Abstract
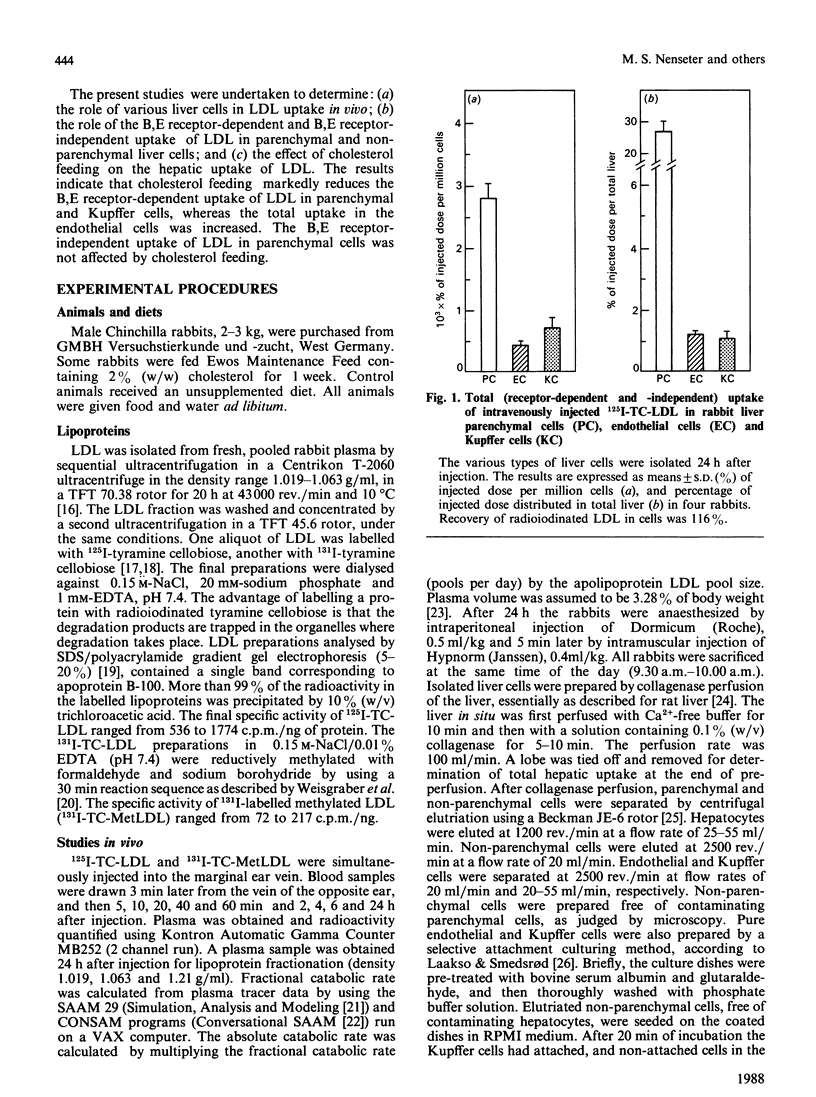
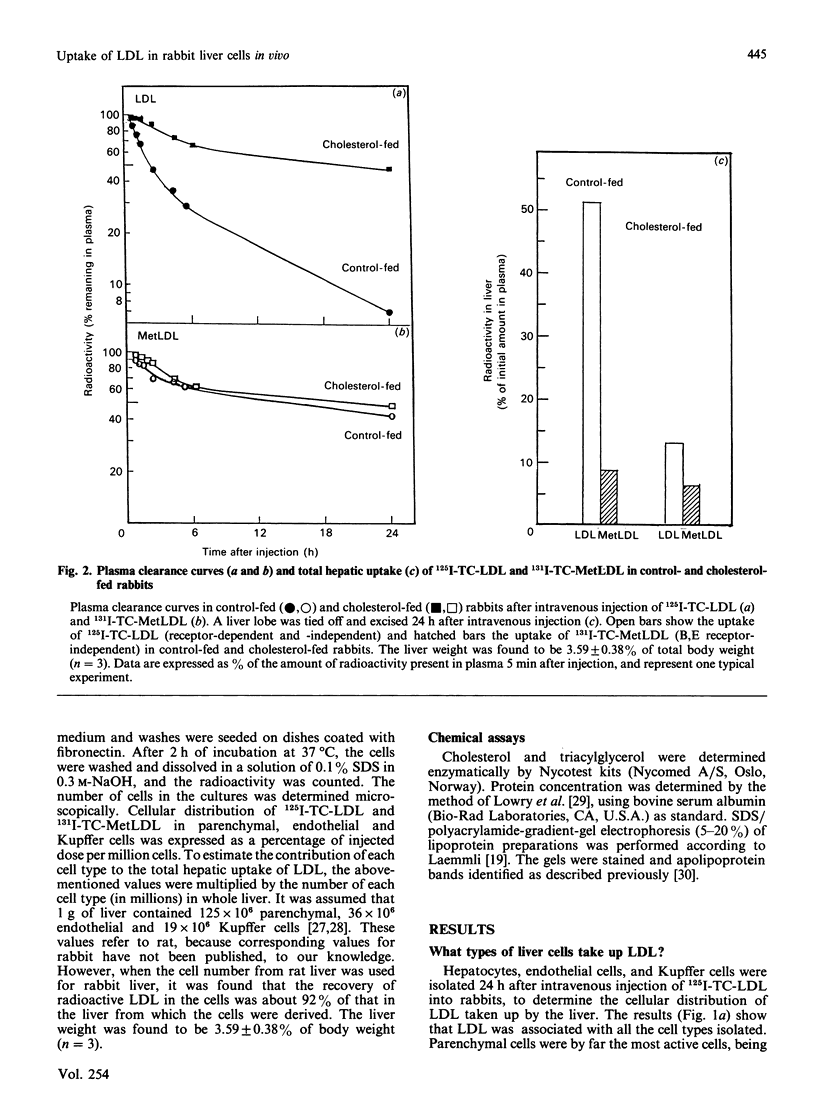
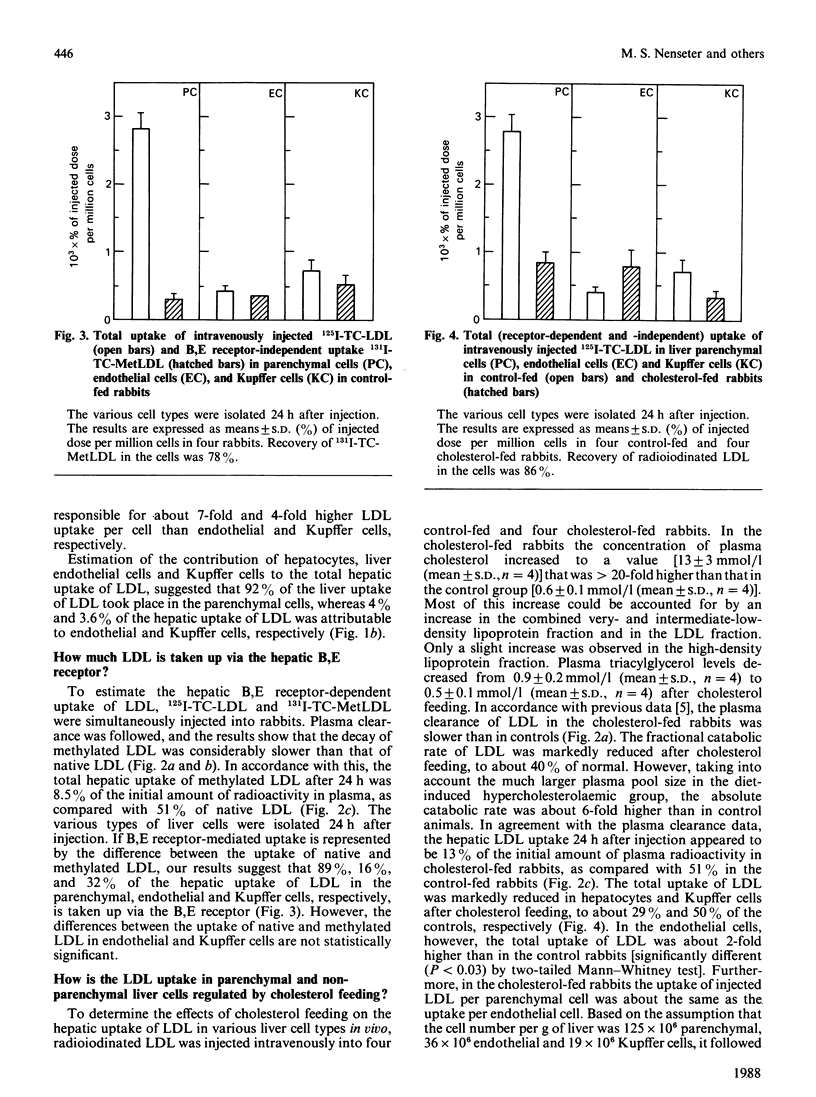
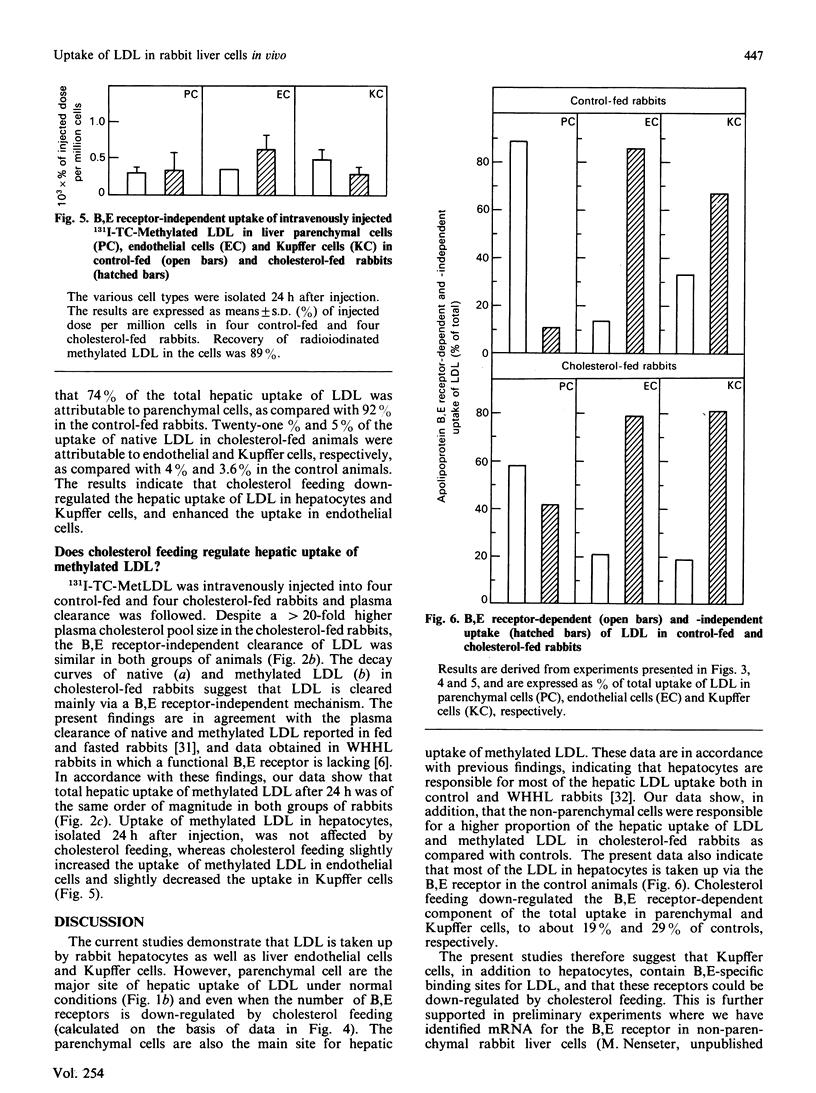
1. Hepatic uptake of low-density lipoprotein (LDL) in parenchymal cells and non-parenchymal cells was studied in control-fed and cholesterol-fed rabbits after intravenous injection of radioiodinated native LDL (125I-TC-LDL) and methylated LDL (131I-TC-MetLDL). 2. LDL was taken up by rabbit liver parenchymal cells, as well as by endothelial and Kupffer cells. Parenchymal cells, however, were responsible for 92% of the hepatic LDL uptake. 3. Of LDL in the hepatocytes, 89% was taken up via the B,E receptor, whereas 16% and 32% of the uptake of LDL in liver endothelial cells and Kupffer cells, respectively, was B,E receptor-dependent. 4. Cholesterol feeding markedly reduced B,E receptor-mediated uptake of LDL in parenchymal liver cells and in Kupffer cells, to 19% and 29% of controls, respectively. Total uptake of LDL in liver endothelial cells was increased about 2-fold. This increased uptake is probably mediated via the scavenger receptor. The B,E receptor-independent association of LDL with parenchymal cells was not affected by the cholesterol feeding. 5. It is concluded that the B,E receptor is located in parenchymal as well as in the non-parenchymal rabbit liver cells, and that this receptor is down-regulated by cholesterol feeding. Parenchymal cells are the main site of hepatic uptake of LDL, both under normal conditions and when the number of B,E receptors is down-regulated by cholesterol feeding. In addition, LDL is taken up by B,E receptor-independent mechanism(s) in rabbit liver parenchymal, endothelial and Kupffer cells. The non-parenchymal liver cells may play a quantitatively important role when the concentration of circulating LDL is maintained at a high level in plasma, being responsible for 26% of hepatic uptake of LDL in cholesterol-fed rabbits as compared with 8% in control-fed rabbits. The proportion of hepatic LDL uptake in endothelial cells was greater than 5-fold higher in the diet-induced hypercholesterolaemic rabbits than in controls.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bilheimer D. W., Watanabe Y., Kita T. Impaired receptor-mediated catabolism of low density lipoprotein in the WHHL rabbit, an animal model of familial hypercholesterolemia. Proc Natl Acad Sci U S A. 1982 May;79(10):3305–3309. doi: 10.1073/pnas.79.10.3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomhoff R., Drevon C. A., Eskild W., Helgerud P., Norum K. R., Berg T. Clearance of acetyl low density lipoprotein by rat liver endothelial cells. Implications for hepatic cholesterol metabolism. J Biol Chem. 1984 Jul 25;259(14):8898–8903. [PubMed] [Google Scholar]
- Blomhoff R., Holte K., Naess L., Berg T. Newly administered [3H]retinol is transferred from hepatocytes to stellate cells in liver for storage. Exp Cell Res. 1984 Jan;150(1):186–193. doi: 10.1016/0014-4827(84)90713-4. [DOI] [PubMed] [Google Scholar]
- Blomhoff R., Smedsrød B., Eskild W., Granum P. E., Berg T. Preparation of isolated liver endothelial cells and Kupffer cells in high yield by means of an enterotoxin. Exp Cell Res. 1984 Jan;150(1):194–204. doi: 10.1016/0014-4827(84)90714-6. [DOI] [PubMed] [Google Scholar]
- Boston R. C., Greif P. C., Berman M. Conversational SAAM--an interactive program for kinetic analysis of biological systems. Comput Programs Biomed. 1981 Mar-Jun;13(1-2):111–119. doi: 10.1016/0010-468x(81)90089-1. [DOI] [PubMed] [Google Scholar]
- Brown M. S., Goldstein J. L. A receptor-mediated pathway for cholesterol homeostasis. Science. 1986 Apr 4;232(4746):34–47. doi: 10.1126/science.3513311. [DOI] [PubMed] [Google Scholar]
- Brown M. S., Goldstein J. L., Krieger M., Ho Y. K., Anderson R. G. Reversible accumulation of cholesteryl esters in macrophages incubated with acetylated lipoproteins. J Cell Biol. 1979 Sep;82(3):597–613. doi: 10.1083/jcb.82.3.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. S., Goldstein J. L. Lipoprotein metabolism in the macrophage: implications for cholesterol deposition in atherosclerosis. Annu Rev Biochem. 1983;52:223–261. doi: 10.1146/annurev.bi.52.070183.001255. [DOI] [PubMed] [Google Scholar]
- Brown M. S., Kovanen P. T., Goldstein J. L. Regulation of plasma cholesterol by lipoprotein receptors. Science. 1981 May 8;212(4495):628–635. doi: 10.1126/science.6261329. [DOI] [PubMed] [Google Scholar]
- Carew T. E., Pittman R. C., Marchand E. R., Steinberg D. Measurement in vivo of irreversible degradation of low density lipoprotein in the rabbit aorta. Predominance of intimal degradation. Arteriosclerosis. 1984 May-Jun;4(3):214–224. doi: 10.1161/01.atv.4.3.214. [DOI] [PubMed] [Google Scholar]
- HAVEL R. J., EDER H. A., BRAGDON J. H. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J Clin Invest. 1955 Sep;34(9):1345–1353. doi: 10.1172/JCI103182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harkes L., Van Berkel J. C. Quantitative role of parenchymal and non-parenchymal liver cells in the uptake of [14C]sucrose-labelled low-density lipoprotein in vivo. Biochem J. 1984 Nov 15;224(1):21–27. doi: 10.1042/bj2240021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriksen T., Mahoney E. M., Steinberg D. Enhanced macrophage degradation of biologically modified low density lipoprotein. Arteriosclerosis. 1983 Mar-Apr;3(2):149–159. doi: 10.1161/01.atv.3.2.149. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Ma P. T., Yamamoto T., Goldstein J. L., Brown M. S. Increased mRNA for low density lipoprotein receptor in livers of rabbits treated with 17 alpha-ethinyl estradiol. Proc Natl Acad Sci U S A. 1986 Feb;83(3):792–796. doi: 10.1073/pnas.83.3.792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahley R. W., Innerarity T. L., Weisgraber K. B., Oh S. Y. Altered metabolism (in vivo and in vitro) of plasma lipoproteins after selective chemical modification of lysine residues of the apoproteins. J Clin Invest. 1979 Sep;64(3):743–750. doi: 10.1172/JCI109518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahley R. W., Weisgraber K. H., Melchior G. W., Innerarity T. L., Holcombe K. S. Inhibition of receptor-mediated clearance of lysine and arginine-modified lipoproteins from the plasma of rats and monkeys. Proc Natl Acad Sci U S A. 1980 Jan;77(1):225–229. doi: 10.1073/pnas.77.1.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munthe-Kaas A. C., Berg T., Seljelid R. Distribution of lysosomal enzymes in different types of rat liver cells. Exp Cell Res. 1976 Apr;99(1):146–154. doi: 10.1016/0014-4827(76)90689-3. [DOI] [PubMed] [Google Scholar]
- Nagelkerke J. F., Bakkeren H. F., Kuipers F., Vonk R. J., van Berkel T. J. Hepatic processing of the cholesteryl ester from low density lipoprotein in the rat. J Biol Chem. 1986 Jul 5;261(19):8908–8913. [PubMed] [Google Scholar]
- Nagelkerke J. F., Barto K. P., van Berkel T. J. In vivo and in vitro uptake and degradation of acetylated low density lipoprotein by rat liver endothelial, Kupffer, and parenchymal cells. J Biol Chem. 1983 Oct 25;258(20):12221–12227. [PubMed] [Google Scholar]
- Nenseter M. S., Blomhoff R., Eskild W., Kindberg G. M., Berg T. Intracellular transport and degradation of chylomicron remnants in rat liver cells after in vivo endocytosis. Biochim Biophys Acta. 1987 Jun 15;929(1):25–33. doi: 10.1016/0167-4889(87)90237-0. [DOI] [PubMed] [Google Scholar]
- Norum K. R., Berg T., Helgerud P., Drevon C. A. Transport of cholesterol. Physiol Rev. 1983 Oct;63(4):1343–1419. doi: 10.1152/physrev.1983.63.4.1343. [DOI] [PubMed] [Google Scholar]
- Pittman R. C., Carew T. E., Attie A. D., Witztum J. L., Watanabe Y., Steinberg D. Receptor-dependent and receptor-independent degradation of low density lipoprotein in normal rabbits and in receptor-deficient mutant rabbits. J Biol Chem. 1982 Jul 25;257(14):7994–8000. [PubMed] [Google Scholar]
- Pittman R. C., Carew T. E., Glass C. K., Green S. R., Taylor C. A., Jr, Attie A. D. A radioiodinated, intracellularly trapped ligand for determining the sites of plasma protein degradation in vivo. Biochem J. 1983 Jun 15;212(3):791–800. doi: 10.1042/bj2120791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittman R. C., Steinberg D. Sites and mechanisms of uptake and degradation of high density and low density lipoproteins. J Lipid Res. 1984 Dec 15;25(13):1577–1585. [PubMed] [Google Scholar]
- Ross A. C., Zilversmit D. B. Chylomicron remnant cholesteryl esters as the major constituent of very low density lipoproteins in plasma of cholesterol-fed rabbits. J Lipid Res. 1977 Mar;18(2):169–181. [PubMed] [Google Scholar]
- Slater H. R., Shepherd J., Packard C. J. Receptor-mediated catabolism and tissue uptake of human low density lipoprotein in the cholesterol-fed, atherosclerotic rabbit. Biochim Biophys Acta. 1982 Nov 12;713(2):435–445. doi: 10.1016/0005-2760(82)90263-6. [DOI] [PubMed] [Google Scholar]
- Spady D. K., Huettinger M., Bilheimer D. W., Dietschy J. M. Role of receptor-independent low density lipoprotein transport in the maintenance of tissue cholesterol balance in the normal and WHHL rabbit. J Lipid Res. 1987 Jan;28(1):32–41. [PubMed] [Google Scholar]
- Stoudemire J. B., Renaud G., Shames D. M., Havel R. J. Impaired receptor-mediated catabolism of low density lipoproteins in fasted rabbits. J Lipid Res. 1984 Jan;25(1):33–39. [PubMed] [Google Scholar]
- Weisgraber K. H., Innerarity T. L., Mahley R. W. Role of lysine residues of plasma lipoproteins in high affinity binding to cell surface receptors on human fibroblasts. J Biol Chem. 1978 Dec 25;253(24):9053–9062. [PubMed] [Google Scholar]


