presence of Ago2 [102a]
The 80 S R3 effector complex whose formation is enhanced by ATP, contains siRNAs, Dcr1, Dcr2, VIG, Tudor staphylococcal nuclease, Ago2, dFXR and R2D2. The R3 complex co-purifies with rRNA from small and large ribosomal subunits, suggesting that it is ribosome-associated [109]. This possibility is supported by the fact that only RNAs undergoing translation are susceptible to RNAi [112], and that dFXR-containing RISC contains ribosomal proteins and RNA [104]. Recently, Tomari et al. [111] reported that an additional, ATP-dependent step occurs in RISC assembly after siRNA duplex unwinding. This process requires the PPD protein Aub, and an ATP-dependent DEA(H/D)-box helicase Armitage [111,113]. Because R3 is so large, and smaller active components of this complex can be purified, it has been proposed that R3 is a ‘holoenzyme’, which includes regulatory factors that are not absolutely necessary for mRNA cleavage in vitro [109].
Slicer
Slicer is the term given to the RISC-associated component that catalyses cleavage of siRNA-targeted mRNAs. The Slicer entity was predicted to be an endoribonuclease that cleaves dsRNA molecules. In their search for Slicer, Hannon's group identified Tudor staphylococcal nuclease in RISC preparations from C. elegans, D. melanogaster and mammalian cells [114]. However, data from other studies were inconsistent with the likelihood that Tudor staphylococcal nuclease was the Slicer in RISC. Specifically, this enzyme is a calcium-dependent nuclease that generates 3′-phosphomono- and di-nucleotides through hydrolysis of DNA or RNA [115]. In contrast, the RISC-associated endonuclease is magnesium-dependent, and its products contain 5′ phosphomonoesters [116,117]. Although the Tudor staphylococcal nuclease inhibitor, 2′-deoxythymidine 5′,3′-biphosphate, was found to inhibit RISC activity in one report [114], a later study found that it had no such effect [117].
Interestingly, Dicer enzymes share a number of characteristics with the RISC Slicer. For example, Dicer cleavage products are similar to those of Slicer in that they contain 3′-hydroxide 5′-phosphate termini [116,117]. In addition to functioning at the initiation stage, Dicer is also essential for the effector phase of RNAi. For example, in D. melanogaster embryos, Dcr2 is required for both siRNA formation and RISC assembly [94]. Similarly, knockdown of Dicer activity in mammalian cells results in failure of siRNA-mediated gene silencing [30]. Further evidence that Dicer associates with RISC stems from the observation that siRNAs can be UV-cross-linked to both Dcr1 and Dcr2 in the D. melanogaster RISC [109].
Recent findings from a number of laboratories revealed that a subset of Argonaute proteins contribute the Slicer activity of RISCs [32,33,45]. Whereas all four human Ago proteins bind to miRNAs, only hAgo2-containing complexes are able to cleave mRNA substrates [6,33]. Furthermore, genetic studies support the biochemical evidence that Ago2 is the Slicer. Specifically, mouse fibroblasts with homozygous null mutations in ago2 are unable to repress gene expression via RNAi [33]. The nuclease activity of Ago2 appears to be highly regulated, since it does not function in the absence of ss siRNAs, nor does it exhibit significant activity in the presence of dsRNA [33]. This suggests that before targeted mRNA cleavage can occur, Ago2 requires an accessory protein, such as Dicer or an R2D2-like protein, to present it with siRNA. It is puzzling that not all hAgo2-associated miRNAs are able to direct cleavage of complementary target mRNAs. This may indicate that some of the Argonaute-protein-containing complexes function in translational repression. Indeed, this scenario is supported by a recent report showing that miRNA-independent tethering of hAgo2, hAgo3 and hAgo4 proteins to the 3′ UTRs of a reporter RNA mediates translational repression without affecting mRNA levels [118]. Although it unknown how PPD proteins mediate translation repression, it seems that the major role of the miRNAs is simply to guide PPD-protein-containing complexes to homologous mRNAs.
Implications of Argonaute structure for the Slicer function
Recent structural studies have greatly advanced our understanding of how PPD proteins function in RNAi. For example, resolution of the PAZ structure indicated that this domain adopts an OB(oligosaccharide/oligonucleotide-binding)-like fold that binds to the 3′ overhangs of siRNAs [29,86,87]. Next, the three-dimensional structure of the archaeon Pyrococcus furiosus Argonaute protein was solved [32], and data from this study further strengthen the argument for the Slicer function of Argonaute proteins. In particular, the finding that the Piwi domain structure is similar to endonucleases such as RNase H and endonuclease V was key to understanding the function of PPD proteins [32,34,35]. The magnesium-dependent activities of RNase H and endonuclease V are in agreement with the reported characteristics of RISC [116,117]. Moreover, two aspartate residues that are required for mRNA target cleavage are spatially conserved between the archaeal Ago protein catalytic centre and the active sites of endonucleases [32,33]. Interestingly, the three human Argonaute proteins hAgo1, hAgo3 and hAgo4, which are unable to form cleavage-competent complexes, contain the same amino acid residues that are found in the catalytic centre of hAgo2. This indicates that in addition to the conserved catalytic carboxylates, other residues (in the Piwi domain) or interacting factors are needed to promote the endonucleolytic activity of hAgo2.
The current model of Argonaute Slicer activity, based on the crystal structure of archaeal Argonaute protein is as follows: the siRNA guide interacts with the PAZ cleft, while the mRNA substrate enters a binding groove formed by the N-terminal, middle and Piwi domains. The 5′ end of the mRNA is predicted to lie between the PAZ and N-terminal domains of Argonaute, the latter domain functioning as an ‘mRNA grip’ [32]. The mRNA is positioned so that the active site, located in the Piwi domain, is 9 nt from the 5′ end of the siRNA–mRNA ds region, allowing for the cleavage of mRNA target between 11 and 12 nt from the 3′ end of the siRNA guide. The predicted length of the groove is enough to accommodate the entire siRNA. A putative hinge region connects the PAZ domain to the rest of the protein. Upon binding to other proteins, this hinge may function to lift PAZ away from the crescent base, allowing binding of ss siRNAs and the completion of RISC assembly [32].
NON-RISC-DEPENDENT FUNCTIONS OF RNAi CORE PROTEINS
RNA-induced initiation of transcriptional gene silencing
Recently, the importance of RNAi in nuclear events such as genome rearrangement [119], chromatin silencing [20,22,48,120] and chromosome segregation [47,121] has come to light. The latter two processes are directly related to one another in that accurate chromosome segregation, which involves cohesin-mediated sister chromatid association [122–125], is contingent upon the assembly of centromeric heterochromatin [126]. In turn, binding of the cohesin complex to silent centromeric sites is dependent upon recruitment of the chromodomain-containing protein Swi6 to these regions [127,128]. Histone H3 Lys9 methylation-dependent Swi6 localization to centromeric, telomeric and the silent mating-type loci requires the function of three core components of the RNAi pathway, Ago1, Dcr1 and RNA-dependent RNA polymerase (Rdp1) [20,22,120].
Targeting of Swi6 to sites of heterochromatin formation in the Schiz. pombe genome is reportedly mediated by a novel type of RNP complex, named the RITS (RNA-induced initiation of transcriptional gene silencing) complex [129]. The functional specificity of the RITS complex requires incorporation of siRNAs derived from centromeric transcripts [49]. Deletion of any one of the core RNAi genes, ago1, dcr1 or rdp1, or genes that encode the RITS-associated proteins Chp1 and Tas1, results in the loss of histone H3 Lys9 methylation and the absence of Swi6 from centromeric chromatin [20,22,47,48,120,129]. This is presumably due to an inability to target the methyltransferase Clr4 to sites of heterochromatin formation. In turn, loss of centromeric heterochromatin leads to chromosomal segregation defects as a result of reduced cohesin binding to centromeres.
Subcellular localization of RITS and other RNAi components
Analysis of the RNAi components in Schiz. pombe using ChIP (chromatin immunoprecipitation) revealed that both Rdp1 and Ago1 are associated with centromeric chromatin [22,130]. In addition, Ago1 can be co-purified with chromatin derived from telomeric and silent mating type loci [130]. Indeed, fluorescence-microscopy studies demonstrated that pools of tagged Rdp1, Ago1 and Chp1 are localized to the nuclei of Schiz. pombe cells [130,131]. Ago1 was also detected in the cytoplasm where it presumably functions in siRNA-directed mRNA degradation. Although Dcr1 is an integral component of chromatin silencing, it was not detected in association with any heterochromatic regions [22]. As yet, the localization of Dcr1 is not known. Whereas it was previously assumed that most siRNA processing and RISC-mediated silencing were restricted to the cytoplasm [132,133], a number of studies are consistent with the possibility that at least some PPD proteins and Dicer homologues are present in the nucleus [20,22,47,48,120,129]. In addition, more direct evidence to support the notion that the initiation and/or effector stages of RNAi take place in the nucleus has recently emerged. For instance, the discovery of Drosha offered a plausible mechanism to account for nucleus-restricted processing of primary miRNAs to precursor miRNAs [72]. Moreover, studies in A. thaliana using nuclear and cytoplasmic variants of the viral suppressor of post-transcriptional gene silencing P19 and DCL1–GFP (green fluorescent protein) fusion proteins provided evidence for nuclear production of siRNAs and miRNAs from precursor molecules [97]. Finally, in D. melanogaster, the nuclearly localized PPD protein Piwi is required for RNA-dependent silencing of specific loci such as gypsy [134].
A recent study reported that miRNA-directed cleavage activity is present in both nuclear and cytoplasmic extracts of HeLa cells [6]. Although cleavage appeared to occur more efficiently in the cytoplasmic fraction, these data suggest that at least a portion of the cytoplasmic miRNA-associated RISC is able to translocate to the nucleus or that there is a stable nuclear pool of RISC. In support of this, another group reported the involvement of RDR2, DCL3, AGO4 and HEN1 in chromatin-modification events in A. thaliana [96]. In this study, DCL3–GFP, HEN-1–GFP and GFP–AGO4 proteins were detected exclusively in the nuclei [96].
Involvement of RNAi core components in cell cycle events
The regulation of chromosome structure and segregation is central to the successful completion of cell cycle events. The heterochromatin-assembly defects observed in Schiz. pombe ago1, dcr1 and rdp1 mutants are associated with frequent lagging DNAs and chromosome loss during mitosis, presumably owing to incorrect sister chromatin orientation and defective kinetichore attachment to spindles [47,121]. While these data support a model in which chromatin architecture is regulated by the RNAi pathway, evidence is also mounting to suggest that RNAi core proteins perform functions that are not directly related to gene silencing [61,135]. In fact, homologues of RNAi core components are now known to regulate cell cycle events in organisms that do not employ RNAi. For example, in the budding yeast Saccharomyces cerevisiae, it was recently discovered that the type III RNase Rnt1p, a Dcr1 homologue, is required for cell-cycle progression [136]. While nuclear localization of Rnt1p is essential for this function, the catalytic activity is dispensable for its role in cell-cycle progression [136]. Although the classical RNAi pathway is not present in S. cerevisiae, this result does support the notion of cell-cycle-related functions for RNase III proteins that are independent of their nuclease activity. By extension, the role of the RNase III Dcr1 in cell cycle events may be independent of its ability to generate siRNAs [137].
In Schiz. pombe, Ago1 and Dcr1, but not Rdp1, are required for regulated hyperphosphorylation of Cdc2 when encountering genotoxic insults [137]. Under normal circumstances, agents that inhibit DNA replication or cause DNA damage activate signalling cascades that lead to inhibitory phosphorylation of the mitotic regulator Cdc2, thereby resulting in cell-cycle arrest [138–140]. After the cells are deemed free of DNA damage or have become replication competent, alleviation of the inhibitory phosphorylation of Cdc2 by the mitotic stimulatory phosphatase Cdc25 initiates resumption of the cell cycle [141,142]. ago1- and dcr1-null mutants fail to block mitosis in response to DNA damage or replication inhibitors such as hydroxyurea, presumably as a result of their inabilities to effect hyperphosphorylation of Cdc2. These cells continue to divide unchecked, resulting in unequal division of genomic material between mother and daughter cells. Interestingly, overexpression of hAgo2 was shown to complement the checkpoint deficiency in Schiz. pombe ago1 mutants [137]. This suggests that, in humans, the core catalytic subunit of RISC may also play a role in cell cycle checkpoint regulation [33]. Moreover, ectopic overexpression of Ago1 in a dcr1-null strain rescued the checkpoint deficiencies, but not the chromosome-segregation defects [137]. Since the biogenesis of siRNAs is completely dependent upon Dcr1 [129], this argues that Ago1 and Dcr1 function in siRNA-independent pathways. Finally, it appears that Ago1 functions downstream of Dcr1 in cell-cycle regulation. Figure 3 depicts a model of how of PPD proteins and Dicer function in the classic RNAi pathway, RNAi-mediated chromatin restructuring and siRNA-independent cell-cycle regulation.
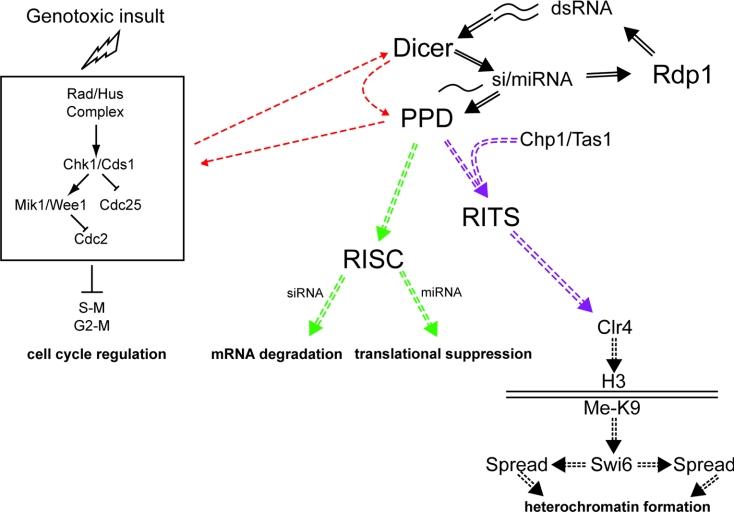
Figure 3. Multiple functions of PPD and Dicer proteins.
PPD proteins and Dicer are proposed to function in three different pathways. On the right-hand side of the diagram, si/miRNA-dependent functions of Dicer and PPD proteins are illustrated. si/miRNA-directed mRNA cleavage or translational repression does not require Chp1 and Tas1-like proteins and is proposed to occur in the cytoplasm. In contrast, the heterochromatin-specific functions take place in the nucleus. On the left-hand side, we outline a model to account for the cell cycle functions of Dicer and PPD proteins. DNA damage or inhibition of replication results in a cascade of signalling events that lead to cell-cycle arrest via a Cdc2-dependent checkpoint. Although the exact points of PPD or Dicer proteins action along this pathway are unknown (red arrows), it is thought that these functions are independent of si/miRNAs. In contrast, RISC-mediated mRNA degradation and translational suppression (green arrows) require Dicer-dependent si/miRNA production and their inclusion into RISC or RISC-like complexes. Likewise, heterochromatin formation (purple arrows) requires the production and inclusion of si/miRNAs into a specialized complex named RITS. Association of RITS with chromatin results in methylation of histone H3 Lys9, followed by Swi6 binding and subsequent spreading of silencing to neighbouring regions.
PPD PROTEINS, DICER AND CANCER
Cancer has often been referred to as a disease caused by the failure of cells to maintain genome stability, which in turn is dependent upon accurate chromosome segregation and cell cycle checkpoints that prevent or delay DNA replication/cell division in response to DNA damage [143]. Not surprisingly, mutations in human genes encoding checkpoint proteins are associated with familial predisposition to cancer [144]. In lower eukaryotes at least, the RNAi apparatus functions to maintain genome stability [119]. Of course, it is not hard to imagine how RNAi effector proteins may indirectly affect cell-cycle progression through their involvement in gene-silencing pathways that regulate expression of transcription factors and other proteins. However, it has recently become evident that PPD proteins and Dicer also function in siRNA-independent pathways that regulate cell cycle events [137]. Accordingly, it is important to consider the aberrant expression of PPD proteins and Dicer in human cancers.
Of particular significance is the observation that seminoma tumours are often associated with increased levels of Hiwi mRNA [145]. Coincidentally, Hiwi was first reported as a gene that is expressed in undifferentiated haematopoietic stem cells, but not in differentiated cells [54]. Likewise, expression of the D. melanogaster orthologue of Hiwi, Piwi, had previously been reported to promote mitosis in stem cells [53]. In addition, the chromosomal locus 12q24.33 that includes the Hiwi gene has been linked to testicular germ cell tumours [146,147]. Conversely, deletion of this region is associated with hypogonadism [148]. Together, these observations are consistent with a scenario in which overexpression of certain PPD proteins is associated with increased mitosis in undifferentiated cells. The role of other PPD protein family members in this process is less clear. For example, we have observed that the expression of Argonaute mRNAs is elevated in multiple human tumour cell lines (K. Jaronczyk, J. B. Carmichael and T. C. Hobman, unpublished work). Paradoxically, there is evidence to suggest that some Argonaute proteins function as tumour suppressors. Specifically, chromosome 1 region p34-35, which is often lost in Wilms' tumours [149,150], contains three human Argonaute genes (hAgo1, hAgo3 and hAgo4) [151]. In addition, miR15 and miR16 are located in chromosome 13q14, a region lost in more than half of B-cell chronic lymphocytic leukaemias. Both genes are deleted or down-regulated in the majority of chronic lymphocytic leukaemia cases [152]. Finally, overexpression of hAgo2 in a Schiz. pombe strain lacking the ago1 gene was shown to correct the cell cycle checkpoint deficiencies of this mutant [137]. These data point to a role for mammalian PPD proteins in enacting checkpoints in response to genotoxic stress.
There is compelling, but indirect, evidence that Dicer may also have a role in human cancer development. For example, in yeast, lack of Dicer function results in chromosome segregation defects [22,121], as well as failure to enact S–M cell cycle checkpoints [137]. Both of these defects would be expected to contribute to the development of cancer in humans. Human Dicer can partially complement the loss of Dcr1 activity in yeast [121], and thus it is possible that the human enzyme also functions in cell-cycle regulation. Interestingly, human Dicer is also known to bind to 5-lipoxygenase [135], an enzyme whose activity is deregulated in pancreatic tumours [153]. Lipoxygenase inhibitors are a promising new class of anticancer reagents [154] that have been shown to prevent lung tumours in mice and slow progression of adenomas to carcinomas [155]. Unfortunately, it is not known if or how Dicer affects the activity of lipoxygenase. In addition, the Burkitt's lymphoma-derived cell line, EB-3, was found to possess 4-fold higher expression of Dicer mRNA than normal human lymphocytes, and 2-fold higher activity of RNA polymerase III [156]. It is also important to note that Burkitt's lymphoma is characterized by increased activity of c-myc, a transcription factor that regulates genes involved in cell-cycle progression and apoptosis [157]. These observations suggest that RNA polymerase III, whose promoter sequences are present in exon1/intron1 of the c-myc gene [157], produces siRNAs that silence the negative-regulatory elements of c-myc expression [156]. Finally, the core RNAi proteins undoubtedly play a role in signalling and cancer that can be attributed to miRNA-regulated alteration of transcription factor expression [158].
OUTLOOK
In a very short period of time, through a combination of genetic and biochemical approaches, we have learned a great deal about how PPD and Dicer family members function in gene-silencing processes. This is particularly true of siRNA-directed cleavage of mRNAs or classical RNAi. The challenges that must be met now are to understand how PPD proteins function in other gene-silencing mechanisms including translational suppression and chromatin remodelling. Moreover, elucidation of how both PPD proteins and Dicer function in cell-cycle modulation, either directly or indirectly, will go a long way to aid our understanding of how the RNAi machinery positively and/or negatively affects tumorigenesis.
Acknowledgments
This work was funded by grants from the Canadian Institutes of Health Research (CIHR) and the Natural Sciences and Engineering Research Council (T.C.H). T.C.H. is the recipient of a Senior Medical Scholarship from the Alberta Heritage Foundation for Medical Research (AHFMR). K. J. is supported by a pre-doctoral studentship award from AHFMR.
References
- 1.Hannon G. J. RNA interference. Nature (London) 2002;418:244–251. doi: 10.1038/418244a. [DOI] [PubMed] [Google Scholar]
- 2.Fire A., Xu S., Montgomery M. K., Kostas S. A., Driver S. E., Mello C. C. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature (London) 1998;391:806–811. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- 3.Kapadia S. B., Brideau-Andersen A., Chisari F. V. Interference of hepatitis C virus RNA replication by short interfering RNAs. Proc. Natl. Acad. Sci. U.S.A. 2003;100:2014–2018. doi: 10.1073/pnas.252783999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wilson J. A., Jayasana S., Khvorova A., Sabatinos S., Rodrigue-Gervais I. G., Arya S., Sarangi F., Harris-Brandts M., Beaulieu S., Richardson C. D. RNA interference blocks gene expression and RNA synthesis from hepatitis C replicons propagated in human liver cells. Proc. Natl. Acad. Sci. U.S.A. 2003;100:2783–2788. doi: 10.1073/pnas.252758799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fuchs U., Damm-Welk C., Borkhardt A. Silencing of disease-related genes by small interfering RNAs. Curr. Mol. Med. 2004;4:507–517. doi: 10.2174/1566524043360492. [DOI] [PubMed] [Google Scholar]
- 6.Meister G., Landthaler M., Dorsett Y., Tuschl T. Sequence-specific inhibition of microRNA- and siRNA-induced RNA silencing. RNA. 2004;10:544–550. doi: 10.1261/rna.5235104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hamilton A., Voinnet O., Chappell L., Baulcombe D. Two classes of short interfering RNA in RNA silencing. EMBO J. 2002;21:4671–4679. doi: 10.1093/emboj/cdf464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee R. C., Ambros V. An extensive class of small RNAs in Caenorhabditis elegans. Science. 2001;294:862–864. doi: 10.1126/science.1065329. [DOI] [PubMed] [Google Scholar]
- 9.Lau N. C., Lim L. P., Weinstein E. G., Bartel D. P. An abundant class of tiny RNAs with probable regulatory roles in Caenorhabditis elegans. Science. 2001;294:858–862. doi: 10.1126/science.1065062. [DOI] [PubMed] [Google Scholar]
- 10.Lagos-Quintana M., Rauhut R., Lendeckel W., Tuschl T. Identification of novel genes coding for small expressed RNAs. Science. 2001;294:853–858. doi: 10.1126/science.1064921. [DOI] [PubMed] [Google Scholar]
- 11.Reinhart B. J., Weinstein E. G., Rhoades M. W., Bartel B., Bartel D. P. MicroRNAs in plants. Genes Dev. 2002;16:1616–1626. doi: 10.1101/gad.1004402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park W., Li J., Song R., Messing J., Chen X. CARPEL FACTORY, a Dicer homolog, and HEN1, a novel protein, act in microRNA metabolism in Arabidopsis thaliana. Curr. Biol. 2002;12:1484–1495. doi: 10.1016/s0960-9822(02)01017-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bernstein E., Caudy A. A., Hammond S. M., Hannon G. J. Role for a bidentate ribonuclease in the initiation step of RNA interference. Nature (London) 2001;409:363–366. doi: 10.1038/35053110. [DOI] [PubMed] [Google Scholar]
- 14.Hammond S. M., Bernstein E., Beach D., Hannon G. J. An RNA-directed nuclease mediates post-transcriptional gene silencing in Drosophila cells. Nature (London) 2000;404:293–296. doi: 10.1038/35005107. [DOI] [PubMed] [Google Scholar]
- 15.Martinez J., Patkaniowska A., Urlaub H., Luhrmann R., Tuschl T. Single-stranded antisense siRNAs guide target RNA cleavage in RNAi. Cell. 2002;110:563–574. doi: 10.1016/s0092-8674(02)00908-x. [DOI] [PubMed] [Google Scholar]
- 16.Tuschl T., Zamore P. D., Lehmann R., Bartel D. P., Sharp P. A. Targeted mRNA degradation by double-stranded RNA in vitro. Genes Dev. 1999;13:3191–3197. doi: 10.1101/gad.13.24.3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zamore P. D., Tuschl T., Sharp P. A., Bartel D. P. RNAi: double-stranded RNA directs the ATP-dependent cleavage of mRNA at 21 to 23 nucleotide intervals. Cell. 2000;101:25–33. doi: 10.1016/S0092-8674(00)80620-0. [DOI] [PubMed] [Google Scholar]
- 18.Hamilton A. J., Baulcombe D. C. A species of small antisense RNA in posttranscriptional gene silencing in plants. Science. 1999;286:950–952. doi: 10.1126/science.286.5441.950. [DOI] [PubMed] [Google Scholar]
- 19.Grishok A., Pasquinelli A. E., Conte D., Li N., Parrish S., Ha I., Baillie D. L., Fire A., Ruvkun G., Mello C. C. Genes and mechanisms related to RNA interference regulate expression of the small temporal RNAs that control C. elegans developmental timing. Cell. 2001;106:23–34. doi: 10.1016/s0092-8674(01)00431-7. [DOI] [PubMed] [Google Scholar]
- 20.Hall I. M., Shankaranarayana G. D., Noma K., Ayoub N., Cohen A., Grewal S. I. Establishment and maintenance of a heterochromatin domain. Science. 2002;297:2232–2237. doi: 10.1126/science.1076466. [DOI] [PubMed] [Google Scholar]
- 21.Pal-Bhadra M., Bhadra U., Birchler J. A. RNAi related mechanisms affect both transcriptional and posttranscriptional transgene silencing in Drosophila. Mol. Cell. 2002;9:315–327. doi: 10.1016/s1097-2765(02)00440-9. [DOI] [PubMed] [Google Scholar]
- 22.Volpe T. A., Kidner C., Hall I. M., Teng G., Grewal S. I., Martienssen R. A. Regulation of heterochromatic silencing and histone H3 lysine-9 methylation by RNAi. Science. 2002;297:1833–1837. doi: 10.1126/science.1074973. [DOI] [PubMed] [Google Scholar]
- 23.Mourelatos Z., Dostie J., Paushkin S., Sharma A., Charroux B., Abel L., Rappsilber J., Mann M., Dreyfuss G. miRNPs: a novel class of ribonucleoproteins containing numerous microRNAs. Genes Dev. 2002;16:720–728. doi: 10.1101/gad.974702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hutvagner G., Zamore P. D. miRNPs: a novel class of ribonucleoproteins containing numerous microRNAs. Science. 2002;297:2056–2060. [Google Scholar]
- 25.Hammond S. M., Boettcher S., Caudy A. A., Kobayashi R., Hannon G. J. Argonaute2, a link between genetic and biochemical analyses of RNAi. Science. 2001;293:1146–1150. doi: 10.1126/science.1064023. [DOI] [PubMed] [Google Scholar]
- 26.Caudy A. A., Myers M., Hannon G. J., Hammond S. M. Fragile X-related protein and VIG associate with the RNA interference machinery. Genes Dev. 2002;16:2491–2496. doi: 10.1101/gad.1025202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dalmay T., Hamilton A., Rudd S., Angell S., Baulcombe D. C. An RNA-dependent RNA polymerase gene in Arabidopsis is required for posttranscriptional gene silencing mediated by a transgene but not by a virus. Cell. 2000;101:543–553. doi: 10.1016/s0092-8674(00)80864-8. [DOI] [PubMed] [Google Scholar]
- 28.Cerutti L., Mian N., Bateman A. Domains in gene silencing and cell differentiation proteins: the novel PAZ domain and redefinition of the Piwi domain. Trends Biochem. Sci. 2000;25:481–482. doi: 10.1016/s0968-0004(00)01641-8. [DOI] [PubMed] [Google Scholar]
- 29.Ma J. B., Ye K., Patel D. J. Structural basis for overhang-specific small interfering RNA recognition by the PAZ domain. Nature (London) 2004;429:318–322. doi: 10.1038/nature02519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Doi N., Zenno S., Ueda R., Ohki-Hamazaki H., UiTei K., Saigo K. Short-interfering-RNA-mediated gene silencing in mammalian cells requires Dicer and eIF2C translation initiation factors. Curr. Biol. 2003;13:41–46. doi: 10.1016/s0960-9822(02)01394-5. [DOI] [PubMed] [Google Scholar]
- 31.Tahbaz N., Kolb F. A., Zhang H., Jaronczyk K., Filipowicz W., Hobman T. C. Characterization of the interactions between mammalian PAZ PIWI domain proteins and Dicer. EMBO Rep. 2004;5:189–194. doi: 10.1038/sj.embor.7400070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Song J. J., Smith S. K., Hannon G. J., Joshua-Tor L. Crystal structure of Argonaute and its implications for RISC slicer activity. Science. 2004;305:1434–1437. doi: 10.1126/science.1102514. [DOI] [PubMed] [Google Scholar]
- 33.Liu J., Carmell M. A., Rivas F. V., Marsden C. G., Thomson J. M., Song J. J., Hammond S. M., Joshua-Tor L., Hannon G. J. Argonaute2 is the catalytic engine of mammalian RNAi. Science. 2004;305:1437–1441. doi: 10.1126/science.1102513. [DOI] [PubMed] [Google Scholar]
- 34.Rand T. A., Ginalski K., Grishin N. V., Wang X. Biochemical identification of Argonaute 2 as the sole protein required for RNA-induced silencing complex activity. Proc. Natl. Acad. Sci. U.S.A. 2004;101:14385–14389. doi: 10.1073/pnas.0405913101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Parker J. S., Roe S. M., Barford D. Crystal structure of a PIWI protein suggests mechanisms for siRNA recognition and slicer activity. EMBO J. 2004;23:4727–4737. doi: 10.1038/sj.emboj.7600488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bohmert K., Camus I., Bellini C., Bouchez D., Caboche M., Benning C. AGO1 defines a novel locus of Arabidopsis controlling leaf development. EMBO J. 1998;17:170–180. doi: 10.1093/emboj/17.1.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moussian B., Schoof H., Haecker A., Juergens G., Laux T. Role of the ZWILLE gene in the regulation of central shoot meristem cell fate during Arabidopsis embryogenesis. EMBO J. 1998;17:1799–1809. doi: 10.1093/emboj/17.6.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lynn K., Fernandez A., Aida M., Sedbrook J., Tasaka M., Masson P., Barton M. K. The PINHEAD/ZWILLE gene acts pleiotropically in Arabidopsis development and has overlapping functions with the ARGONAUTE1 gene. Development. 1999;126:469–481. doi: 10.1242/dev.126.3.469. [DOI] [PubMed] [Google Scholar]
- 39.Boutet S., Vazquez F., Liu J., Beclin C., Fagard M., Gratias A., Morel J. B., Crete P., Chen X., Vaucheret H. Arabidopsis HEN1: a genetic link between endogenous miRNA controlling development and siRNA controlling transgene silencing and virus resistance. Curr. Biol. 2003;13:843–848. doi: 10.1016/s0960-9822(03)00293-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fagard M., Boutet S., Morel J. B., Bellini C., Vaucheret H. AGO1, QDE-2, and RDE-1 are related proteins required for post-transcriptional gene silencing in plants, quelling in fungi, and RNA interference in animals. Proc. Natl. Acad. Sci. U.S.A. 2000;97:11650–11654. doi: 10.1073/pnas.200217597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vaucheret H., Vazquez F., Crete P., Bartel D. P. The action of ARGONAUTE1 in the miRNA pathway and its regulation by the miRNA pathway are crucial for plant development. Genes Dev. 2004;18:1187–1197. doi: 10.1101/gad.1201404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tijsterman M., Okihara K. L., Thijssen K., Plasterk R. H. PPW-1, a PAZ/PIWI protein required for efficient germline RNAi, is defective in a natural isolate of C. elegans. Curr. Biol. 2002;12:1535–1540. doi: 10.1016/s0960-9822(02)01110-7. [DOI] [PubMed] [Google Scholar]
- 43.Tabara H., Sarkissian M., Kelly W. G., Fleenor J., Grishok A., Timmons L., Fire A., Mello C. C. The rde-1 gene, RNA interference, and transposon silencing in C. elegans. Cell. 1999;99:123–132. doi: 10.1016/s0092-8674(00)81644-x. [DOI] [PubMed] [Google Scholar]
- 44.Okamura K., Ishizuka A., Siomi H., Siomi M. C. Distinct roles for Argonaute proteins in small RNA-directed RNA cleavage pathways. Genes Dev. 2004;18:1655–1666. doi: 10.1101/gad.1210204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Meister G., Landthaler M., Patkaniowska A., Dorsett Y., Teng G., Tuschl T. Human Argonaute2 mediates RNA cleavage targeted by miRNAs and siRNAs. Mol. Cell. 2004;15:185–197. doi: 10.1016/j.molcel.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 46.Ekwall K. The RITS complex – a direct link between small RNA and heterochromatin. Mol. Cell. 2004;13:304–305. doi: 10.1016/s1097-2765(04)00057-7. [DOI] [PubMed] [Google Scholar]
- 47.Hall I., Noma K., Grewal S. I. RNA interference machinery regulates chromosome dynamics during mitosis and meiosis in fission yeast. Proc. Natl. Acad. Sci. U.S.A. 2003;100:193–198. doi: 10.1073/pnas.232688099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Volpe T., Schramke V., Hamilton G. L., White S. A., Teng G., Martienssen R. A., Allshire R. C. RNA interference is required for normal centromere function in fission yeast. Chromosome Res. 2003;11:137–146. doi: 10.1023/a:1022815931524. [DOI] [PubMed] [Google Scholar]
- 49.Reinhart B. J., Bartel D. P. Small RNAs correspond to centromere heterochromatic repeats. Science. 2002;297:1831. doi: 10.1126/science.1077183. [DOI] [PubMed] [Google Scholar]
- 50.Sigova A., Rhind N., Zamore P. D. A single Argonaute protein mediates both transcriptional and posttranscriptional silencing in Schizosaccharomyces pombe. Genes Dev. 2004;18:2359–2367. doi: 10.1101/gad.1218004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cox D. N., Chao A., Baker J., Chang L., Qiao D., Lin H. A novel class of evolutionarily conserved genes defined by piwi are essential for stem cell self-renewal. Genes Dev. 1998;12:3715–3727. doi: 10.1101/gad.12.23.3715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kataoka Y., Takeichi M., Uemura T. Developmental roles and molecular characterization of a Drosophila homologue of Arabidopsis Argonaute1, the founder of a novel gene superfamily. Genes Cells. 2001;6:313–325. doi: 10.1046/j.1365-2443.2001.00427.x. [DOI] [PubMed] [Google Scholar]
- 53.Cox D. N., Chao A., Lin H. piwi encodes a nucleoplasmic factor whose activity modulates the number and division rate of germline stem cells. Development. 2000;127:503–514. doi: 10.1242/dev.127.3.503. [DOI] [PubMed] [Google Scholar]
- 54.Sharma A. K., Nelson M. C., Brandt J. E., Wessman M., Mahmud N., Weller K. P., Hoffman R. Human CD34+ stem cells express the hiwi gene, a human homologue of the Drosophila gene piwi. Blood. 2001;97:426–434. doi: 10.1182/blood.v97.2.426. [DOI] [PubMed] [Google Scholar]
- 55.Kuramochi-Miyagawa S., Kimura T., Yomogida K., Kuroiwa A., Tadokoro Y., Fujita Y., Sato M., Matsuda Y., Nakano T. Two mouse piwi-related genes: miwi and mili. Mech. Dev. 2001;108:121–133. doi: 10.1016/s0925-4773(01)00499-3. [DOI] [PubMed] [Google Scholar]
- 56.Findley S. D., Tamanaha M., Clegg N. J., Ruohola-Baker H. Maelstrom, a Drosophila spindle-class gene, encodes a protein that colocalizes with Vasa and RDE1/AGO1 homolog, Aubergine, in nuage. Development. 2003;130:859–871. doi: 10.1242/dev.00310. [DOI] [PubMed] [Google Scholar]
- 57.Yoon C., Kawakami K., Hopkins N. Zebrafish vasa homologue RNA is localized to the cleavage planes of 2- and 4-cell-stage embryos and is expressed in the primordial germ cells. Development. 1997;124:3157–3165. doi: 10.1242/dev.124.16.3157. [DOI] [PubMed] [Google Scholar]
- 58.Knaut H., Pelegri F., Bohmann K., Schwarz H., Nusslein-Volhard C. Zebrafish vasa RNA but not its protein is a component of the germ plasm and segregates asymmetrically before germline specification. J. Cell Biol. 2000;149:875–888. doi: 10.1083/jcb.149.4.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang J. F., Zhou H., Chen Y. Q., Luo Q. J., Qu L. H. Identification of 20 microRNAs from Oryza sativa. Nucleic Acids Res. 2004;32:1688–1695. doi: 10.1093/nar/gkh332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rhoades M. W., Reinhart B. J., Lim L. P., Burge C. B., Bartel B., Bartel D. P. Prediction of plant microRNA targets. Cell. 2002;110:513–520. doi: 10.1016/s0092-8674(02)00863-2. [DOI] [PubMed] [Google Scholar]
- 61.Morel J. B., Godon C., Mourrain P., Beclin C., Boutet S., Feuerbach F., Proux F., Vaucheret H. Fertile hypomorphic ARGONAUTE (ago1) mutants impaired in post-transcriptional gene silencing and virus resistance. Plant Cell. 2002;14:629–639. doi: 10.1105/tpc.010358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.King F. J., Szakmary A., Cox D. N., Lin H. Yb modulates the divisions of both germline and somatic stem cells through piwi- and hh-mediated mechanisms in the Drosophila ovary. Mol. Cell. 2001;7:497–508. doi: 10.1016/s1097-2765(01)00197-6. [DOI] [PubMed] [Google Scholar]
- 63.Barnes E. A., Kong M., Ollendorff V., Donoghue D. J. Patched1 interacts with cyclin B1 to regulate cell cycle progression. EMBO J. 2001;20:2214–2223. doi: 10.1093/emboj/20.9.2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Roy S., Ingham P. W. Hedgehogs tryst with the cell cycle. J. Cell Sci. 2002;115:4393–4397. doi: 10.1242/jcs.00158. [DOI] [PubMed] [Google Scholar]
- 65.Tabata T., Kornberg T. B. Hedgehog is a signaling protein with a key role in patterning Drosophila imaginal discs. Cell. 1994;76:89–102. doi: 10.1016/0092-8674(94)90175-9. [DOI] [PubMed] [Google Scholar]
- 66.Lamontagne B., Larose S., Boulanger J., Elela S. A. The RNase III family: a conserved structure and expanding functions in eukaryotic dsRNA metabolism. Curr. Issues Mol. Biol. 2001;3:71–78. [PubMed] [Google Scholar]
- 67.Nicholson A. W. Function, mechanism and regulation of bacterial ribonucleases. FEMS Microbiol. Rev. 1999;23:371–390. doi: 10.1111/j.1574-6976.1999.tb00405.x. [DOI] [PubMed] [Google Scholar]
- 68.Kay B. K., Williamson M. P., Sudol M. The importance of being proline: the interaction of proline-rich motifs in signaling proteins with their cognate domains. FASEB J. 2000;14:231–241. [PubMed] [Google Scholar]
- 69.Filippov V., Solovyev V., Filippova M., Gill S. S. A novel type of RNase III family proteins in eukaryotes. Gene. 2000;245:213–221. doi: 10.1016/s0378-1119(99)00571-5. [DOI] [PubMed] [Google Scholar]
- 70.Fortin K. R., Nicholson R. H., Nicholson A. W. Mouse ribonuclease III. cDNA structure, expression analysis, and chromosomal location. BMC Genomics. 2002;3:26. doi: 10.1186/1471-2164-3-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wu H., Xu H., Miraglia L. J., Crooke S. T. Human RNase III is a 160-kDa protein involved in preribosomal RNA processing. J. Biol. Chem. 2000;275:36957–36965. doi: 10.1074/jbc.M005494200. [DOI] [PubMed] [Google Scholar]
- 72.Lee Y., Ahn C., Han J., Choi H., Kim J., Yim J., Lee J., Provost P., Radmark O., Kim S., Kim V. N. The nuclear RNase III Drosha initiates microRNA processing. Nature (London) 2003;425:415–419. doi: 10.1038/nature01957. [DOI] [PubMed] [Google Scholar]
- 73.Gregory R. I., Yan K. P., Amuthan G., Chendrimada T., Doratotaj B., Cooch N., Shiekhattar R. The Microprocessor complex mediates the genesis of microRNAs. Nature (London) 2004;432:235–240. doi: 10.1038/nature03120. [DOI] [PubMed] [Google Scholar]
- 74.Denli A. M., Tops B. B., Plasterk R. H., Ketting R. F., Hannon G. J. Processing of primary microRNAs by the Microprocessor complex. Nature (London) 2004;432:231–235. doi: 10.1038/nature03049. [DOI] [PubMed] [Google Scholar]
- 75.Han J., Lee Y., Yeom K. H., Kim Y. K., Jin H., Kim V. N. The Drosha-DGCR8 complex in primary microRNA processing. Genes Dev. 2004;18:3016–3027. doi: 10.1101/gad.1262504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yi R., Qin Y., Macara I. G., Cullen B. R. Exportin-5 mediates the nuclear export of pre-microRNAs and short hairpin RNAs. Genes Dev. 2003;17:3011–3016. doi: 10.1101/gad.1158803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bohnsack M. T., Czaplinski K., Gorlich D. Exportin 5 is a RanGTP-dependent dsRNA-binding protein that mediates nuclear export of pre-miRNAs. RNA. 2004;10:185–191. doi: 10.1261/rna.5167604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lund E., Guttinger S., Calado A., Dahlberg J. E., Kutay U. Nuclear export of microRNA precursors. Science. 2004;303:95–98. doi: 10.1126/science.1090599. [DOI] [PubMed] [Google Scholar]
- 79.Bernstein E., Kim S. Y., Carmell M. A., Murchison E. P., Alcorn H., Li M. Z., Mills A. A., Elledge S. J., Anderson K. V., Hannon G. J. Dicer is essential for mouse development. Nat. Genet. 2003;35:215–217. doi: 10.1038/ng1253. [DOI] [PubMed] [Google Scholar]
- 80.Ketting R. F., Fischer S. E., Bernstein E., Sijen T., Hannon G. J., Plasterk R. H. Dicer functions in RNA interference and in synthesis of small RNA involved in developmental timing in C. elegans. Genes Dev. 2001;15:2654–2659. doi: 10.1101/gad.927801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Liu Q., Rand T. A., Kalidas S., Du F., Kim H. E., Smith D. P., Wang X. R2D2, a bridge between the initiation and effector steps of the Drosophila RNAi pathway. Science. 2003;301:1921–1925. doi: 10.1126/science.1088710. [DOI] [PubMed] [Google Scholar]
- 82.Nykanen A., Haley B., Zamore P. D. ATP requirements and small interfering RNA structure in the RNA interference pathway. Cell. 2001;107:309–321. doi: 10.1016/s0092-8674(01)00547-5. [DOI] [PubMed] [Google Scholar]
- 83.Zhang H., Kolb F., Brondani V., Billy E., Filipowicz W. Human Dicer preferentially cleaves dsRNAs at their termini without a requirement for ATP. EMBO J. 2002;21:5875–5885. doi: 10.1093/emboj/cdf582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Xie Z., Kasschau K. D., Carrington J. C. Negative feedback regulation of Dicer-Like1 in Arabidopsis by microRNA-guided mRNA degradation. Curr. Biol. 2003;13:784–789. doi: 10.1016/s0960-9822(03)00281-1. [DOI] [PubMed] [Google Scholar]
- 85.Zhang H., Kolb F. A., Jaskiewicz L., Westhof E., Filipowicz W. Single processing center models for human Dicer and bacterial RNase III. Cell. 2004;118:57–68. doi: 10.1016/j.cell.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 86.Lingel A., Simon B., Izaurralde E., Sattler M. Structure and nucleic-acid binding of the Drosophila Argonaute 2 PAZ domain. Nature (London) 2003;426:465–469. doi: 10.1038/nature02123. [DOI] [PubMed] [Google Scholar]
- 87.Song J. J., Liu J., Tolia N. H., Schneiderman J., Smith S. K., Martienssen R. A., Hannon G. J., Joshua-Tor L. The crystal structure of the Argonaute2 PAZ domain reveals an RNA binding motif in RNAi effector complexes. Nat. Struct. Biol. 2003;10:1026–1032. doi: 10.1038/nsb1016. [DOI] [PubMed] [Google Scholar]
- 88.Yan K. S., Yan S., Farooq A., Han A., Zeng L., Zhou M. M. Structure and conserved RNA binding of the PAZ domain. Nature (London) 2003;426:468–474. doi: 10.1038/nature02129. [DOI] [PubMed] [Google Scholar]
- 89.Wienholds E., Koudijs M. J., van Eeden F. J., Cuppen E., Plasterk R. H. The microRNA-producing enzyme Dicer1 is essential for zebrafish development. Nat. Genet. 2003;35:217–218. doi: 10.1038/ng1251. [DOI] [PubMed] [Google Scholar]
- 90.Knight S. W., Bass B. L. A role for the RNase III enzyme DCR-1 in RNA interference and germ line development in Caenorhabditis elegans. Science. 2001;293:2269–2271. doi: 10.1126/science.1062039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hutvagner G., McLachlan J., Pasquinelli A. E., Balint E., Tuschl T., Zamore P. D. A cellular function for the RNA-interference enzyme Dicer in the maturation of the let-7 small temporal RNA. Science. 2001;293:834–838. doi: 10.1126/science.1062961. [DOI] [PubMed] [Google Scholar]
- 92.Fukagawa T., Nogami M., Yoshikawa M., Ikeno M., Okazaki T., Takami Y., Nakayama T., Oshimura M. Dicer is essential for formation of the heterochromatin structure in vertebrate cells. Nat. Cell Biol. 2004;6:784–791. doi: 10.1038/ncb1155. [DOI] [PubMed] [Google Scholar]
- 93.Catalanotto C., Pallotta M., ReFalo P., Sachs M. S., Vayssie L., Macino G., Cogoni C. Redundancy of the two dicer genes in transgene-induced posttranscriptional gene silencing in Neurospora crassa. Mol. Cell. Biol. 2004;24:2536–2545. doi: 10.1128/MCB.24.6.2536-2545.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lee Y. S., Nakahara K., Pham J. W., Kim K., He Z., Sontheimer E. J., Carthew R. W. Distinct roles for Drosophila Dicer-1 and Dicer-2 in the siRNA/miRNA silencing pathways. Cell. 2004;117:69–81. doi: 10.1016/s0092-8674(04)00261-2. [DOI] [PubMed] [Google Scholar]
- 95.Schauer S. E., Jacobsen S. E., Meinke D. W., Ray A. DICER-LIKE1: blind men and elephants in Arabidopsis development. Trends Plant Sci. 2002;7:487–491. doi: 10.1016/s1360-1385(02)02355-5. [DOI] [PubMed] [Google Scholar]
- 96.Xie Z., Johansen L. K., Gustafson A. M., Kasschau K. D., Lellis A. D., Zilberman D., Jacobsen S. E., Carrington J. C. Genetic and functional diversification of small RNA pathways in plants. PLoS Biol. 2004;2:E104. doi: 10.1371/journal.pbio.0020104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Papp I., Mette M. F., Aufsatz W., Daxinger L., Schauer S. E., Ray A., van der Winden J., Matzke M., Matzke A. J. Evidence for nuclear processing of plant micro RNA and short interfering RNA precursors. Plant Physiol. 2003;132:1382–1390. doi: 10.1104/pp.103.021980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Baulcombe D. Diced defence. Nature (London) 2001;409:295–296. doi: 10.1038/35053256. [DOI] [PubMed] [Google Scholar]
- 99.Tahbaz N., Carmichael J. B., Hobman T. C. GERp95 belongs to a family of signal-transducing proteins and requires Hsp90 activity for stability and Golgi localization. J. Biol. Chem. 2001;276:43294–43299. doi: 10.1074/jbc.M107808200. [DOI] [PubMed] [Google Scholar]
- 100.Tabara H., Yigit E., Siomi H., Mello C. C. The dsRNA binding protein RDE-4 interacts with RDE-1, DCR-1, and a DExH-box helicase to direct RNAi in C. elegans. Cell. 2002;109:861–871. doi: 10.1016/s0092-8674(02)00793-6. [DOI] [PubMed] [Google Scholar]
- 101.Vazquez F., Gasciolli V., Crete P., Vaucheret H. The nuclear dsRNA binding protein HYL1 is required for microRNA accumulation and plant development, but not posttranscriptional transgene silencing. Curr. Biol. 2004;14:346–351. doi: 10.1016/j.cub.2004.01.035. [DOI] [PubMed] [Google Scholar]
- 102.Parrish S., Fire A. Distinct roles for RDE-1 and RDE-4 during RNA interference in Caenorhabditis elegans. RNA. 2001;7:1397–1402. [PMC free article] [PubMed] [Google Scholar]
- 102a.Tomari Y., Matranga C., Haley B., Martinez N., Zamore P. D. A protein sensor for siRNA asymmetry. Science. 2004;306:1377–1380. doi: 10.1126/science.1102755. [DOI] [PubMed] [Google Scholar]
- 103.Grishok A., Tabara H., Mello C. C. Genetic requirements for inheritance of RNAi in C. elegans. Science. 2000;287:2494–2497. doi: 10.1126/science.287.5462.2494. [DOI] [PubMed] [Google Scholar]
- 104.Ishizuka A., Siomi M. C., Siomi H. A Drosophila fragile X protein interacts with components of RNAi and ribosomal proteins. Genes Dev. 2002;16:2497–2508. doi: 10.1101/gad.1022002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Schwarz D. S., Hutvagner G., Haley B., Zamore P. D. Evidence that siRNAs function as guides, not primers, in the Drosophila and human RNAi pathways. Mol. Cell. 2002;10:537–548. doi: 10.1016/s1097-2765(02)00651-2. [DOI] [PubMed] [Google Scholar]
- 106.Nelson P. T., Hatzigeorgiou A. G., Mourelatos Z. miRNP:mRNA association in polyribosomes in a human neuronal cell line. RNA. 2004;10:387–394. doi: 10.1261/rna.5181104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Doench J. G., Petersen C. P., Sharp P. A. siRNAs can function as miRNAs. Genes Dev. 2003;17:438–442. doi: 10.1101/gad.1064703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zeng Y., Yi R., Cullen B. R. MicroRNAs and small interfering RNAs can inhibit mRNA expression by similar mechanisms. Proc. Natl. Acad. Sci. U.S.A. 2003;100:9779–9784. doi: 10.1073/pnas.1630797100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Pham J. W., Pellino J. L., Lee Y. S., Carthew R. W., Sontheimer E. J. A Dicer-2-dependent 80s complex cleaves targeted mRNAs during RNAi in Drosophila. Cell. 2004;117:83–94. doi: 10.1016/s0092-8674(04)00258-2. [DOI] [PubMed] [Google Scholar]
- 110.Schwarz D. S., Hutvagner G., Du T., Xu Z., Aronin N., Zamore P. D. Asymmetry in the assembly of the RNAi enzyme complex. Cell. 2003;115:199–208. doi: 10.1016/s0092-8674(03)00759-1. [DOI] [PubMed] [Google Scholar]
- 111.Tomari Y., Du T., Haley B., Schwarz D. S., Bennett R., Cook H. A., Koppetsch B. S., Theurkauf W. E., Zamore P. D. RISC assembly defects in the Drosophila RNAi mutant armitage. Cell. 2004;116:831–841. doi: 10.1016/s0092-8674(04)00218-1. [DOI] [PubMed] [Google Scholar]
- 112.Kennerdell J. R., Yamaguchi S., Carthew R. W. RNAi is activated during Drosophila oocyte maturation in a manner dependent on aubergine and spindle-E. Genes Dev. 2002;16:1884–1889. doi: 10.1101/gad.990802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Cook H. A., Koppetsch B. S., Wu J., Theurkauf W. E. The Drosophila SDE3 homolog armitage is required for oskar mRNA silencing and embryonic axis specification. Cell. 2004;116:817–829. doi: 10.1016/s0092-8674(04)00250-8. [DOI] [PubMed] [Google Scholar]
- 114.Caudy A. A., Ketting R. F., Hammond S. M., Denli A. M., Bathoorn A. M., Tops B. B., Silva J. M., Myers M. M., Hannon G. J., Plasterk R. H. A micrococcal nuclease homologue in RNAi effector complexes. Nature (London) 2003;425:411–414. doi: 10.1038/nature01956. [DOI] [PubMed] [Google Scholar]
- 115.Reddi K. K. Mode of action of micrococcal phosphodiesterase. Nature (London) 1960;187:74–75. doi: 10.1038/187074a0. [DOI] [PubMed] [Google Scholar]
- 116.Martinez J., Tuschl T. RISC is a 5′ phosphomonoester-producing RNA endonuclease. Genes Dev. 2004;18:975–980. doi: 10.1101/gad.1187904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Schwarz D. S., Tomari Y., Zamore P. D. The RNA-induced silencing complex is a Mg2+-dependent endonuclease. Curr. Biol. 2004;14:787–791. doi: 10.1016/j.cub.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 118.Pillai R. S., Artus C. G., Filipowicz W. Tethering of human Ago proteins to mRNA mimics the miRNA-mediated repression of protein synthesis. RNA. 2004;10:1518–1525. doi: 10.1261/rna.7131604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mochizuki K., Fine N. A., Fujisawa T., Gorovsky M. A. Analysis of a piwi-related gene implicates small RNAs in genome rearrangement in Tetrahymena. Cell. 2002;110:689–699. doi: 10.1016/s0092-8674(02)00909-1. [DOI] [PubMed] [Google Scholar]
- 120.Pal-Bhadra M., Leibovitch B. A., Gandhi S. G., Rao M., Bhadra U., Birchler J. A., Elgin S. C. Heterochromatic silencing and HP1 localization in Drosophila are dependent on the RNAi machinery. Science. 2004;303:669–672. doi: 10.1126/science.1092653. [DOI] [PubMed] [Google Scholar]
- 121.Provost P., Silverstein R., Dishart D., Walfridsson J., Djupedal I., Kniola B., Wright A., Samuelsson B., Radmark O., Ekwall K. Dicer is required for chromosome segregation and gene silencing in fission yeast cells. Proc. Natl. Acad. Sci. U.S.A. 2002;99:16648–16653. doi: 10.1073/pnas.212633199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ciosk R., Zachariae W., Michaelis C., Shevchenko A., Mann M., Nasmyth K. An ESP1/PDS1 complex regulates loss of sister chromatid cohesion at the metaphase to anaphase transition in yeast. Cell. 1998;93:1067–1076. doi: 10.1016/s0092-8674(00)81211-8. [DOI] [PubMed] [Google Scholar]
- 123.Guacci V., Koshland D., Strunnikov A. A direct link between sister chromatid cohesion and chromosome condensation revealed through the analysis of MCD1 in S. cerevisiae. Cell. 1997;91:47–57. doi: 10.1016/s0092-8674(01)80008-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Michaelis C., Ciosk R., Nasmyth K. Cohesins: chromosomal proteins that prevent premature separation of sister chromatids. Cell. 1997;91:35–45. doi: 10.1016/s0092-8674(01)80007-6. [DOI] [PubMed] [Google Scholar]
- 125.Lee J. Y., Orr-Weaver T. L. The molecular basis of sister-chromatid cohesion. Annu. Rev. Cell Dev. Biol. 2001;17:753–777. doi: 10.1146/annurev.cellbio.17.1.753. [DOI] [PubMed] [Google Scholar]
- 126.Grewal S. I., Elgin S. C. Heterochromatin: new possibilities for the inheritance of structure. Curr. Opin. Genet. Dev. 2002;12:178–187. doi: 10.1016/s0959-437x(02)00284-8. [DOI] [PubMed] [Google Scholar]
- 127.Bernard P., Maure J. F., Partridge J. F., Genier S., Javerzat J. P., Allshire R. C. Requirement of heterochromatin for cohesion at centromeres. Science. 2001;294:2539–2542. doi: 10.1126/science.1064027. [DOI] [PubMed] [Google Scholar]
- 128.Nonaka N., Kitajima T., Yokobayashi S., Xiao G., Yamamoto M., Grewal S. I., Watanabe Y. Recruitment of cohesin to heterochromatic regions by Swi6/HP1 in fission yeast. Nat. Cell Biol. 2002;4:89–93. doi: 10.1038/ncb739. [DOI] [PubMed] [Google Scholar]
- 129.Verdel A., Jia S., Gerber S., Sugiyama T., Gygi S., Grewal S. I., Moazed D. RNAi-mediated targeting of heterochromatin by the RITS complex. Science. 2004;303:672–676. doi: 10.1126/science.1093686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Noma K., Sugiyama T., Cam H., Verdel A., Zofall M., Jia S., Moazed D., Grewal S. I. RITS acts in cis to promote RNA interference-mediated transcriptional and post-transcriptional silencing. Nat. Genet. 2004;36:1141–1142. doi: 10.1038/ng1452. [DOI] [PubMed] [Google Scholar]
- 131.Motamedi M. R., Verdel A., Colmenares S. U., Gerber S. A., Gygi S. P., Moazed D. Two RNAi complexes, RITS and RDRC, physically interact and localize to noncoding centromeric RNAs. Cell. 2004;119:789–802. doi: 10.1016/j.cell.2004.11.034. [DOI] [PubMed] [Google Scholar]
- 132.Zeng Y., Cullen B. R. RNA interference in human cells is restricted to the cytoplasm. RNA. 2002;8:855–860. doi: 10.1017/s1355838202020071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Billy E., Brondani V., Zhang H., Muller U., Filipowicz W. Specific interference with gene expression induced by long, double-stranded RNA in mouse embryonal teratocarcinoma cell lines. Proc. Natl. Acad. Sci. U.S.A. 2001;98:14428–14433. doi: 10.1073/pnas.261562698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Sarot E., Payen-Groschene G., Bucheton A., Pelisson A. Evidence for a piwi-dependent RNA silencing of the gypsy endogenous retrovirus by the Drosophila melanogaster flamenco gene. Genetics. 2004;166:1313–1321. doi: 10.1534/genetics.166.3.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Provost P., Samuelsson B., Radmark O. Interaction of 5-lipoxygenase with cellular proteins. Proc. Natl. Acad. Sci. U.S.A. 1999;96:1881–1885. doi: 10.1073/pnas.96.5.1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Catala M., Lamontagne B., Larose S., Ghazal G., Elela S. A. Cell cycle-dependent nuclear localization of yeast RNase III is required for efficient cell division. Mol. Biol. Cell. 2004;15:3015–3030. doi: 10.1091/mbc.E04-03-0183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Carmichael J. B., Provost P., Ekwall K., Hobman T. C. ago1 and dcr1, two core components of the RNA interference pathway, functionally diverge from rdp1 in regulating cell cycle events in Schizosaccharomyces pombe. Mol. Biol. Cell. 2004;15:1425–1435. doi: 10.1091/mbc.E03-06-0433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Raleigh J. M., O'Connell M. J. The G2 DNA damage checkpoint targets both Wee1 and Cdc25. J. Cell Sci. 2000;113:1727–1736. doi: 10.1242/jcs.113.10.1727. [DOI] [PubMed] [Google Scholar]
- 139.Rhind N., Furnari B., Russell P. Cdc2 tyrosine phosphorylation is required for the DNA damage checkpoint in fission yeast. Genes Dev. 1997;11:504–511. doi: 10.1101/gad.11.4.504. [DOI] [PubMed] [Google Scholar]
- 140.Rhind N., Russell P. Tyrosine phosphorylation of cdc2 is required for the replication checkpoint in Schizosaccharomyces pombe. Mol. Cell. Biol. 1998;18:3782–3787. doi: 10.1128/mcb.18.7.3782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Russell P., Nurse P. cdc25+ functions as an inducer in the mitotic control of fission yeast. Cell. 1986;45:145–153. doi: 10.1016/0092-8674(86)90546-5. [DOI] [PubMed] [Google Scholar]
- 142.Millar J. B., McGowan C. H., Lenaers G., Jones R., Russell P. p80cdc25 mitotic inducer is the tyrosine phosphatase that activates p34cdc2 kinase in fission yeast. EMBO J. 1991;10:4301–4309. doi: 10.1002/j.1460-2075.1991.tb05008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Nyberg K. A., Michelson R. J., Putnam C. W., Weinert T. A. Toward maintaining the genome: DNA damage and replication checkpoints. Annu. Rev. Genet. 2002;36:617–656. doi: 10.1146/annurev.genet.36.060402.113540. [DOI] [PubMed] [Google Scholar]
- 144.Hoeijmakers J. H. Genome maintenance mechanisms for preventing cancer. Nature (London) 2001;411:366–374. doi: 10.1038/35077232. [DOI] [PubMed] [Google Scholar]
- 145.Qiao D., Zeeman A. M., Deng W., Looijenga L. H., Lin H. Molecular characterization of hiwi, a human member of the piwi gene family whose overexpression is correlated to seminomas. Oncogene. 2002;21:3988–3999. doi: 10.1038/sj.onc.1205505. [DOI] [PubMed] [Google Scholar]
- 146.Skotheim R. I., Kraggerud S. M., Fossa S. D., Stenwig A. E., Gedde-Dahl T., Jr, Danielsen H. E., Jakobsen K. S., Lothe R. A. Familial/bilateral and sporadic testicular germ cell tumors show frequent genetic changes at loci with suggestive linkage evidence. Neoplasia. 2001;3:196–203. doi: 10.1038/sj.neo.7900153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Summersgill B., Osin P., Lu Y. J., Huddart R., Shipley J. Chromosomal imbalances associated with carcinoma in situ and associated testicular germ cell tumours of adolescents and adults. Br. J. Cancer. 2001;85:213–220. doi: 10.1054/bjoc.2001.1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Sathya P., Tomkins D. J., Freeman V., Paes B., Nowaczyk M. J. De novo deletion 12q: report of a patient with 12q24.31q24.33 deletion. Am. J. Med. Genet. 1999;84:116–119. doi: 10.1002/(sici)1096-8628(19990521)84:2<116::aid-ajmg6>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 149.Dome J. S., Coppes M. J. Recent advances in Wilms tumor genetics. Curr. Opin. Pediatr. 2002;14:5–11. doi: 10.1097/00008480-200202000-00002. [DOI] [PubMed] [Google Scholar]
- 150.Koesters R., Ridder R., Kopp-Schneider A., Betts D., Adams V., Niggli F., Briner J., von Knebel Doeberitz M. Mutational activation of the β-catenin proto-oncogene is a common event in the development of Wilms' tumors. Cancer Res. 1999;59:3880–3882. [PubMed] [Google Scholar]
- 151.Carmell M., Xuan Z., Zhang M., Hannon G. The Argonaute family: tentacles that reach into RNAi, developmental control, stem cell maintenance, and tumorigenesis. Genes Dev. 2002;16:2733–2742. doi: 10.1101/gad.1026102. [DOI] [PubMed] [Google Scholar]
- 152.Calin G. A., Dumitru C. D., Shimizu M., Bichi R., Zupo S., Noch E., Aldler H., Rattan S., Keating M., Rai K., et al. Frequent deletions and down-regulation of micro-RNA genes miR15 and miR16 at 13q14 in chronic lymphocytic leukemia. Proc. Natl. Acad. Sci. U.S.A. 2002;99:15524–15529. doi: 10.1073/pnas.242606799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Ding X. Z., Hennig R., Adrian T. E. Lipoxygenase and cyclooxygenase metabolism: new insights in treatment and chemoprevention of pancreatic cancer. Mol. Cancer. 2003;2:10. doi: 10.1186/1476-4598-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Kennedy T. J., Chan C. Y., Ding X. Z., Adrian T. E. Lipoxygenase inhibitors for the treatment of pancreatic cancer. Expert Rev. Anticancer Ther. 2003;3:525–536. doi: 10.1586/14737140.3.4.525. [DOI] [PubMed] [Google Scholar]
- 155.Gunning W. T., Kramer P. M., Steele V. E., Pereira M. A. Chemoprevention by lipoxygenase and leukotriene pathway inhibitors of vinyl carbamate-induced lung tumors in mice. Cancer Res. 2002;62:4199–4201. [PubMed] [Google Scholar]
- 156.Kaul D., Sikand K. Defective RNA-mediated c-myc gene silencing pathway in Burkitt's lymphoma. Biochem. Biophys. Res. Commun. 2004;313:552–554. doi: 10.1016/j.bbrc.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 157.Hecht J. L., Aster J. C. Molecular biology of Burkitt's lymphoma. J. Clin. Oncol. 2000;18:3707–3721. doi: 10.1200/JCO.2000.18.21.3707. [DOI] [PubMed] [Google Scholar]
- 158.Lewis B. P., Shih I. H., Jones-Rhoades M. W., Bartel D. P., Burge C. B. Prediction of mammalian microRNA targets. Cell. 2003;115:787–798. doi: 10.1016/s0092-8674(03)01018-3. [DOI] [PubMed] [Google Scholar]