Abstract
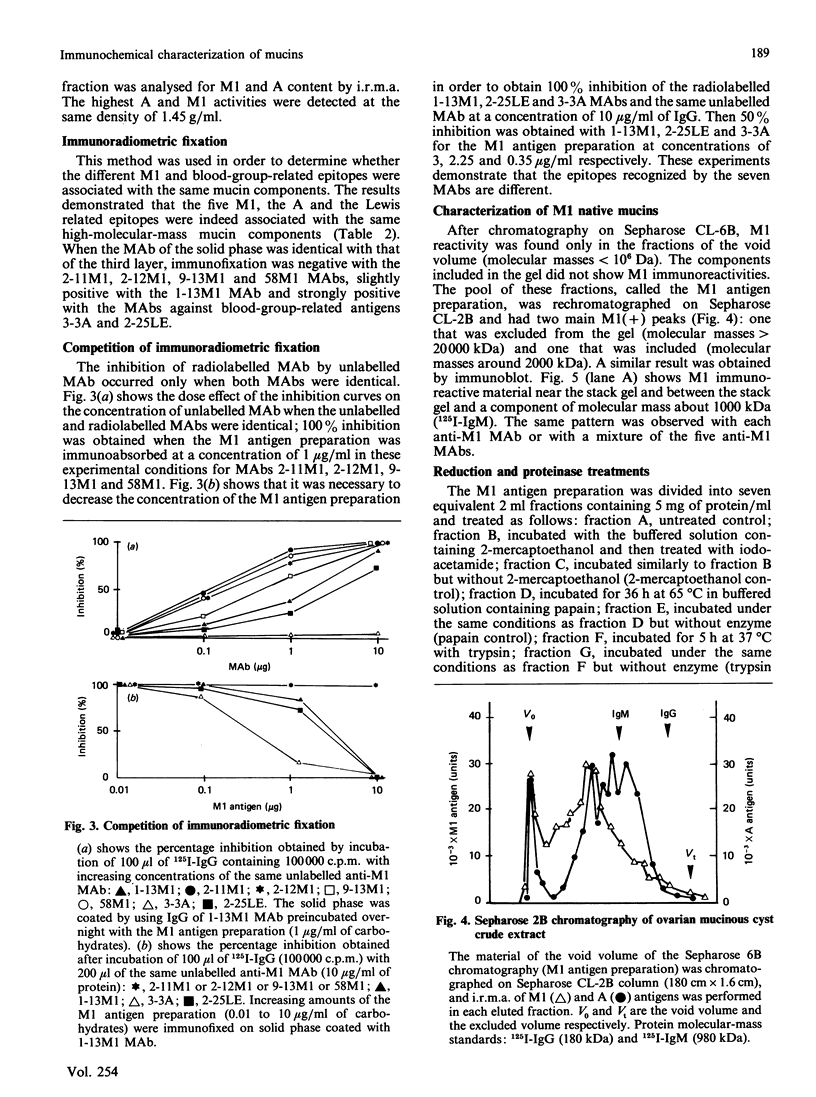
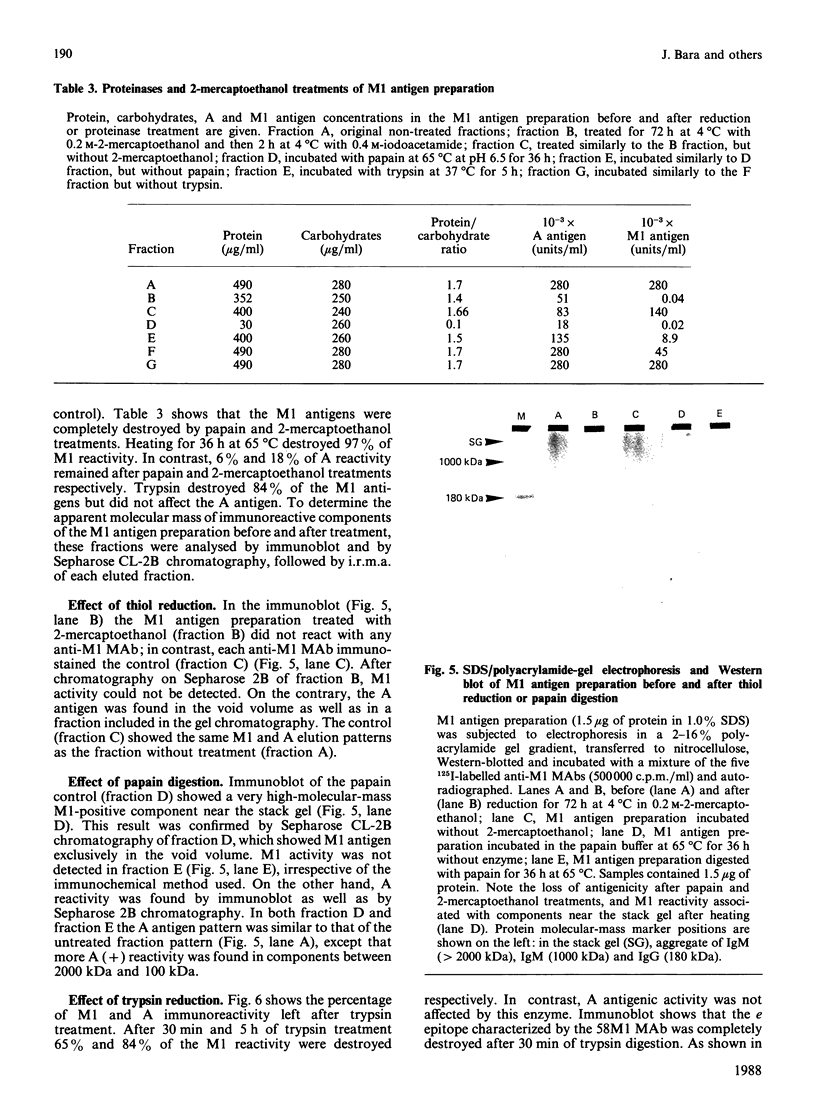
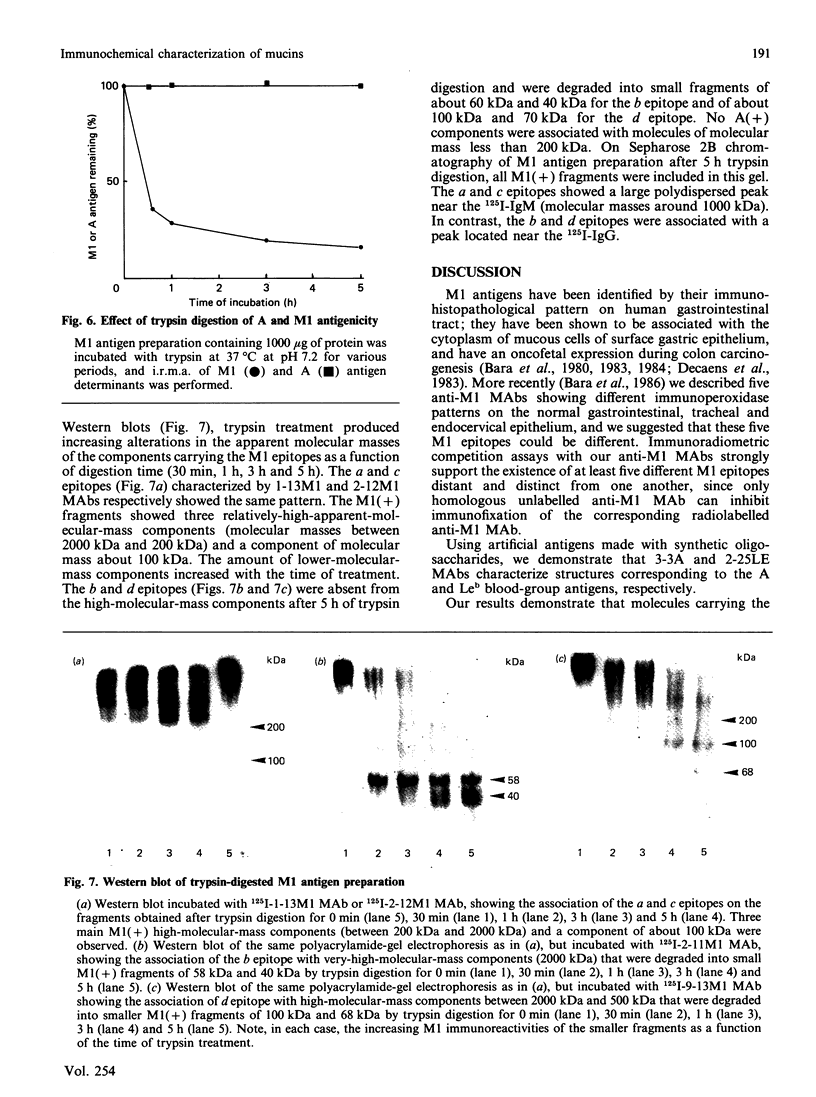
Seven monoclonal antibodies (MAbs) reacting with high-molecular-mass components (greater than 20,000 kDa) isolated from an ovarian mucinous cyst of an A Le(a-b+) patient are described. By the use of immunoradiometric methods, these MAbs characterized seven different epitopes associated with components having a density of 1.45 g/ml by CsCl-density-gradient ultracentrifugation, like mucins. Two MAbs reacted with A and Lewis blood-group antigens respectively (polysaccharide epitopes). The five other MAbs characterized five M1 epitopes (called a, b, c, d and e), mainly associated with components of more than 20,000 kDa and 2000 kDa. They were completely destroyed by papain and 2-mercaptoethanol treatment (polypeptide epitopes). Moreover, timed trypsin digestion of native mucin resulted in a progressive loss of M1 activity and degraded these mucins into smaller M1-positive fragments. The a and c epitopes were partially degraded from relatively high-molecular-mass fragments (2000 kDa to 500 kDa) into a 100 kDa fragment. The b and d epitopes were completely degraded into smaller fragments ranging from 100 kDa to 40 kDa. The e epitope was completely destroyed by trypsin. These different pathways of M1 antigen degradation suggest the occurrence of different epitopes located in separate regions of the mucin molecules.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bara J., André J., Gautier R., Burtin P. Abnormal pattern of mucus-associated M1 antigens in histologically normal mucosa adjacent to colonic adenocarcinomas. Cancer Res. 1984 Sep;44(9):4040–4045. [PubMed] [Google Scholar]
- Bara J., Gautier R., Daher N., Zaghouani H., Decaens C. Monoclonal antibodies against oncofetal mucin M1 antigens associated with precancerous colonic mucosae. Cancer Res. 1986 Aug;46(8):3983–3989. [PubMed] [Google Scholar]
- Bara J., Languille O., Gendron M. C., Daher N., Martin E., Burtin P. Immunohistological study of precancerous mucus modification in human distal colonic polyps. Cancer Res. 1983 Aug;43(8):3885–3891. [PubMed] [Google Scholar]
- Bara J., Loisillier F., Burtin P. Antigens of gastric and intestinal mucous cells in human colonic tumours. Br J Cancer. 1980 Feb;41(2):209–221. doi: 10.1038/bjc.1980.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bara J., Mollicone R., Herrero-Zabaleta E., Gautier R., Daher N., Oriol R. Ectopic expression of the Y (Ley) antigen defined by monoclonal antibody 12-4LE in distal colonic adenocarcinomas. Int J Cancer. 1988 May 15;41(5):683–689. doi: 10.1002/ijc.2910410508. [DOI] [PubMed] [Google Scholar]
- Carlstedt I., Lindgren H., Sheehan J. K. The macromolecular structure of human cervical-mucus glycoproteins. Studies on fragments obtained after reduction of disulphide bridges and after subsequent trypsin digestion. Biochem J. 1983 Aug 1;213(2):427–435. doi: 10.1042/bj2130427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlstedt I., Sheehan J. K., Corfield A. P., Gallagher J. T. Mucous glycoproteins: a gel of a problem. Essays Biochem. 1985;20:40–76. [PubMed] [Google Scholar]
- Creeth J. M., Denborough M. A. The use of equilibrium-density-gradient methods for the preparation and characterization of blood-group-specific glycoproteins. Biochem J. 1970 May;117(5):879–891. doi: 10.1042/bj1170879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decaens C., Bara J., Rosa B., Daher N., Burtin P. Early oncofetal antigenic modifications during rat colonic carcinogenesis. Cancer Res. 1983 Jan;43(1):355–362. [PubMed] [Google Scholar]
- Ey P. L., Prowse S. J., Jenkin C. R. Isolation of pure IgG1, IgG2a and IgG2b immunoglobulins from mouse serum using protein A-sepharose. Immunochemistry. 1978 Jul;15(7):429–436. doi: 10.1016/0161-5890(78)90070-6. [DOI] [PubMed] [Google Scholar]
- Fahim R. E., Specian R. D., Forstner G. G., Forstner J. F. Characterization and localization of the putative 'link' component in rat small-intestinal mucin. Biochem J. 1987 May 1;243(3):631–640. doi: 10.1042/bj2430631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feizi T., Gooi H. C., Childs R. A., Picard J. K., Uemura K., Loomes L. M., Thorpe S. J., Hounsell E. F. Tumour-associated and differentiation antigens on the carbohydrate moieties of mucin-type glycoproteins. Biochem Soc Trans. 1984 Aug;12(4):591–596. doi: 10.1042/bst0120591. [DOI] [PubMed] [Google Scholar]
- Fenoglio C. M., Ferenczy A., Richart R. M. Mucinous tumors of the ovary. Ultrastructural studies of mucinous cystadenomas with histogenetic considerations. Cancer. 1975 Nov;36(5):1709–1722. doi: 10.1002/1097-0142(197511)36:5<1709::aid-cncr2820360526>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Le Pendu J., Fredman P., Richter N. D., Magnani J. L., Willingham M. C., Pastan I., Oriol R., Ginsburg V. Monoclonal antibody 101 that precipitates the glycoprotein receptor for epidermal growth factor is directed against the Y antigen, not the H type 1 antigen. Carbohydr Res. 1985 Sep 1;141(2):347–349. doi: 10.1016/s0008-6215(00)90468-3. [DOI] [PubMed] [Google Scholar]
- Mantle M., Forstner G. G., Forstner J. F. Antigenic and structural features of goblet-cell mucin of human small intestine. Biochem J. 1984 Jan 1;217(1):159–167. doi: 10.1042/bj2170159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantle M., Mantle D., Allen A. Polymeric structure of pig small-intestinal mucus glycoprotein. Dissociation by proteolysis or by reduction of disulphide bridges. Biochem J. 1981 Apr 1;195(1):277–285. doi: 10.1042/bj1950277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monday L. A., Lemieux B. Etude audiovestibulaire dans l'ataxie de Friedreich. J Otolaryngol. 1978 Oct;7(5):415–423. [PubMed] [Google Scholar]
- Pearson J. P., Allen A., Parry S. A 70000-molecular-weight protein isolated from purified pig gastric mucus glycoprotein by reduction of disulphide bridges and its implication in the polymeric structure. Biochem J. 1981 Jul 1;197(1):155–162. doi: 10.1042/bj1970155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shochat D., Archey R. L., Pant K. D., Dahlman H. L., Gold D. V., Goldenberg D. M. Characterization of colon-specific antigen-p and isolation of immunologically active tryptic peptides. J Immunol. 1981 Jun;126(6):2284–2289. [PubMed] [Google Scholar]
- Shochat D., Pant K. D., Goldenberg D. M. Colon-specific antigen-p (CSAp). II. Further characterization in colorectal and pancreatic cancer. Cancer. 1982 Sep 1;50(5):927–931. doi: 10.1002/1097-0142(19820901)50:5<927::aid-cncr2820500521>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Slayter H. S., Lamblin G., Le Treut A., Galabert C., Houdret N., Degand P., Roussel P. Complex structure of human bronchial mucus glycoprotein. Eur J Biochem. 1984 Jul 16;142(2):209–218. doi: 10.1111/j.1432-1033.1984.tb08273.x. [DOI] [PubMed] [Google Scholar]
- Slomiany B. L., Zdebska E., Slomiany A. Structural characterization of neutral oligosaccharides of human H+Leb+ gastric mucin. J Biol Chem. 1984 Mar 10;259(5):2863–2869. [PubMed] [Google Scholar]
- Wesley A., Mantle M., Man D., Qureshi R., Forstner G., Forstner J. Neutral and acidic species of human intestinal mucin. Evidence for different core peptides. J Biol Chem. 1985 Jul 5;260(13):7955–7959. [PubMed] [Google Scholar]