Abstract
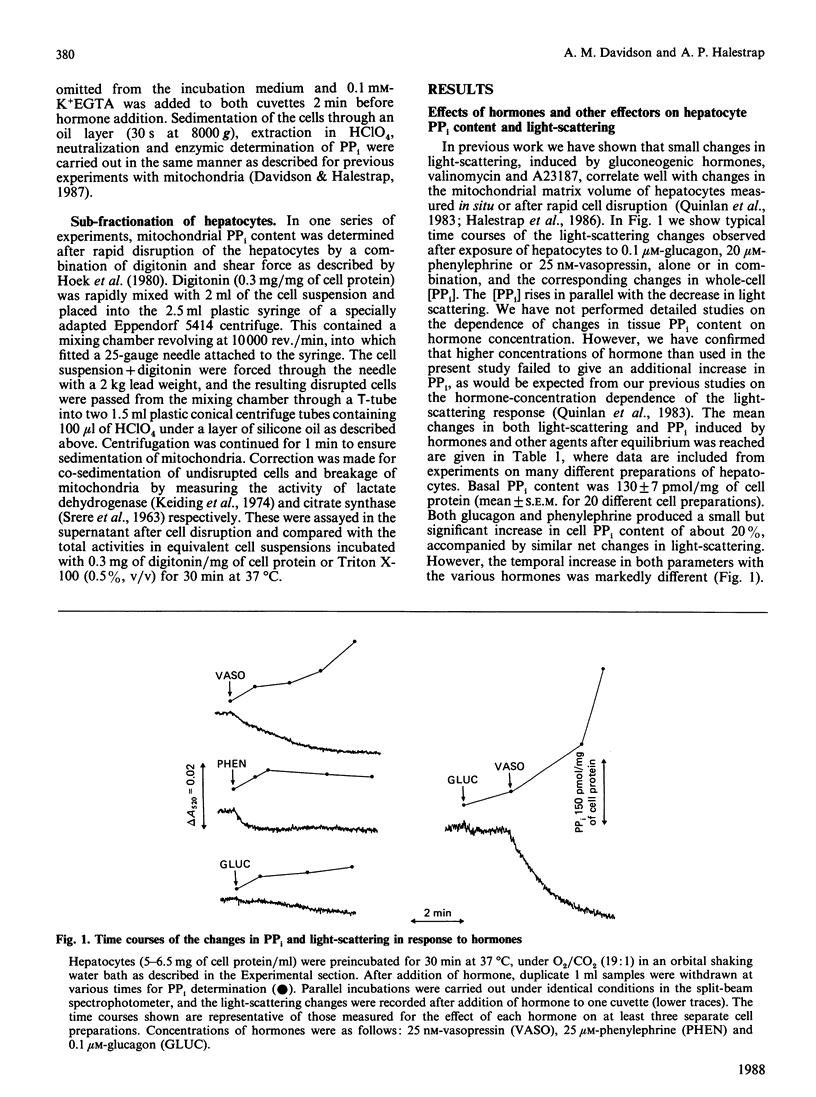
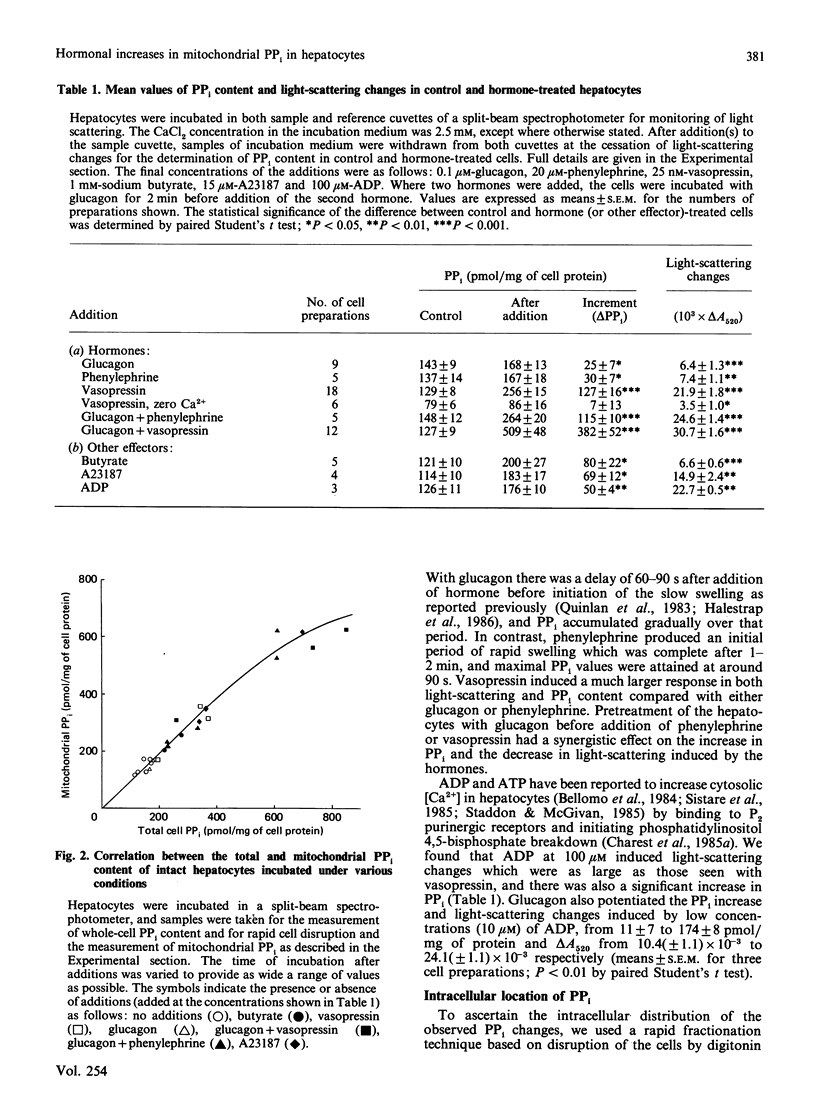
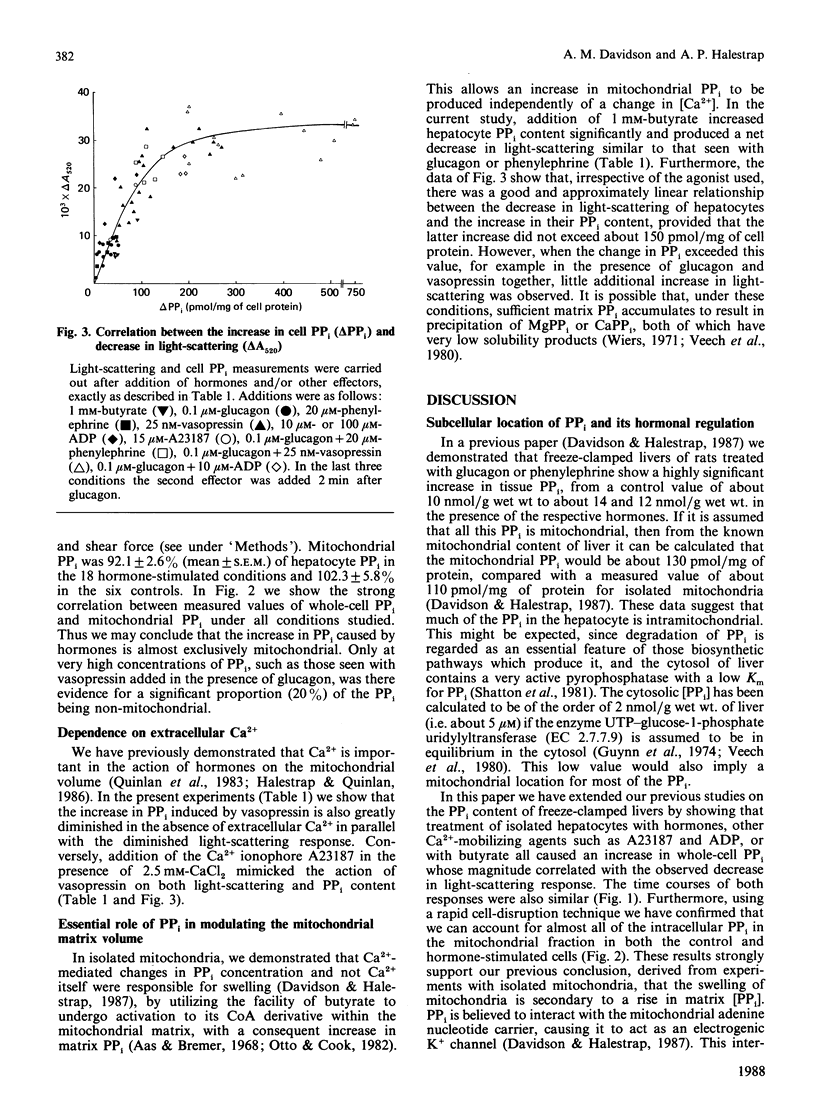
1. The effects of a variety of hormones on the PPi content and light-scattering of isolated rat liver cells was studied. 2. The basal PPi content was about 130 pmol/mg of cell protein, and increased after hormone addition, in parallel with a decrease in light-scattering which we have observed previously [Quinlan, Thomas, Armston & Halestrap (1983) Biochem. J. 214, 395-404]. 3. The mean increases in PPi content with the agonists shown (as pmol/mg of protein) were: 0.1 microM-glucagon, 25; 20 microM-phenylephrine, 30; 25 nM-vasopressin, 127; glucagon + phenylephrine, 115; glucagon + vasopressin, 382; 100 microM-ADP, 50; 15 microM-A23187, 72; 1 mM-butyrate, 80. 4. In the absence of extracellular Ca2+, vasopressin had little effect on either the PPi content or the light-scattering of hepatocytes. 5. The magnitude of the increase in PPi content correlated with that of the decrease in light-scattering irrespective of the stimulating agent, provided that the PPi did not exceed 300 pmol/mg of protein. Above this value little additional change in light-scattering was observed. 6. Subcellular fractionation showed that over 90% of the cellular PPi was intramitochondrial in both control and stimulated cells. 7. The data support the conclusions of previous experiments using isolated liver mitochondria [Davidson & Halestrap (1987) Biochem. J. 246, 715-723] that hormones increase the mitochondrial matrix volume through a Ca2+-induced rise in matrix [PPi]. 8. It is further proposed that this increase in mitochondrial [PPi] allows entry of ADP into the mitochondria in exchange for PPi and is therefore responsible for the increase in total mitochondrial adenine nucleotides observed after hormone treatment.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aas M., Bremer J. Short-chain fatty acid activation in rat liver. A new assay procedure for the enzymes and studies on their intracellular localization. Biochim Biophys Acta. 1968 Oct 22;164(2):157–166. doi: 10.1016/0005-2760(68)90142-2. [DOI] [PubMed] [Google Scholar]
- Altin J. G., Bygrave F. L. Ca2+ uptake stimulated by the synergistic action of glucagon and Ca2+-mobilizing agents in the perfused rat liver occurs through the activation of a unidirectional Ca2+ influx pathway. Biochem Biophys Res Commun. 1987 Feb 13;142(3):745–753. doi: 10.1016/0006-291x(87)91477-x. [DOI] [PubMed] [Google Scholar]
- Altin J. G., Bygrave F. L. Synergistic stimulation of Ca2+ uptake by glucagon and Ca2+-mobilizing hormones in the perfused rat liver. A role for mitochondria in long-term Ca2+ homoeostasis. Biochem J. 1986 Sep 15;238(3):653–661. doi: 10.1042/bj2380653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altin J. G., Bygrave F. L. The Ca2+-mobilizing actions of vasopressin and angiotensin differ from those of the alpha-adrenergic agonist phenylephrine in the perfused rat liver. Biochem J. 1985 Dec 15;232(3):911–917. doi: 10.1042/bj2320911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aprille J. R., Nosek M. T., Brennan W. A., Jr Adenine nucleotide content of liver mitochondria increases after glucagon treatment of rats or isolated hepatocytes. Biochem Biophys Res Commun. 1982 Sep 30;108(2):834–839. doi: 10.1016/0006-291x(82)90905-6. [DOI] [PubMed] [Google Scholar]
- Assimacopoulos-Jeannet F., McCormack J. G., Jeanrenaud B. Vasopressin and/or glucagon rapidly increases mitochondrial calcium and oxidative enzyme activities in the perfused rat liver. J Biol Chem. 1986 Jul 5;261(19):8799–8804. [PubMed] [Google Scholar]
- Barritt G. J., Thorne R. F., Hughes B. P. Effects of hormones and N6O2'-dibutyryl-adenosine 3' :5'-cyclic monophosphate, administered in vivo, on phosphate transport and metabolism in isolated rat liver mitochondria. Biochem J. 1978 Jun 15;172(3):577–585. doi: 10.1042/bj1720577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellomo G., Nicotera P., Orrenius S. Alterations in intracellular calcium compartmentation following inhibition of calcium efflux from isolated hepatocytes. Eur J Biochem. 1984 Oct 1;144(1):19–23. doi: 10.1111/j.1432-1033.1984.tb08425.x. [DOI] [PubMed] [Google Scholar]
- Bryla J., Harris E. J., Plumb J. A. The stimulatory effect of glucagon and dibutyryl cyclic AMP on ureogenesis and gluconeogenesis in relation to the mitochondrial ATP content. FEBS Lett. 1977 Aug 15;80(2):443–448. doi: 10.1016/0014-5793(77)80494-8. [DOI] [PubMed] [Google Scholar]
- Charest R., Blackmore P. F., Exton J. H. Characterization of responses of isolated rat hepatocytes to ATP and ADP. J Biol Chem. 1985 Dec 15;260(29):15789–15794. [PubMed] [Google Scholar]
- Charest R., Prpić V., Exton J. H., Blackmore P. F. Stimulation of inositol trisphosphate formation in hepatocytes by vasopressin, adrenaline and angiotensin II and its relationship to changes in cytosolic free Ca2+. Biochem J. 1985 Apr 1;227(1):79–90. doi: 10.1042/bj2270079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson A. M., Halestrap A. P. Liver mitochondrial pyrophosphate concentration is increased by Ca2+ and regulates the intramitochondrial volume and adenine nucleotide content. Biochem J. 1987 Sep 15;246(3):715–723. doi: 10.1042/bj2460715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Exton J. H. Mechanisms involved in alpha-adrenergic phenomena. Am J Physiol. 1985 Jun;248(6 Pt 1):E633–E647. doi: 10.1152/ajpendo.1985.248.6.E633. [DOI] [PubMed] [Google Scholar]
- Guynn R. W., Veloso D., Lawson J. W., Veech R. L. The concentration and control of cytoplasmic free inorganic pyrophosphate in rat liver in vivo. Biochem J. 1974 Jun;140(3):369–375. doi: 10.1042/bj1400369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halestrap A. P., Dunlop J. L. Intramitochondrial regulation of fatty acid beta-oxidation occurs between flavoprotein and ubiquinone. A role for changes in the matrix volume. Biochem J. 1986 Nov 1;239(3):559–565. doi: 10.1042/bj2390559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halestrap A. P., Quinlan P. T., Whipps D. E., Armston A. E. Regulation of the mitochondrial matrix volume in vivo and in vitro. The role of calcium. Biochem J. 1986 Jun 15;236(3):779–787. doi: 10.1042/bj2360779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoek J. B., Nicholls D. G., Williamson J. R. Determination of the mitochondrial protonmotive force in isolated hepatocytes. J Biol Chem. 1980 Feb 25;255(4):1458–1464. [PubMed] [Google Scholar]
- Joseph S. K., Coll K. E., Thomas A. P., Rubin R., Williamson J. R. The role of extracellular Ca2+ in the response of the hepatocyte to Ca2+-dependent hormones. J Biol Chem. 1985 Oct 15;260(23):12508–12515. [PubMed] [Google Scholar]
- Mauger J. P., Poggioli J., Claret M. Synergistic stimulation of the Ca2+ influx in rat hepatocytes by glucagon and the Ca2+-linked hormones vasopressin and angiotensin II. J Biol Chem. 1985 Sep 25;260(21):11635–11642. [PubMed] [Google Scholar]
- Mauger J. P., Poggioli J., Guesdon F., Claret M. Noradrenaline, vasopressin and angiotensin increase Ca2+ influx by opening a common pool of Ca2+ channels in isolated rat liver cells. Biochem J. 1984 Jul 1;221(1):121–127. doi: 10.1042/bj2210121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack J. G. Evidence that adrenaline activates key oxidative enzymes in rat liver by increasing intramitochondrial [Ca2+]. FEBS Lett. 1985 Jan 28;180(2):259–264. doi: 10.1016/0014-5793(85)81082-6. [DOI] [PubMed] [Google Scholar]
- McCormack J. G. Studies on the activation of rat liver pyruvate dehydrogenase and 2-oxoglutarate dehydrogenase by adrenaline and glucagon. Role of increases in intramitochondrial Ca2+ concentration. Biochem J. 1985 Nov 1;231(3):597–608. doi: 10.1042/bj2310597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan N. G., Blackmore P. F., Exton J. H. Modulation of the alpha 1-adrenergic control of hepatocyte calcium redistribution by increases in cyclic AMP. J Biol Chem. 1983 Apr 25;258(8):5110–5116. [PubMed] [Google Scholar]
- Morgan N. G., Charest R., Blackmore P. F., Exton J. H. Potentiation of alpha 1-adrenergic responses in rat liver by a cAMP-dependent mechanism. Proc Natl Acad Sci U S A. 1984 Jul;81(13):4208–4212. doi: 10.1073/pnas.81.13.4208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto D. A., Cook G. A. Role of Ca2+ in regulating the level of mitochondrial pyrophosphate. Effect on butyrate oxidation. FEBS Lett. 1982 Dec 13;150(1):172–176. doi: 10.1016/0014-5793(82)81328-8. [DOI] [PubMed] [Google Scholar]
- Poggioli J., Mauger J. P., Claret M. Effect of cyclic AMP-dependent hormones and Ca2+-mobilizing hormones on the Ca2+ influx and polyphosphoinositide metabolism in isolated rat hepatocytes. Biochem J. 1986 May 1;235(3):663–669. doi: 10.1042/bj2350663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan P. T., Halestrap A. P. The mechanism of the hormonal activation of respiration in isolated hepatocytes and its importance in the regulation of gluconeogenesis. Biochem J. 1986 Jun 15;236(3):789–800. doi: 10.1042/bj2360789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan P. T., Thomas A. P., Armston A. E., Halestrap A. P. Measurement of the intramitochondrial volume in hepatocytes without cell disruption and its elevation by hormones and valinomycin. Biochem J. 1983 Aug 15;214(2):395–404. doi: 10.1042/bj2140395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recommended methods for the determination of four enzymes in blood. Scand J Clin Lab Invest. 1974 Jun;33(4):291–306. doi: 10.1080/00365517409082499. [DOI] [PubMed] [Google Scholar]
- Reinhart P. H., Taylor W. M., Bygrave F. L. The role of calcium ions in the mechanism of action of alpha-adrenergic agonists in rat liver. Biochem J. 1984 Oct 1;223(1):1–13. doi: 10.1042/bj2230001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shatton J. B., Shah H., Williams A., Morris H. P., Weinhouse S. Activities and properties of inorganic pyrophosphate in normal tissues and hepatic tumors of the rat. Cancer Res. 1981 May;41(5):1866–1872. [PubMed] [Google Scholar]
- Siess E. A., Brocks D. G., Lattke H. K., Wieland O. H. Effect of glucagon on metabolite compartmentation in isolated rat liver cells during gluconeogenesis from lactate. Biochem J. 1977 Aug 15;166(2):225–235. doi: 10.1042/bj1660225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sistare F. D., Picking R. A., Haynes R. C., Jr Sensitivity of the response of cytosolic calcium in Quin-2-loaded rat hepatocytes to glucagon, adenine nucleosides, and adenine nucleotides. J Biol Chem. 1985 Oct 15;260(23):12744–12747. [PubMed] [Google Scholar]
- Soboll S., Scholz R. Control of energy metabolism by glucagon and adrenaline in perfused rat liver. FEBS Lett. 1986 Sep 1;205(1):109–112. doi: 10.1016/0014-5793(86)80875-4. [DOI] [PubMed] [Google Scholar]
- Staddon J. M., Hansford R. G. 4 beta-Phorbol 12-myristate 13-acetate attenuates the glucagon-induced increase in cytoplasmic free Ca2+ concentration in isolated rat hepatocytes. Biochem J. 1986 Sep 15;238(3):737–743. doi: 10.1042/bj2380737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staddon J. M., Hansford R. G. The glucagon-induced activation of pyruvate dehydrogenase in hepatocytes is diminished by 4 beta-phorbol 12-myristate 13-acetate. A role for cytoplasmic Ca2+ in dehydrogenase regulation. Biochem J. 1987 Feb 1;241(3):729–735. doi: 10.1042/bj2410729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staddon J. M., McGivan J. D. Ca2+-dependent activation of oxoglutarate dehydrogenase by vasopressin in isolated hepatocytes. Biochem J. 1985 Jan 15;225(2):327–333. doi: 10.1042/bj2250327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titheradge M. A., Haynes R. C., Jr The hormonal stimulation of ureogenesis in isolated hepatocytes through increases in mitochondrial ATP production. Arch Biochem Biophys. 1980 Apr 15;201(1):44–55. doi: 10.1016/0003-9861(80)90485-3. [DOI] [PubMed] [Google Scholar]
- Titheradge M. A., Stringer J. L., Haynes R. C., Jr The stimulation of the mitochondrial uncoupler-dependent ATPase in isolated hepatocytes by catecholamines and glucagon and its relationship to gluconeogenesis. Eur J Biochem. 1979 Dec;102(1):117–124. doi: 10.1111/j.1432-1033.1979.tb06271.x. [DOI] [PubMed] [Google Scholar]
- Veech R. L., Cook G. A., King M. T. Relationship of free cytoplasmic pyrophosphate to liver glucose content and total pyrophosphate to cytoplasmic phosphorylation potential. FEBS Lett. 1980 Aug 25;117 (Suppl):K65–K72. doi: 10.1016/0014-5793(80)80571-0. [DOI] [PubMed] [Google Scholar]


