Abstract
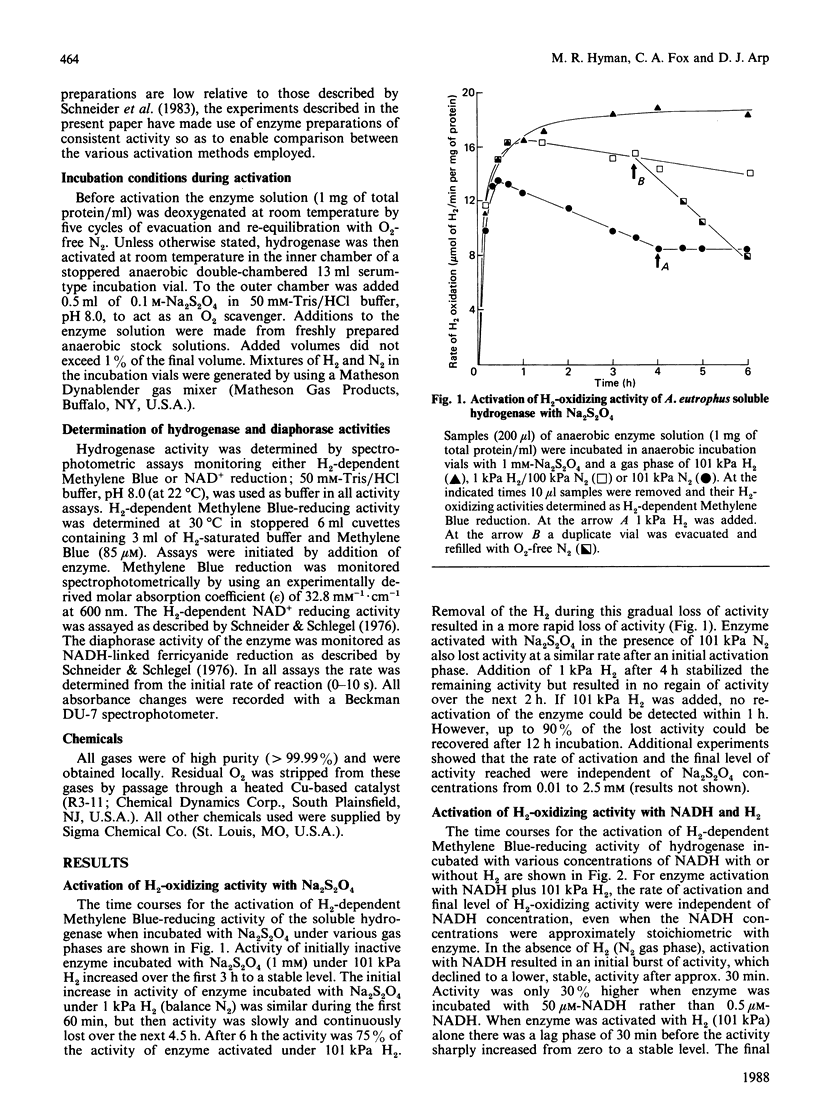
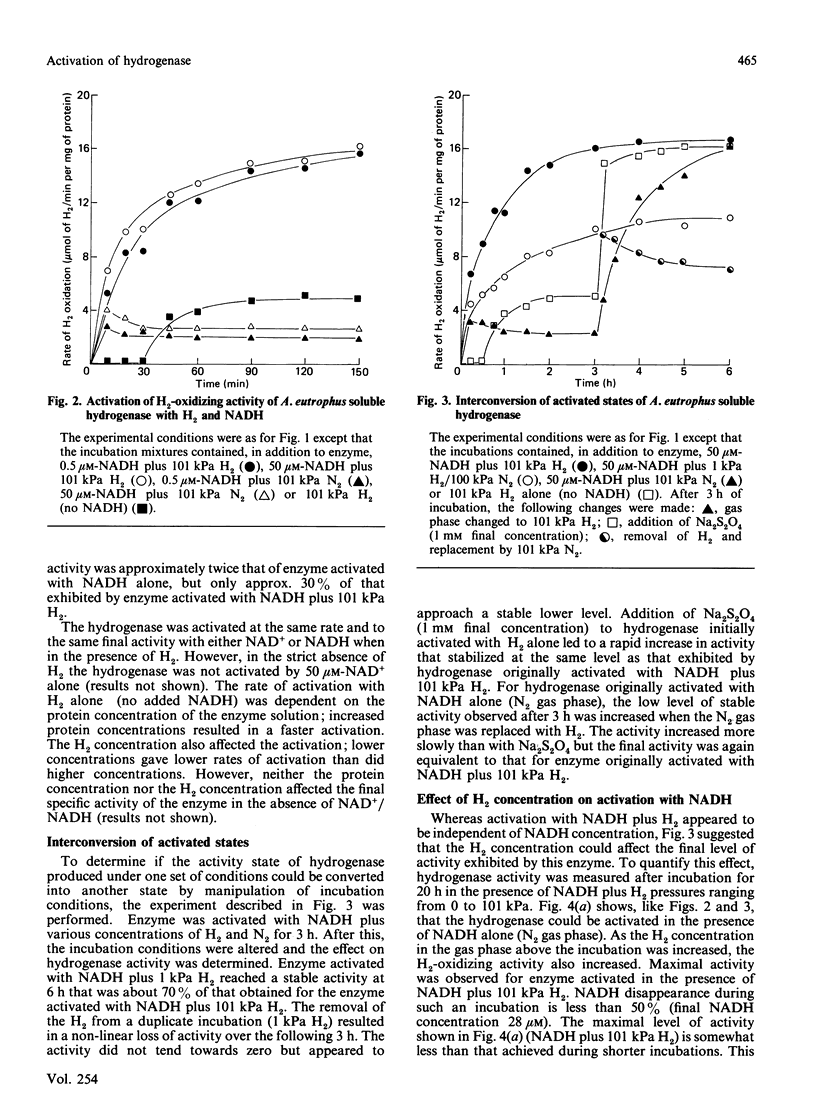
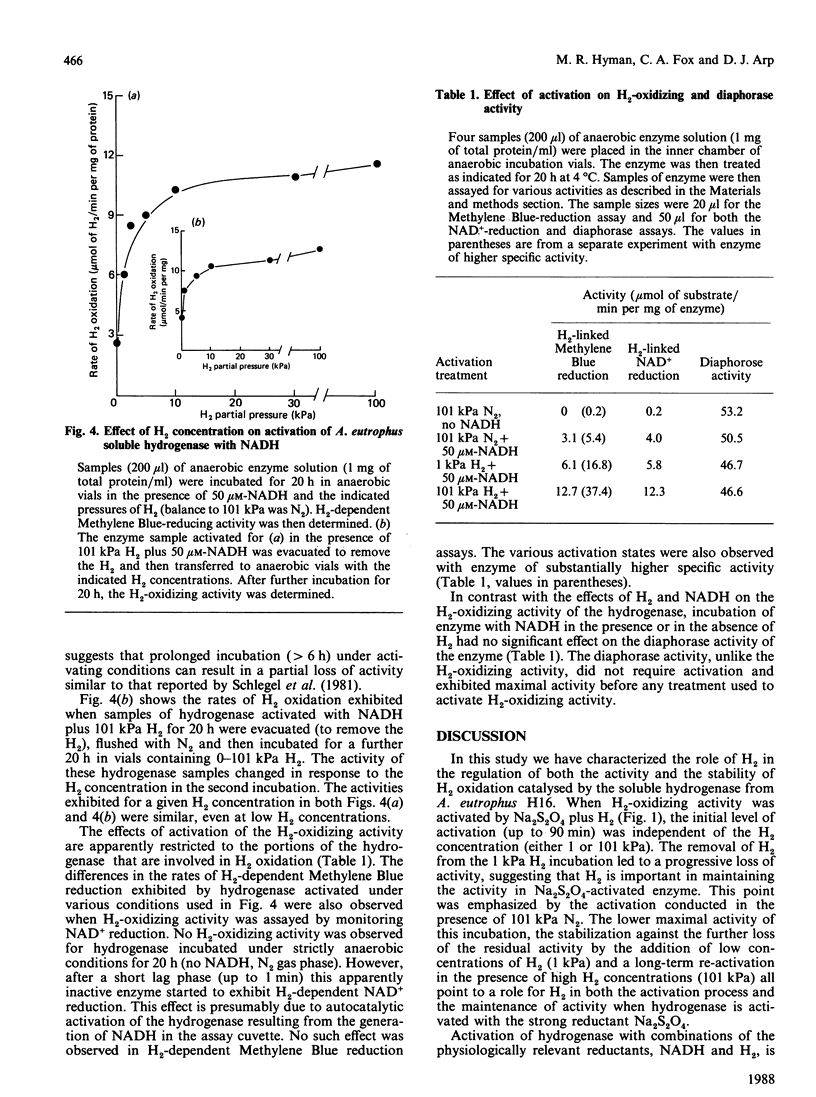
The activation kinetics of the H2-oxidizing activity of the soluble hydrogenase from Alcaligenes eutrophus H16 were investigated. Activation with Na2S2O4 plus 101 kPa H2 resulted in a rapid increase in activity over 1 h and constant activity after 3 h incubation. Less-stable activations were achieved if enzyme was incubated with Na2S2O4 under 1 kPa H2 or 101 kPa N2. The enzyme could also be partly activated either with NADH alone or with H2 alone. The level of activity obtained with both 101 kPa H2 and NADH present was greater than that obtained with either 101 kPa H2 or NADH alone. Activation with H2 plus NADH was virtually independent of NADH concentration but highly dependent on H2 concentration. The effects of various concentrations of H2 and constant concentration of NADH on the level of activation were the same whether H2 oxidation was assayed by H2-dependent Methylene Blue or NAD+ reduction. Diaphorase activity did not require activation and was little affected by the treatments that activated H2-oxidizing activity. The results suggest that H2 plays an important role in regulating the level of H2-oxidizing activity in this soluble hydrogenase.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berlier Y. M., Fauque G., Lespinat P. A., Le Gall J. Activation, reduction and proton-deuterium exchange reaction of the periplasmic hydrogenase from Desulfovibrio gigas in relation with the role of cytochrome c3. FEBS Lett. 1982 Apr 19;140(2):185–188. doi: 10.1016/0014-5793(82)80890-9. [DOI] [PubMed] [Google Scholar]
- Egerer P., Günther H., Simon H. On the hydrogen-deuterium exchange reaction catalyzed by the soluble hydrogenase from Alcaligenes eutrophus H16 in the free and immobilized state. Biochim Biophys Acta. 1982 May 3;703(2):149–157. doi: 10.1016/0167-4838(82)90043-7. [DOI] [PubMed] [Google Scholar]
- Hornhardt S., Schneider K., Schlegel H. G. Characterization of a native subunit of the NAD-linked hydrogenase isolated from a mutant of Alcaligenes eutrophus H16. Biochimie. 1986 Jan;68(1):15–24. doi: 10.1016/s0300-9084(86)81063-x. [DOI] [PubMed] [Google Scholar]
- Hyman M. R., Arp D. J. Reversible and irreversible effects of nitric oxide on the soluble hydrogenase from Alcaligenes eutrophus H16. Biochem J. 1988 Sep 1;254(2):469–475. doi: 10.1042/bj2540469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lissolo T., Pulvin S., Thomas D. Reactivation of the hydrogenase from Desulfovibrio gigas by hydrogen. Influence of redox potential. J Biol Chem. 1984 Oct 10;259(19):11725–11729. [PubMed] [Google Scholar]
- Schneider K., Cammack R., Schlegel H. G., Hall D. O. The iron-sulphur centres of soluble hydrogenase from Alcaligenes eutrophus. Biochim Biophys Acta. 1979 Jun 19;578(2):445–461. doi: 10.1016/0005-2795(79)90175-2. [DOI] [PubMed] [Google Scholar]
- Schneider K., Piechulla B. Isolation and immunological characterization of the four non-identical subunits of the soluble NAD-linked hydrogenase from Alcaligenes eutrophus H16. Biochimie. 1986 Jan;68(1):5–13. doi: 10.1016/s0300-9084(86)81062-8. [DOI] [PubMed] [Google Scholar]
- Schneider K., Pinkwart M., Jochim K. Purification of hydrogenases by affinity chromatography on Procion Red-agarose. Biochem J. 1983 Aug 1;213(2):391–398. doi: 10.1042/bj2130391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider K., Schlegel H. G. Production of superoxide radicals by soluble hydrogenase from Alcaligenes eutrophus H16. Biochem J. 1981 Jan 1;193(1):99–107. doi: 10.1042/bj1930099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider K., Schlegel H. G. Purification and properties of soluble hydrogenase from Alcaligenes eutrophus H 16. Biochim Biophys Acta. 1976 Nov 8;452(1):66–80. doi: 10.1016/0005-2744(76)90058-9. [DOI] [PubMed] [Google Scholar]


