Abstract
SMG (submucosal gland) secretions are a major component of the airway surface liquid, are associated with innate immunity in the lung, and have been reported to be altered in lung disease. Changes in lung mucosal glycosylation have been reported in CF (cystic fibrosis), which may be responsible for differential bacterial binding to glycosylated components in the lung mucosa and hence increased pre-disposition to pulmonary infection. Glycoproteomic analysis was performed on SMG secretions collected from explanted bronchial tissue of subjects with severe lung disease, with and without CF, and controls without lung disease. Mucins MUC5B and MUC5AC were shown to be the dominant high-molecular-mass glycoprotein components, with a minor non-mucin glycoprotein component, gp-340, also present. Oligosaccharides containing blood-group determinants corresponding to subjects' blood type were abundant on MUC5B/MUC5AC, as were Lewis-type epitopes and their sialylated analogues, which are ligands for pathogens and leucocytes. No significant differences were found in the glycosylation of MUC5B/MUC5AC or gp-340 between CF and non-CF subjects with severe lung disease, implying that CF does not influence SMG secretion mucin glycosylation in end-stage lung disease. There were also no significant differences found in the glycosylation of these components in severe lung disease compared with non-diseased lungs. This suggests that previously reported changes in the glycosylation of respiratory glycoconjugates in CF, and other pulmonary conditions, are not due to the glycosylation of components in SMG secretions, but may involve other secretions, responses or extracellular factors.
Keywords: cystic fibrosis, glycosylation, inflammation, mass spectrometry (MS), mucin, submucosal gland secretions
Abbreviations: CF, cyotic fibrosis; CFTR, cystic fibrosis transmembrane conductance regulator protein; 1D SDS/Ag/PAGE, one-dimensional SDS/agarose/PAGE; GlycoSuiteDB®, GlycoComp™, BioinformatIQ™ and GlycosidIQ™ are trademarks of Proteome Systems Limited, Sydney, Australia; LC-ESI-MS, liquid chromatography–electrospray ionization–MS; Lea, Lewis a; Leb, Lewis b; Lex, Lewis x; Ley, Lewis y; MALDI-TOF-MS, matrix-assisted laser-desorption ionization–time-of-flight MS; MS/MS, tandem MS; PMF, peptide mass fingerprinting; SMG, submucosal gland
INTRODUCTION
SMG (submucosal gland) secretions, together with secretions from epithelial goblet cells, contribute the bulk of mucous secretions in the lung [1]. With the periciliary liquid, this forms the airway surface liquid. Mucociliary clearance of the airway surface liquid and entrapped particles, including pathogens, is a primary innate defence mechanism in the lung [2,3]. SMG secretions themselves are thought to be important in the innate immune response, by contributing antimicrobial components such as lysozyme, lactoferrin, secretory IgA, antimicrobial peptides and defensins to the airway surface liquid as well as several high-molecular-mass highly glycosylated mucins [4]. Glandular hypertrophy and hyperplasia of SMGs have been associated with chronic lung disease, including asthma [5], chronic obstructive pulmonary disease [5] and cystic fibrosis (CF) [6]. SMGs are of particular interest in CF lung disease, as serous cells of the SMG epithelium are the predominant site of expression of CFTR (CF transmembrane conductance regulator protein) in the lung [7]. Lack of CFTR function causes CF [8]. The lack of functional CFTR in CF contributes directly to the increased viscosity of SMG secretions [9], which in turn probably contributes significantly to CF lung disease by reducing mucus clearance in the lung [6,10].
Chronic pulmonary infection by organisms such as Pseudomonas aeruginosa is a primary pathology of CF, resulting in permanent lung tissue damage, progressive deterioration in lung condition, and often, eventually, death (reviewed in [11]). Mucus in the airway surface liquid is the primary location of pulmonary infection in CF [12], and mucins are an important component of mucus. Mucin glycosylation involves a wide diversity of oligosaccharide structures that have not been extensively studied, except in CF, where alterations have been reported (reviewed in [13]). It is proposed that these differences in glycosylation may cause increased bacterial adhesion, colonization and infection [12]. Investigations of glycoconjugates present on lung epithelial cell-surface proteins have suggested increased fucosylation and decreased sialylation in the absence of functional CFTR [14]. This pattern does not appear to occur in the glycosylation of secreted airway mucins and other glycoconjugates of CF subjects [13]. However, several reports have described increased sulphation in secreted respiratory mucins from CF subjects [13], whereas increased levels of sialylation and sulphation have also been associated with the severity of infection in CF and chronic bronchitis [15]. These changes may be important for the biological function of glycoconjugates in the lung, as they affect terminal oligosaccharide epitopes involved in protein–protein interactions, thereby potentially altering the adhesion and localization of various components and cells in the respiratory mucosa. The mucous cells of the SMGs, together with epithelial goblet cells, produce MUC5B and MUC5AC, which are the dominant secreted mucins in the lung [16,17]. Immunohistochemical studies have shown that glycoconjugates in SMG mucous cells are heavily O-glycosylated, and contain Lewis-type and blood-group antigens [18,19]. It is therefore possible that SMG secretions contribute to the reported alterations in the glycosylation of components of the lung mucosa associated with lung infection.
Given the previously observed changes to SMGs in various lung diseases, it is possible that differences in the composition of SMG secretions may be associated with, or contribute to, particular conditions. While some of the protein constituents of SMG secretions have been investigated previously [4], the present paper describes the first differential analysis of the high-molecular-mass proteins in SMG secretions from humans with CF and non-CF lung disease, and also controls without lung disease, incorporating protein identification and characterization of the glycosylation of the major mucins. As in vitro collection of SMG secretions from tissue cultures of explanted human bronchial tissue yields only limited sample quantities (≈1 μl of secretion per subject), we utilized an MS-based fragmentation approach for sample analysis, after separation of protein components by gel electrophoresis, which has been most efficient in simultaneous detailed characterization of a broad range of protein and carbohydrate species.
EXPERIMENTAL
SMG secretion collection
Human bronchial explants were received after lung transplantation surgery (subjects with CF and non-CF lung disease) or from cadavers without lung disease (controls). Consent was obtained for accessing all lung-explant samples after appropriate ethics approval by the institutions involved. In the case of cadavers, subjects had provided consent prior to their death [e.g. on driver's licences (U.S.A.)] for use of their organs for research purposes. Explants were kept at 4 °C throughout the course of sample collection. Collection of SMG secretions was performed within 1–3 h of explantation, using methods similar to those described previously [20]. Briefly, bronchial tissue segments were dissected, cleaned, dried, mounted mucosal-side-up on to silica gel and covered with mineral oil. Gland secretion was stimulated by addition of about 2 ml of Krebs buffer containing 250 μM pilocarpine hydrochloride underneath the tissue section to cover the lower surface. Tissue sections were incubated in a humidified environment at 37 °C for 45 min, and then SMG secretion droplets were collected using fine glass rods. Droplets were subsequently pooled under mineral oil to prevent evaporation and placed on ice. Typically, three to five bronchial sections (size about 2 cm×2 cm) were prepared from each subject, resulting in the collection of about 1 μl of secretion. Secretions were stored under mineral oil at −80 °C before further processing. Subject information is summarized in Table 1. Information regarding the subjects' Lewis or secretor status was not available.
Table 1. Summary of subjects from which SMG secretions were analysed.
| Group | Subject | Condition | Age | Gender | Blood group |
|---|---|---|---|---|---|
| CF | 1 | CF | 31 | M | A+ |
| 2 | CF | 31 | F | O− | |
| 3 | CF | 26 | F | A+ | |
| 4 | CF | 22 | M | A− | |
| 5 | CF | 36 | M | O+ | |
| Non-CF | 1 | Emphysema | 59 | M | A+ |
| 2 | Emphysema | 63 | M | O+ | |
| 3 | Bronchiectasis | 27 | M | O− | |
| 4 | Emphysema | 57 | M | O+ | |
| 5 | Emphysema | 52 | F | B+ | |
| Control | 1 | Head trauma | 37 | F | O+ |
| 2 | Head trauma | 22 | M | A− | |
| 3 | Meningitis | 50 | M | O+ |
Sample preparation and gel electrophoresis
Mineral oil was removed from the ≈1 μl SMG secretion samples before they were diluted in 100 μl of sample buffer, reduced and alkylated, concentrated, separated by 1D SDS/Ag/PAGE (one-dimensional SDS/agarose/PAGE), electroblotted to Immobilon-PSQ PVDF membrane (Millipore) and stained for the presence of acidic oligosaccharides with Alcian Blue, as described previously [21]. Each entire sample was loaded into a single gel lane. The molecular-mass spread of the intensity of glycoprotein staining, normalized to the total amount of staining within each subject, was determined from spot-densitometry data of adjoining bands down the region of staining, and was obtained with FluorChem software (Alpha Innotech, San Leandro, CA, U.S.A.).
Protein identification
Protein bands on PVDF membrane were digested with trypsin, and the peptide mixture was analysed using an Axima CFR™ instrument (Kratos, Manchester, U.K.) for MALDI-TOF-MS (matrix-assisted laser-desorption ionization–time-of-flight MS) as described previously [22]. Protein identities were assigned by PMF (peptide mass fingerprinting) with BioinformatIQ™ (Proteome Systems). Some tryptic-digest samples were also analysed by LC-ESI-MS/MS (liquid chromatography coupled to electrospray-ionization tandem MS) using an LCQ DECA system (Thermo Electron Corp., Waltham, MA, U.S.A.) as described previously [23], with protein identities confirmed by SEQUEST database searching (Thermo Electron).
Oligosaccharide analysis
O-linked oligosaccharides were released from protein bands off PVDF membranes by reductive β-elimination, desalted with cation-exchange beads and analysed by LC-ESI-MS/MS with an LCQ DECA XP system (Thermo Electron) as described previously [21,24]. Oligosaccharide compositions were assigned from the parent ion using GlycoComp™ (Proteome Systems). It was assumed that all deoxyhexoses were fucose, and the reduced terminal N-acetylgalactosamine was included as an N-acetylhexosamine. Oligosaccharide structural characterization was performed using MS/MS fragmentation data, the GlycoSuiteDB® glycan database (http://www.glycosuite.com) and the associated glycan mass fingerprinting tool GlycosidIQ™ [25] (Proteome Systems). Assigned structures were also manually validated. The relative intensity of each oligosaccharide was determined from integrated single-ion chromatograms normalized to the total intensity of oligosaccharide ions within each sample. Weighted average occurrences of each monosaccharide or substructure were determined using oligosaccharide intensities calculated from single-ion chromatograms, together with the number of times each monosaccharide or substructure was present in each particular oligosaccharide. Oligosaccharides previously reported from respiratory secretions in humans were determined from those reported in GlycoSuiteDB®.
RESULTS
The components or composition of SMG secretions may be affected by the lack of functional CFTR in CF and may also exhibit changes caused by chronic severe lung disease. Any changes that are specific to individuals with CF may reduce or alter any contribution to innate immunity of SMG secretions in the lung, and thereby contribute to the progression of CF lung disease. In addition, any changes in SMG secretions associated with chronic lung disease may reflect or contribute to the chronic inflammation typical of such conditions. As the small amount of material (about 1 μl) able to be collected from individuals limited the biochemical characterization that was possible, and high-molecular-mass glycoproteins are abundant in SMG secretions [17,26], we focused on protein and glycosylation analysis of these components.
Gel electrophoresis
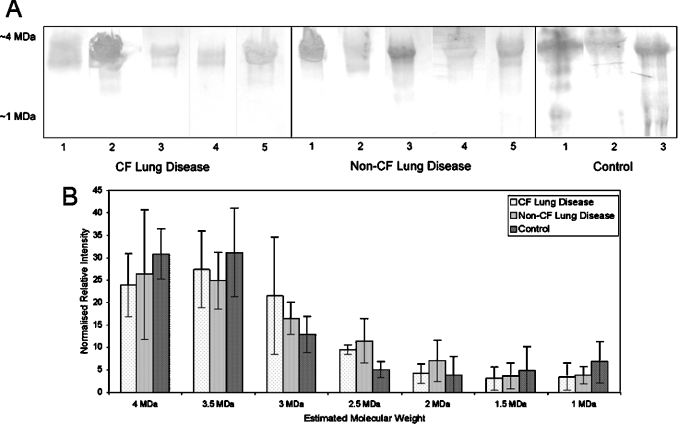
To separate and display the high-molecular-mass proteins present in SMG secretions from diseased and non-diseased explanted bronchial tissue, reduced/alkylated SMG secretion samples were separated by 1D SDS/Ag/PAGE, electroblotted to PVDF membrane and stained with Alcian Blue, which is an effective stain for mucins and highly glycosylated proteins. The molecular-mass range containing stained proteins, together with the molecular-mass spread of normalized protein abundances, is shown in Figure 1. SMG secretions from CF and non-CF subjects with lung disease, and from controls without lung disease, showed the presence of several high-molecular-mass proteins at about 4 MDa. The observed molecular masses should only be regarded as reference points rather than actual molecular masses of mucins. The migration of mucins by electrophoresis is influenced strongly by levels of sialylation and sulphation, as well as the size of the mucins. Owing to the lack of available molecular-mass markers in the high-molecular-mass region, the observed molecular mass is estimated by extrapolation. Intense Alcian Blue staining suggested that these were negatively charged highly glycosylated proteins. Low-intensity bands at about 1 MDa were more abundant in control subjects, although this difference was not statistically significant. The protein profile, as detected by 1D SDS/Ag/PAGE, did not show any significant differences in expression of the high-molecular-mass glycoproteins between SMG secretions from CF and non-CF diseased lungs, nor between these diseased samples and SMG secretions from controls (Figure 1).
Figure 1. High-molecular-mass glycoproteins in SMG secretions collected from explanted bronchial tissue from non-CF and CF subjects with lung disease and controls.
SMG secretions were collected from human explanted bronchial tissue, reduced and alkylated, separated by 1D SDS/Ag/PAGE, electroblotted to PVDF membrane and (A) stained for acidic oligosaccharides with Alcian Blue. All samples contained major components at ≈4 MDa, with minor components present down to ≈1 MDa. (B) Normalized intensity of Alcian Blue staining down the molecular-mass range containing glycoproteins. Error bars show S.E.M. values.
Protein identification
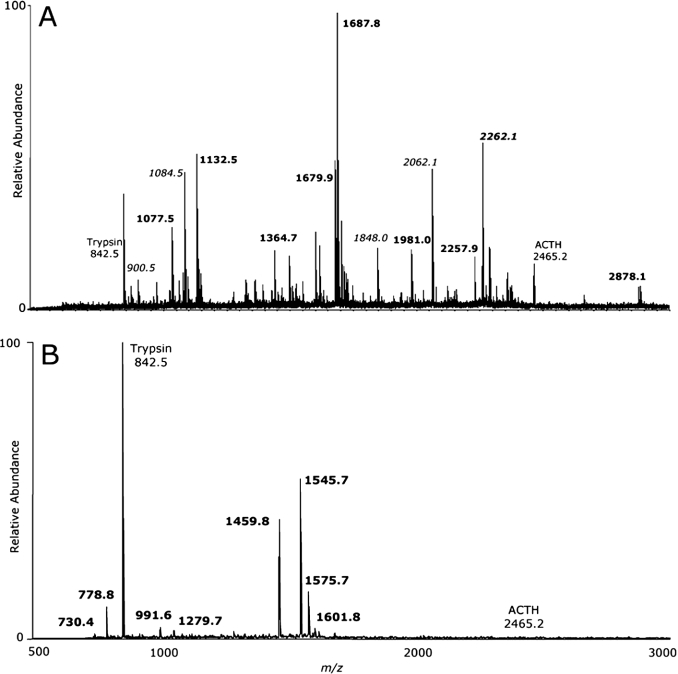
To determine the identities of the high-molecular-mass proteins separated by gel electrophoresis, protein bands which stained with Alcian Blue were excised, digested with trypsin and analysed by MALDI-TOF-MS. PMF was performed for these spectra, identifying several components. LC-ESI-MS/MS analysis also confirmed the identity of these protein components. Figure 2 shows MALDI-TOF-MS spectra representative of the ≈4 MDa and ∼1 MDa protein bands, and Table 2 shows a summary of proteins identified in all samples. The details of the PMF protein identifications, in which the peptides mapped to the non-glycosylated regions of the mucin proteins, are shown in Supplementary Tables S1 and S2 (http://www.BiochemJ.org/bj/387/bj3870911add.htm). The proteins identified in the high-molecular-mass range of SMG secretions from all subjects were the highly glycosylated mucins MUC5B and MUC5AC (≈1–4 MDa) and the mucin-like protein gp-340 (≈1 MDa). The number and relative intensity of peptide hits indicates that the most intense band in all CF and non-CF samples, at ≈4 MDa, is dominated by MUC5B, with MUC5AC also present. This is in agreement with previous immunohistochemical studies, which showed that the mucous cells of SMGs produce predominantly MUC5B [16], together with small amounts of MUC5AC [16,17,26], in contrast with lung epithelial goblet cells, which produce both MUC5B and MUC5AC. MUC5B and MUC5AC were also detected in the bands seen between ≈1 MDa and ≈4 MDa. The protein gp-340 was detected in the ≈1 MDa band in two of five CF lung diseases, all non-CF lung disease and all control samples (Table 2). Although not statistically significant, gel image analysis (Figure 1B) suggested that MUC5B and MUC5AC are more abundant, relative to gp-340, in SMG secretions from subjects with severe lung disease compared with controls without lung disease. This may be due to a decrease in the amount of gp-340, or relative increases in the amount of MUC5AC and MUC5B, present in SMG secretions from subjects with lung disease.
Figure 2. MALDI-TOF-MS spectra of tryptic digests from SMG secretions from explanted bronchial tissue.
Major protein components were excised from the PVDF membrane, digested with trypsin, and the resulting peptide mixture was analysed by MALDI-TOF-MS. (A) Spectrum from the ≈4 MDa protein band from CF subject 1. Peptide ions shown in boldface, MUC5B peptides; peptide ions shown in italics, MUC5AC peptides; Trypsin, autodigest peak; ACTH (adrenocorticotropic hormone), internal calibration peptide. (B) Spectrum from the ≈1 MDa protein band from non-CF subject 3. Bold peptide ions, gp-340 peptides. Spectra are typical of all subjects.
Table 2. High-molecular-mass glycoproteins in SMG secretions.
Proteins were separated by 1D SDS/Ag/PAGE, digested with trypsin, and identified by MALDI-TOF-MS and PMF. MUC5B and MUC5AC were detected in a protein band at ≈4 MDa, and gp-340 variant protein was detected in a protein band at ≈1 MDa. The peptide coverage (%) for each protein is shown for each subject.
| Peptide coverage (%) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CF lung disease | Non-CF lung disease | Control | |||||||||||||
| Protein band | Identified protein | SwissProt accession no. | 1 | 2 | 3 | 4 | 5 | 1 | 2 | 3 | 4 | 5 | 1 | 2 | 3 |
| ≈4 MDa | Mucin 5B | Q9HC84 | 10.6 | 7.1 | 7.1 | 9.2 | 7.2 | 10.3 | 7.7 | 13.2 | 9.2 | 8.7 | 9.9 | 10.3 | 14.7 |
| Mucin 5AC | Q8WWQ4 | 14.7 | N.D.* | 8.0 | 12.6 | 11.0 | 14.0 | 4.8 | 6.4 | 9.3 | 8.0 | 6.4 | 12.2 | 9.2 | |
| ≈1 MDa | gp-340 | Q9UKJ4 | 9.7 | 6.9 | N.D. | N.D. | 12.0 | 11.4 | 20.7 | 19.9 | 22.8 | 23.5 | 29.2 | 17.2 | 28.8 |
* N.D., not detected.
Oligosaccharide compositional characterization
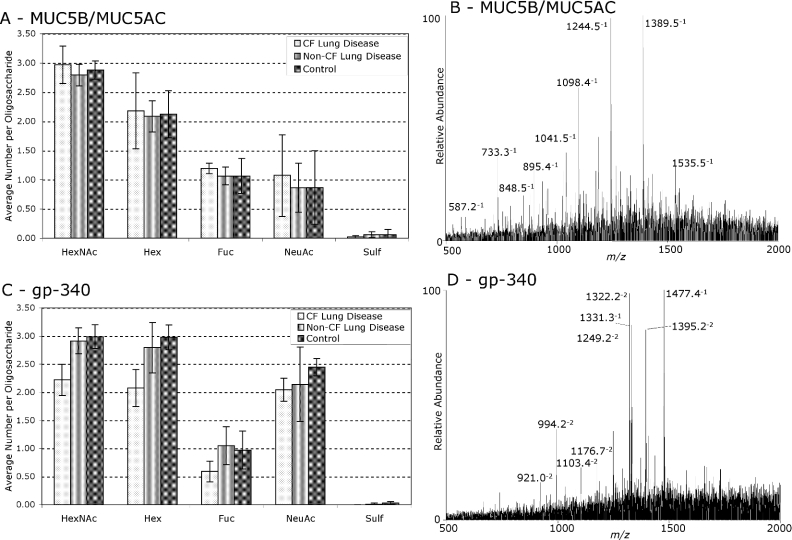
O-linked oligosaccharides are the major post-translational modification of mucins, and account for many of their physical and biological properties. Oligosaccharides were released from Alcian-Blue-staining protein bands by reductive β-elimination and analysed by LC-ESI-MS. The monosaccharide composition of oligosaccharides was determined on the basis of the mass of detected ions, and their corresponding relative intensities were determined from single-ion chromatograms. This compositional analysis can be performed even with limiting amounts of material or when MS/MS spectra are not of sufficient quality to unambiguously assign complete structures to oligosaccharide components. Oligosaccharide ions from MUC5B/MUC5AC and gp-340 from SMG secretions from CF and non-CF subjects with lung disease, and controls, are shown in Supplementary Tables S3 and S4 (http://www.BiochemJ.org/bj/387/bj3870911add.htm). Figure 3 shows the weighted average monosaccharide compositions for these components, together with typical LC-ESI-MS spectra of released oligosaccharide alditols. In the cases where gp-340 was not detected by MALDI-TOF-MS and PMF, oligosaccharides were analysed from the molecular-mass region of the gel where gp-340 was predicted to be localized. Visual inspection of the LC-ESI-MS spectra of released oligosaccharide alditols from the lower-molecular-mass MUC5B/MUC5AC-containing bands (≈1–4 MDa) showed that they were not substantially different from the major ≈4 MDa band, in each subject (results not shown). This compositional analysis did not show any statistically significant differences in the monosaccharide compositions of oligosaccharides from the major glycoproteins in SMG secretions between subjects with CF lung disease, non-CF lung disease or controls.
Figure 3. Monosaccharide compositions of O-linked oligosaccharides from MUC5B/MUC5AC and gp-340 in SMG secretions from non-CF and CF subjects with lung disease and controls.
The gp-340 containing band at ≈1 MDa and the MUC5B/MUC5AC containing band at ≈4 MDa were excised and O-linked oligosaccharides were released by reductive β-elimination. The resulting oligosaccharide alditols were analysed by LC-ESI-MS, and their relative intensities were determined based on single-ion chromatograms. GlycoComp™ was used to determine the monosaccharide composition of the detected oligosaccharides. Weighted average monosaccharide compositions, with error bars representing S.E.M. values, and typical MS spectra, are shown for (A and B) MUC5B/MUC5AC and (C and D) gp-340. MUC5B/MUC5AC was found to have a glycosylation pattern different from that of gp-340 variant protein, specifically with regard to fucosylation and sialylation, but there were no significant differences between CF and non-CF subjects with lung disease or controls. Abbreviations: HexNAc, N-acetylhexosamine; Hex, hexose; Fuc, fucose; NeuAc, N-acetylneuraminic acid; Sulf, sulphate.
MUC5B/MUC5AC and gp-340 from SMG secretions have significantly different O-linked glycosylation profiles, both with low sulphation, but with gp-340 showing more sialylation relative to fucosylation than MUC5B/MUC5AC (Figure 3). The low sulphation of glycoproteins in SMG secretions from both CF and non-CF subjects with severe lung disease is in contrast with the increased sulphation of whole lung mucus previously reported in CF (reviewed in [13]). It is possible that very large highly sulphated oligosaccharides may have not been detected by our methods, which only detected components with an m/z of less than 2000 (including doubly charged ion components of <4000 Da). However, using this methodology we have also detected abundant sulphated oligosaccharides in saliva [21], sputum (results not shown) and epithelial-cell-secreted mucins [27]. Therefore, it is probable that the differences in glycosylation reported in whole lung mucus are due to contributions from sources other than SMGs or to regulated changes in glycosylation as a response to infection or inflammation.
Oligosaccharide structural characterization of MUC5B/MUC5AC
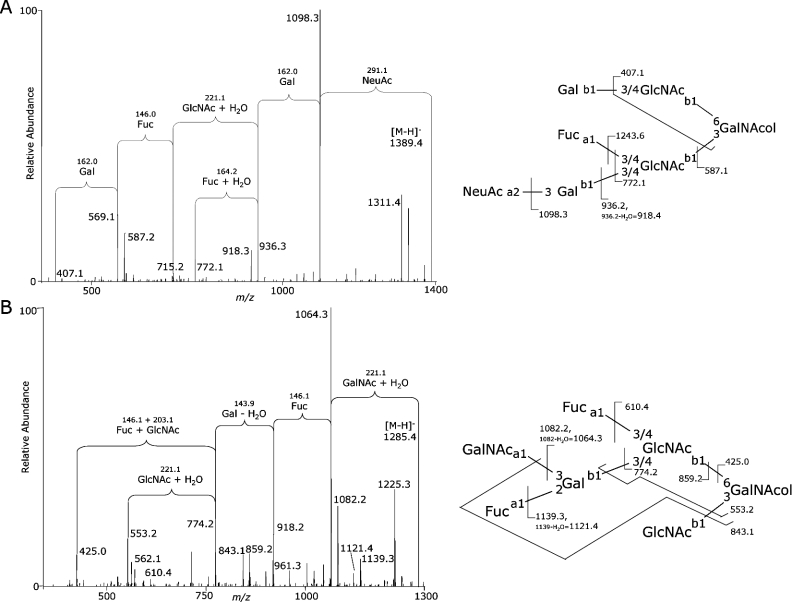
While monosaccharide composition is a useful description of overall oligosaccharide properties, biologically important interactions mediated by oligosaccharides are actually determined by their structural terminal epitopes. Released O-linked oligosaccharides from the high-molecular-mass bands of MUC5B and MUC5AC were therefore fragmented by LC-ESI-MS/MS to determine their structures. Structural characterization of oligosaccharides by MS/MS requires high-quality spectra. The lower-molecular-mass MUC5B, MUC5AC and gp-340-containing Alcian Blue-staining protein bands were not sufficiently abundant for this structural characterization. Empirically determined rules can be used to interpret MS/MS fragmentation data and assign structural epitopes to the corresponding components [28]. Figure 4 shows several typical features of oligosaccharide MS/MS fragmentation that were used to assign various structural elements of oligosaccharides. The bioinformatic tool GlycosidIQ™ [25] automates this process, and was used to interpret MS/MS spectra for components from all subjects, which were also manually validated. Over 90 oligosaccharides ranging from tri- to nonasaccharides were sequenced, with each structure typically detected from several individuals (Supplementary Table S5; http://www.BiochemJ.org/bj/387/bj3870911add.htm). Structural characterization was in agreement with the assigned monosaccharide compositions and showed the presence of a high diversity of structures. Qualitatively, Lewis-type epitopes, blood-group antigens and sialylated structures were found to be the dominant terminal epitopes, with all common N-acetyl-lactosamine chain types and core structures also detected.
Figure 4. Example of structural characterization of typical O-linked oligosaccharides from MUC5B/MUC5AC in SMG secretions by MS/MS.
Oligosaccharides were released by reductive β-elimination from high-molecular-mass glycoproteins in SMG secretions from non-CF subject 4 and analysed by LC-ESI-MS/MS. Structures were assigned from fragment ions using the GlycosidIQ™ glycan mass-fingerprinting tool. (A) Example of a sialyl-Le-type structure. (B) Example of blood-group A/Le type structure. Abbreviations: Gal, galactose; GlcNAc, N-acetylglucosamine; GalNAc, N-acetylgalactosamine; GalNAcol, N-acetylgalactosaminitol; Fuc, fucose; NeuAc, N-acetylneuraminic acid; a, α linkage; b, β linkage; 1, 2, 3, 4, 6, linked carbon position.
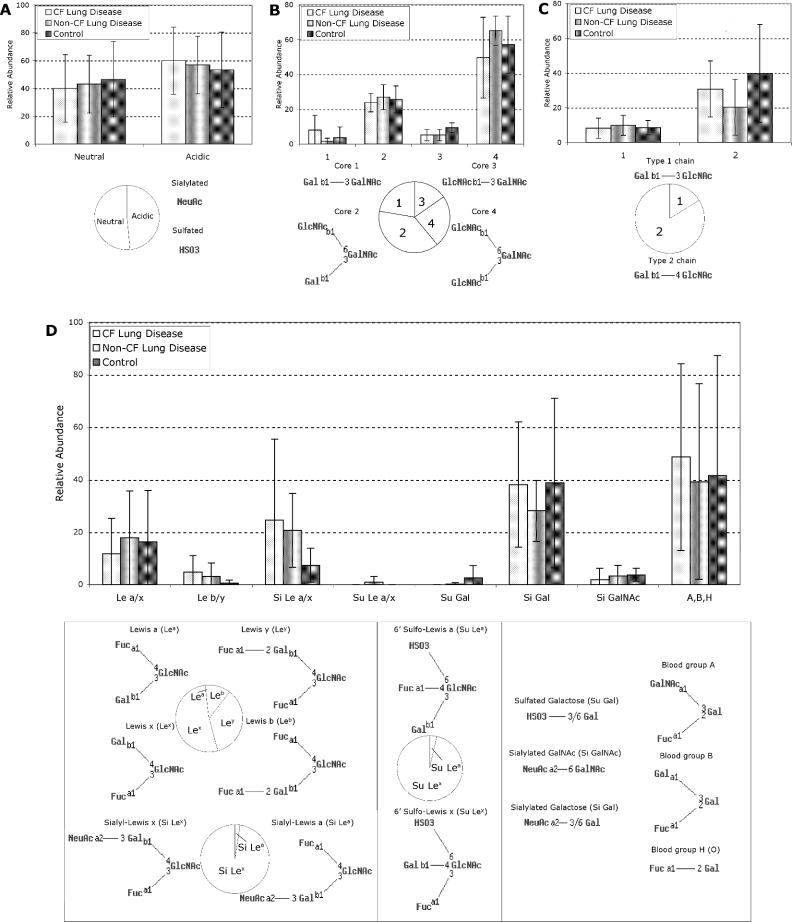
To describe more accurately the glycosylation of oligosaccharides from MUC5B/MUC5AC in SMG secretions from subjects with CF and non-CF lung disease, and from controls, the relative abundance of the different structural features was semi-quantitatively determined, and the three subject groups were statistically compared (Figure 5). The relative abundances of these structural features were in general agreement with the occurrence of previously reported oligosaccharides from human respiratory glycoproteins containing these structural epitopes, as determined by querying GlycoSuiteDB®. Several features of the oligosaccharide structures were compared, including charge, core structures, N-acetyl-lactosamine chain-extension types and terminal epitopes (summarized in Figure 5). We did not find any significant differences in the relative abundances of any of the oligosaccharide internal substructures or terminal epitopes detected in SMG secretions between subjects with CF lung disease, non-CF lung disease or controls, but instead found large individual variation that overshadowed any differences between these groups (Figure 5).
Figure 5. Epitopes and substructures of O-linked oligosaccharides from MUC5B/MUC5AC in SMG secretions from explanted bronchial tissue from CF and non-CF subjects with lung disease and controls.
The weighted average abundances of each structural epitope in oligosaccharides from MUC5B/MUC5AC (Supplementary Table S5; http://www.BiochemJ.org/bj/387/bj3870911add.htm) are shown as bar charts, with error bars representing S.E.M. values. There were no significant differences between CF lung disease, non-CF lung disease or control subjects in any of the structural epitopes detected. The occurrences of previously characterized oligosaccharides from human respiratory glycoproteins containing these epitopes were determined from GlycoSuiteDB® and are shown as pie charts. (A) Oligosaccharide charge: neutral, non-sialylated and non-sulphated; acidic, sialylated or sulphated. (B) O-linked core type (1, 2, 3 or 4). (C) N-Acetyl-lactosamine chain elongation type (1 or 2). (D) Terminal epitopes: Le a/x, Lea or Lex; Le b/y, Leb or Ley; Si Le a/x, sialyl-Lea or sialyl-Lex; Su Le a/x, sulpho-Lea or sulpho-Lex; A,B,H, blood-group A, B or H (O) antigen; Si Gal, sialylated galactose; Si GalNAc, sialylated core N-acetylgalactosamine; Su Gal, sulphated galactose. Abbreviations: Gal, galactose; GlcNAc, N-acetylglucosamine; GalNAcol, N-acetylgalactosaminitol; Fuc, fucose; NeuAc, N-acetylneuraminic acid; a, α linkage; b, β linkage; 1, 2, 3, 4, 6, linked carbon position.
Several of the terminal oligosaccharide epitopes that we detected on mucins in SMG secretions have been reported to be high-affinity ligands for binding by leucocytes [29] and pathogens such as P. aeruginosa [12], particularly Lex (Lewis x) and sialyl-Lex structures [30]. It was not possible to unambiguously differentiate between Lea (Lewis a) and Lex, nor between Leb (Lewis b) and Ley (Lewis y) by MS/MS data of individual oligosaccharide components (Figure 5D). However, the greater relative abundance of type 2 chains over type 1 chains (Figure 5C) suggests that these epitopes are predominantly Lex, Ley and sialyl-Lex, since these terminal epitopes are extensions of the dominant type 2 N-acetyl-lactosamine chains. This suggests that the oligosaccharides present on the major mucins in SMG secretions from CF and non-CF subjects with lung disease, and from controls, are high-affinity ligands for cells of the immune system, as well as for potential pathogens.
DISCUSSION
The high-molecular-mass protein components of SMG secretions from explanted human bronchial tissue from patients with severe lung disease are very similar in subjects with CF lung disease, non-CF lung disease and controls without lung disease. The reproducibility between samples indicates that the SMG secretion sample analysis procedure, including tissue dissection, collection of secretions, sample preparation and protein separation, is reproducible and robust. The apparent molecular mass and abundance of MUC5B, the major high-molecular-mass protein component of SMG secretions, shows no differences between subjects with CF lung disease, non-CF lung disease or controls. Although both MUC5AC and MUC5B were detected in SMG secretions, PMF data suggest that MUC5B is the dominant mucin present, in agreement with previous immunohistochemical studies [16,17].
The gp-340 glycoprotein is a minor component of SMG secretions and appears to be present at a lower abundance in subjects with severe lung disease compared with controls without lung disease. This may represent either a down-regulation of gp-340 or an overproduction of MUC5B in SMG secretions from these subjects. Immunohistochemistry has shown that gp-340 in the lung is mainly produced by serous cells of the SMGs, with lower amounts also produced by glandular mucous cells and epithelial goblet cells [31]. In contrast, MUC5B is produced by glandular mucous cells and epithelial goblet cells, but not glandular serous cells [16]. The results presented here suggest that, in subjects with severe lung disease, serous cell products (including gp-340) contribute relatively less, and mucous cell products (mucins, including MUC5B) contribute relatively more, to total SMG secretions. This may be caused by increased mucin secretion by the glandular mucous cells in the disease or inflammation, suggested by glandular hypertrophy [6] and glandular-cell culture mucin hypersecretion [32]. Another contributing factor in CF subjects may be that a lack of functional CFTR causes changes in general protein secretion from glandular serous cells, where CFTR is normally present at high levels. It has been reported that inhibition of ion transport reduces lysozyme secretion in glandular serous cells [33], and it is therefore likely that secretion of other glandular serous-cell products, including gp-340, is similarly reduced in the absence of functional CFTR.
O-linked oligosaccharides from high-molecular-mass glycoprotein gp-340 and MUC5B/MUC5AC mucins in SMG secretions show no statistically significant differences between CF and non-CF subjects with lung disease, nor between these subjects and controls. Whereas the monosaccharide composition of gp-340 is different from that of MUC5B, there are nonetheless no significant differences in the composition of oligosaccharides from either protein between CF and non-CF subjects with lung disease, and in controls. O-linked oligosaccharide structures and substructures present on MUC5B/MUC5AC in SMG secretions were also found to be very similar in all subjects (CF lung disease, non-CF lung disease and controls). Le, sialyl-Le and related oligosaccharide epitopes were found to be abundant on mucins in SMG secretions, in agreement with previously reported oligosaccharides in human respiratory mucosa. These epitopes are important in interactions with leucocytes [34] and bacteria [30,35]. Blood-group structures (A, B and H/O) are also abundant as terminal epitopes on MUC5B from CF and non-CF SMG secretions. There are large S.E.M. values associated with the relative abundances of the various terminal epitopes present. This suggests a high degree of population diversity in these terminal epitopes, which is probably due to the importance of heritable Le-blood-group- and ABO-blood-group-antigen determining enzymes in the biosynthesis of these substructures. Whilst we acknowledge the small population size used in the present study, the similar mean values and high variation evident in the abundances of terminal epitopes nevertheless suggest that these differences are not sufficient to account for different pathogen interactions which may be responsible for the increased bacterial colonization that occurs in CF subjects.
Several previous studies in epithelial-cell culture [36,37] and tracheal xenograph [38] models have shown increased sulphation associated with CF. However, another study [27] of mucins from epithelial-cell culture showed no glycosylation differences in CF compared with non-diseased samples. In addition, sulphation of whole lung mucus has also been shown to be higher in CF than in chronic bronchitis, and also to show increases in sialylation and sulphation with increased severity of pulmonary infection [15]. Our observations indicate that these reported increases in sulphation on CF glycoconjugates are not due to differences in the glycosylation of SMG secretion components of the lung mucosa.
The similarity in the glycosylation of high-molecular-mass glycoproteins in SMG secretions from all subjects is striking, given the range of conditions analysed, including severe congenital disease (CF), severe acquired lung disease (bronchiectasis and emphysema) and non-pulmonary-related conditions (physical trauma and meningitis). Unfortunately, it is exceedingly difficult to study SMG secretions in a truly in vivo or healthy condition, and it is therefore possible that this analysis of control subjects does not reflect truly ‘healthy’ SMG secretions. However, previous immunohistochemical analysis in diseased and non-diseased subjects show results similar to those presented here, with MUC5B as the main mucin produced by SMGs, along with small amounts of MUC5AC [16,17] and blood-group determinants, Le-type structures and sialylation characterizing the glycosylation of SMG secretion components [18,19]. Together, this suggests that there are no large or significant differences in the glycosylation of high-molecular-mass glycoproteins in SMG secretions between subjects with severe lung disease and controls, and also that CF does not influence the glycosylation of secreted high-molecular-mass proteins in SMG secretions in severe lung disease. This is in contrast with the reported increased sialylation of glycoconjugates in sputum during infection and inflammation ([13] and our ongoing studies on CF sputum (results not shown)). However, sputum comprises secretions from a mixture of sources, including SMGs and epithelial goblet cells, suggesting that sources of mucus other than SMGs are important in determining the physical and biological properties, including glycosylation, of whole-lung mucus in relation to infection and inflammatory responses.
Mucin oligosaccharide structural epitopes with high affinity for bacteria such as P. aeruginosa are abundant in SMG secretions. Previous studies suggest that micro-organisms tend to adhere strongly to sialylated and fucosylated oligosaccharide structures [35,39]. P. aeruginosa produces lectins with affinity to a wide spectrum of glycoconjugate epitopes, and these affinities have been studied with a variety of experimental models: (i) PA-IL shows high affinity for galactose [40]; (ii) PA-IIL has a very high affinity for fucose [39]; (iii) flagellin [41], as well as pili [42], bind non-sialylated gangliotetraosyl ceramide (asialo-GM1); (iv) FliD, the flagellar cap protein, binds sialyl-Lex and sialyl-sulpho-Lex [35]. In addition, different strains of P. aeruginosa have different lectin expression patterns and appear to show different affinities for various oligosaccharides [30]. This diversity of adhesion molecules means that it is difficult to predict specific bacterial binding in vivo based on literature reports. However, it appears that pathogens such as P. aeruginosa do display differential adhesion to different oligosaccharide epitopes, and this may play an important role in the subsequent progression of infection in both CF and non-CF lung disease.
Glycoproteomic analysis has revealed that the same major high-molecular-mass glycoproteins and mucins are present in CF and non-CF SMG secretions from patients with severe lung disease and also in controls without lung disease. Additionally, the oligosaccharides of these major glycoconjugates in SMG secretions are not significantly different between these subject groups. Lack of functional CFTR in CF must therefore result in changes in physical and biological properties of the lung, other than the glycosylation of secreted glycoconjugates from SMGs, which lead inexorably to chronic lung infection. These changes may be biological, including decreased concentrations of antimicrobial proteins such as gp-340, or altered cell-surface glycoconjugates, or physical, such as increased mucous viscosity and reduced mucociliary clearance. The glycosylation of mucins and glycoproteins in SMG secretions with epitopes which display affinity for leucocytes and pathogens, together with the presence of antimicrobial proteins such as gp-340, lactoferrin and lysozyme, are in agreement with previous suggestions of an important role for SMG secretions in the innate immune response in the lung [4]. It is possible that, once secreted from SMGs into the lung mucosa, these antimicrobial proteins remain associated with the mucins in SMG secretions, to which leucocytes and pathogens may be recruited. These might then provide a localized, but intense, antimicrobial attack, while protecting the bulk of the lung mucosa from an excessive inflammatory response. Increased mucous viscosity and decreased mucous clearance could then predispose not only to bacterial infection, but also to chronic inflammation, in the CF lung.
Online data
Acknowledgments
This project was supported by the CFFT (Cystic Fibrosis Foundation Therapeutics), Bethesda, MD, U.S.A. We gratefully acknowledge the input throughout the course of this work from Dr Melissa Ashlock (CFFT), Dr Preston Campbell III, Dr Christopher Penland and Dr Robert Beall [CFF (Cystic Fibrosis Foundation), Bethesda, MD, U.S.A.] and from project steering committee members Dr Richard Moss (Department of Pathology, Stanford University Medical Center, Palo Alto, CA, U.S.A.), Dr Ronald Gibson (Department of Pediatrics, University of Washington, School of Medicine, Seattle, WA, U.S.A.) and Dr Harvey Pollard [USUHS (Uniformed Services University of the Health Sciences), Bethesda, MD, U.S.A.].
References
- 1.Sharma P., Dudus L., Nielsen P. A., Clausen H., Yankaskas J. R., Hollingsworth M. A., Engelhardt J. F. MUC5B and MUC7 are differentially expressed in mucous and serous cells of submucosal glands in human bronchial airways. Am. J. Respir. Cell Mol. Biol. 1998;19:30–37. doi: 10.1165/ajrcmb.19.1.3054. [DOI] [PubMed] [Google Scholar]
- 2.Bals R., Weiner D. J., Wilson J. M. The innate immune system in cystic fibrosis lung disease. J. Clin. Invest. 1999;103:303–307. doi: 10.1172/JCI6277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Knowles M. R., Boucher R. C. Mucus clearance as a primary innate defense mechanism for mammalian airways. J. Clin. Invest. 2002;109:571–577. doi: 10.1172/JCI15217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Finkbeiner W. E. Physiology and pathology of tracheobronchial glands. Respir. Physiol. 1999;118:77–83. doi: 10.1016/s0034-5687(99)00080-8. [DOI] [PubMed] [Google Scholar]
- 5.Jeffery P. K. Remodeling in asthma and chronic obstructive lung disease. Am. J. Respir. Crit. Care Med. 2001;164:S28–S38. doi: 10.1164/ajrccm.164.supplement_2.2106061. [DOI] [PubMed] [Google Scholar]
- 6.Verkman A. S., Song Y., Thiagarajah J. R. Role of airway surface liquid and Submucosal glands in cystic fibrosis lung disease. Am. J. Physiol. Cell Physiol. 2003;284:C2–C15. doi: 10.1152/ajpcell.00417.2002. [DOI] [PubMed] [Google Scholar]
- 7.Engelhardt J. F., Yankaskas J. R., Ernst S. A., Yang Y., Marino C. R., Boucher R. C., Cohn J. A., Wilson J. M. Submucosal glands are the predominant site of CFTR expression in the human bronchus. Nat. Genet. 1992;2:240–248. doi: 10.1038/ng1192-240. [DOI] [PubMed] [Google Scholar]
- 8.Riordan J. R., Rommens J. M., Kerem B., Alon N., Rozmahel R., Grzelczak Z., Zielenski J., Lok S., Plavsic N., Chou J. L. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science. 1989;245:1066–1073. doi: 10.1126/science.2475911. [DOI] [PubMed] [Google Scholar]
- 9.Thiagarajah J. R., Song Y., Haggie P. M., Verkman A. S. A small molecule CFTR inhibitor produces cystic fibrosis-like submucosal gland fluid secretions in normal airways. FASEB J. 2004;18:875–877. doi: 10.1096/fj.03-1248fje. [DOI] [PubMed] [Google Scholar]
- 10.Wine J. J., Joo N. S. Submucosal glands and airway defense. Proc. Am. Thorac. Soc. 2004;1:47–53. doi: 10.1513/pats.2306015. [DOI] [PubMed] [Google Scholar]
- 11.Gibson R. L., Burns J. L., Ramsey B. W. Pathophysiology and management of pulmonary infections in cystic fibrosis. Am. J. Respir. Crit. Care Med. 2003;168:918–951. doi: 10.1164/rccm.200304-505SO. [DOI] [PubMed] [Google Scholar]
- 12.Ramphal R., Arora S. K. Recognition of mucin components by Pseudomonas aeruginosa. Glycoconj. J. 2001;18:709–713. doi: 10.1023/a:1020823406840. [DOI] [PubMed] [Google Scholar]
- 13.Lamblin G., Degroote S., Perini J. M., Delmotte P., Scharfman A. E., Davril M., Lo-Guidice J. M., Houdret N., Dumur V., Klein A., Roussel P. Human airway mucin glycosylation: A combinatory of carbohydrate determinants which vary in cystic fibrosis. Glycoconj. J. 2001;18:661–684. doi: 10.1023/a:1020867221861. [DOI] [PubMed] [Google Scholar]
- 14.Rhim A. D., Stoykova L., Glick M. C., Scanlin T. F. Terminal glycosylation in cystic fibrosis (CF): a review emphasizing the airway epithelial cell. Glycoconj. J. 2001;18:649–659. doi: 10.1023/a:1020815205022. [DOI] [PubMed] [Google Scholar]
- 15.Davril M., Degroote S., Humbert P., Galabert C., Dumur V., Lafitte J. J., Lamblin G., Roussel P. The sialylation of bronchial mucins secreted by patients suffering from cystic fibrosis or from chronic bronchitis is related to the severity of airway infection. Glycobiology. 1999;9:311–321. doi: 10.1093/glycob/9.3.311. [DOI] [PubMed] [Google Scholar]
- 16.Groneberg D. A., Peiser C., Dinh Q. T., Matthias J., Eynott P. R., Heppt W., Carlstedt I., Witt C., Fischer A., Chung K. F. Distribution of respiratory mucin proteins in human nasal mucosa. Laryngoscope. 2003;113:520–524. doi: 10.1097/00005537-200303000-00023. [DOI] [PubMed] [Google Scholar]
- 17.Hovenberg H. W., Davies J. R., Carlstedt I. Different mucins are produced by the surface epithelium and the submucosa in human trachea: identification of MUC5AC as a major mucin from the goblet cells. Biochem. J. 1996;318:319–324. doi: 10.1042/bj3180319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bals R., Woeckel W., Welsch U. Use of antibodies directed against blood group substances and lectins together with glycosidase digestion to study the composition and cellular distribution of glycoproteins in the large human airways. J. Anat. 1996;190:73–84. doi: 10.1046/j.1469-7580.1997.19010073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tsokos M., Anders S., Paulsen F. Lectin binding patterns of alveolar epithelium and subepithelial seromucous glands of the bronchi in sepsis and controls – an approach to characterize the nonspecific immunological response of the human lung to sepsis. Virchows Arch. 2002;440:181–186. doi: 10.1007/s004280100488. [DOI] [PubMed] [Google Scholar]
- 20.Jayaraman S., Joo N. S., Reitz B., Wine J. J., Verkman A. S. Submucosal gland secretions in airways from cystic fibrosis patients have normal [Na+] and pH but elevated viscosity. Proc. Natl. Acad. Sci. U.S.A. 2001;98:8119–8123. doi: 10.1073/pnas.131087598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schulz B. L., Packer N. H., Karlsson N. G. Small-scale analysis of O-linked oligosaccharides from glycoproteins and mucins separated by gel electrophoresis. Anal. Chem. 2002;74:6088–6097. doi: 10.1021/ac025890a. [DOI] [PubMed] [Google Scholar]
- 22.Wilson N. L., Schulz B. L., Karlsson N. G., Packer N. H. Sequential analysis of N- and O-linked glycosylation of 2D-PAGE separated glycoproteins. J. Proteome Res. 2002;1:521–529. doi: 10.1021/pr025538d. [DOI] [PubMed] [Google Scholar]
- 23.Grinyer J., McKay M., Nevalainen H., Herbert B. R. Fungal proteomics: initial mapping of biological control strain Trichoderma harzianum. Curr. Genet. 2004;45:163–169. doi: 10.1007/s00294-003-0474-4. [DOI] [PubMed] [Google Scholar]
- 24.Karlsson N. G., Wilson N. L., Wirth H.-L., Dawes P., Joshi H., Packer N. H. Negative ion graphitised carbon nano-liquid chromatography/mass spectrometry increases sensitivity for glycoprotein oligosaccharide analysis. Rapid Commun. Mass Spectrom. 2004;18:2282–2292. doi: 10.1002/rcm.1626. [DOI] [PubMed] [Google Scholar]
- 25.Joshi H. J., Harrison M. J., Schulz B. L., Cooper C. A., Packer N. H., Karlsson N. G. Developent of a mass fingerprinting tool for automated interpretation of oligosaccharide fragmentation data. Proteomics. 2004;4:1650–1664. doi: 10.1002/pmic.200300784. [DOI] [PubMed] [Google Scholar]
- 26.Groneberg D. A., Eynott P. R., Oates T., Lim S., Wu R., Carlstedt I., Nicholson A. G., Chung K. F. Expression of MUC5AC and MUC5B mucins in normal and cystic fibrosis lung. Respir. Med. 2002;96:81–86. doi: 10.1053/rmed.2001.1221. [DOI] [PubMed] [Google Scholar]
- 27.Holmen J. M., Karlsson N. G., Abdullah L. H., Randell S. H., Sheehan J. K., Hansson G. C., Davis C. W. Mucins and their O-glycans from human bronchial epithelial cell cultures. Am. J. Physiol. Lung Cell Mol. Physiol. 2004;287:L824–L834. doi: 10.1152/ajplung.00108.2004. [DOI] [PubMed] [Google Scholar]
- 28.Karlsson N. G., Schulz B. L., Packer N. H. Structural determination of neutral O-linked oligosaccharide alditols by negative ion LC-electrospray-MS. J. Am. Soc. Mass Spectrom. 2004;15:659–672. doi: 10.1016/j.jasms.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 29.Zak I., Lewandowska E., Gnyp W. Selectin glycoprotein ligands. Acta Biochim. Pol. 2000;47:393–412. [PubMed] [Google Scholar]
- 30.Scharfman A., Delmotte P., Beau J., Lamblin G., Roussel P., Mazurier J. Sialyl-Lex and sulfo-sialyl-Lex determinants are receptors for P. aeruginosa. Glycoconj. J. 2000;17:735–740. doi: 10.1023/a:1011091112884. [DOI] [PubMed] [Google Scholar]
- 31.Thornton D. J., Davies J. R., Kirkham S., Gautrey A., Khan N., Richardson P. S., Sheehan J. K. Identification of a nonmucin glycoprotein (gp-340) from a purified respiratory mucin preparation: evidence for an association involving the MUC5B mucin. Glycobiology. 2001;11:969–977. doi: 10.1093/glycob/11.11.969. [DOI] [PubMed] [Google Scholar]
- 32.Merten M. D., Figarella C. Constitutive hypersecretion and insensitivity to neurotransmitters by cystic fibrosis tracheal gland cells. Am. J. Physiol. 1993;264:93–99. doi: 10.1152/ajplung.1993.264.2.L93. [DOI] [PubMed] [Google Scholar]
- 33.Duszyk M. CFTR and lysozyme secretion in human airway epithelial cells. Pflugers Arch. 2001;443:S45–S49. doi: 10.1007/s004240100643. [DOI] [PubMed] [Google Scholar]
- 34.Lowe J. B. Glycosylation in the control of selectin counter-receptor structure and function. Immunol. Rev. 2002;186:19–36. doi: 10.1034/j.1600-065x.2002.18603.x. [DOI] [PubMed] [Google Scholar]
- 35.Scharfman A., Arora S. K., Delmotte P., Van Brussel E., Mazurier J., Ramphal R., Roussel P. Recognition of Lewis x derivatives present on mucins by flagellar components of Pseudomonas aeruginosa. Infect. Immun. 2001;69:5243–5248. doi: 10.1128/IAI.69.9.5243-5248.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Frates R. C., Kaizu T. T., Last J. A. Mucus glycoproteins secreted by respiratory epithelial tissue from cystic fibrosis patients. Pediatr. Res. 1983;17:30–34. doi: 10.1203/00006450-198301000-00006. [DOI] [PubMed] [Google Scholar]
- 37.Mendicino J., Sangadala S. Synthesis of sulfated oligosaccharides by cystic fibrosis trachea epithelial cells. Mol. Cell. Biochem. 1999;201:141–149. doi: 10.1023/a:1007014613768. [DOI] [PubMed] [Google Scholar]
- 38.Zhang Y., Doranz B., Yankaskas J. R., Engelhardt J. F. Genotypic analysis of respiratory mucous sulfation defects in cystic fibrosis. J. Clin. Invest. 1995;96:2997–3004. doi: 10.1172/JCI118372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mitchell E., Houles C., Sudakevitz D., Wimmerova M., Gautier C., Perez S., Wu A. M., Gilboa-Garber N., Imberty A. Structural basis for oligosaccharide-mediated adhesion of Pseudomonas aeruginosa in the lungs of cystic fibrosis patients. Nat. Struct. Biol. 2002;9:918–921. doi: 10.1038/nsb865. [DOI] [PubMed] [Google Scholar]
- 40.Chen C. P., Song S. C., Gilboa-Garber N., Chang K. S. S., Wu A. M. Studies on the binding site of the galactose-specific agglutinin PA-IL from Pseudomonas aeruginosa. Glycobiology. 1998;8:7–16. doi: 10.1093/glycob/8.1.7. [DOI] [PubMed] [Google Scholar]
- 41.Feldman M., Bryan R., Rajan S., Scheffler L., Brunnert S., Tang H., Prince A. Role of flagella in pathogenesis of Pseudomonas aeruginosa pulmonary infection. Infect. Immun. 1998;66:43–51. doi: 10.1128/iai.66.1.43-51.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee K. K., Sheth H. B., Wong W. Y., Sherburne R., Paranchych W., Hodges R. S., Lingwood C. A., Krivan H., Irvin R. T. The binding of Pseudomonas aeruginosa pili to glycosphingolipids is a tip-associated event involving the C-terminal region of the structural pilin subunit. Mol. Microbiol. 1994;11:705–713. doi: 10.1111/j.1365-2958.1994.tb00348.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.