Abstract
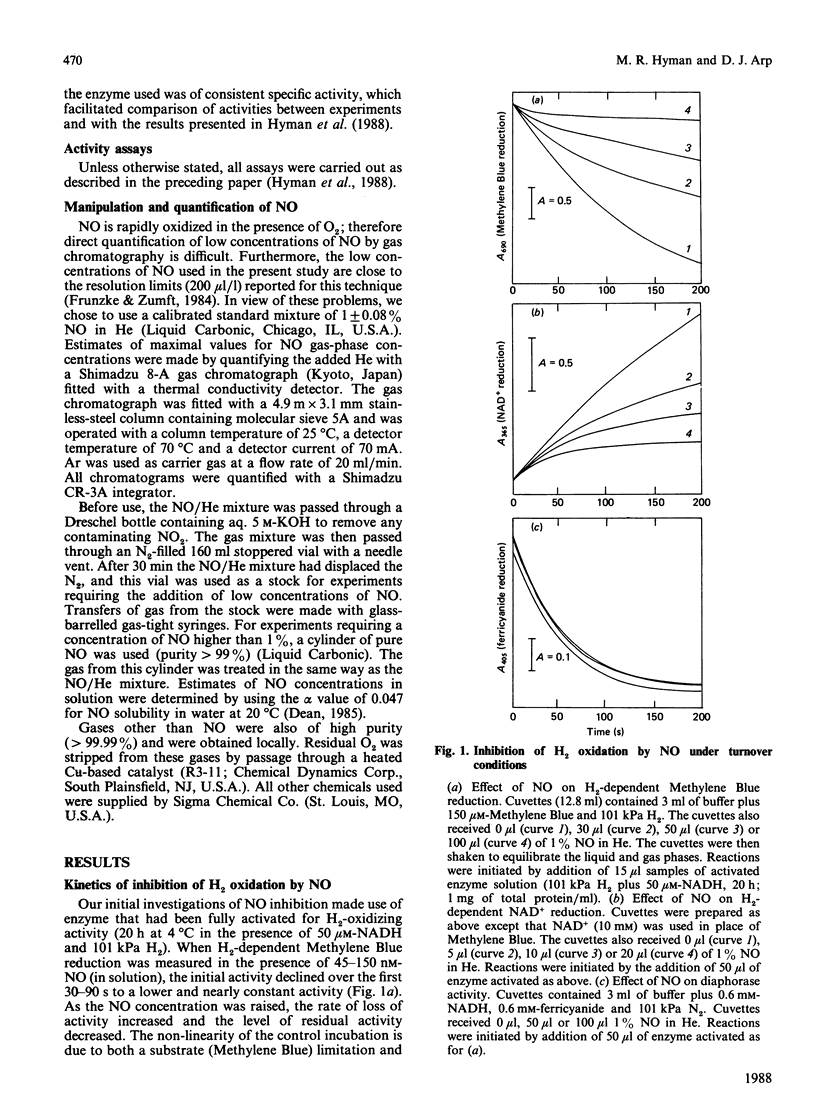
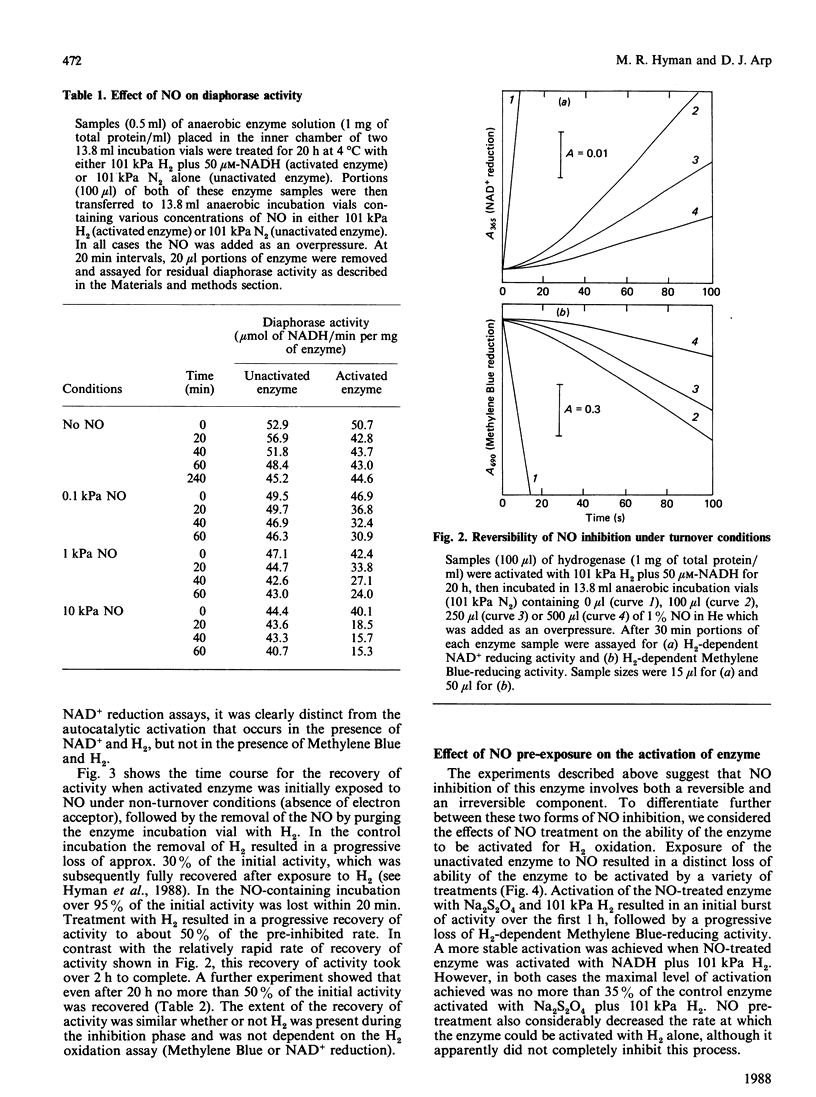
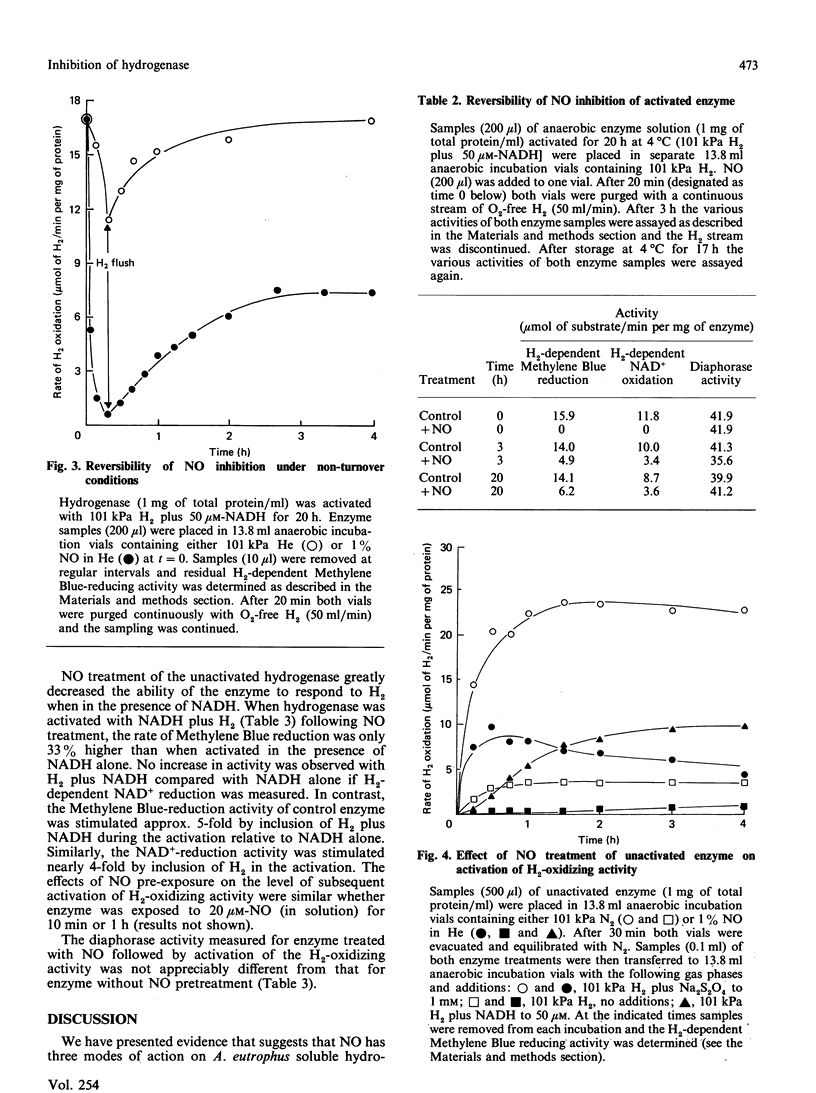
The effects of NO on the H2-oxidizing and diaphorase activities of the soluble hydrogenase from Alcaligenes eutrophus H16 were investigated. With fully activated enzyme, NO (8-150 nM in solution) inhibited H2 oxidation in a time- and NO-concentration-dependent process. Neither H2 nor NAD+ appeared to protect the enzyme against the inhibition. Loss of activity in the absence of an electron acceptor was about 10 times slower than under turnover conditions. The inhibition was partially reversible; approx. 50% of full activity was recoverable after removal of the NO. Recovery was slower in the absence of an electron acceptor than in the presence of H2 plus an electron acceptor. The diaphorase activity of the unactivated hydrogenase was not affected by NO concentrations of up to 200 microM in solution. Exposure of the unactivated hydrogenase to NO irreversibly inhibited the ability of the enzyme to be fully activated for H2-oxidizing activity. The enzyme also lost its ability to respond to H2 during activation in the presence of NADH. The results are interpreted in terms of a complex inhibition that displays elements of (1) a reversible slow-binding inhibition of H2-oxidizing activity, (2) an irreversible effect on H2-oxidizing activity and (30 an irreversible inhibition of a regulatory component of the enzyme. Possible sites of action for NO are discussed.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berlier Y., Fauque G. D., LeGall J., Choi E. S., Peck H. D., Jr, Lespinat P. A. Inhibition studies of three classes of Desulfovibrio hydrogenase: application to the further characterization of the multiple hydrogenases found in Desulfovibrio vulgaris Hildenborough. Biochem Biophys Res Commun. 1987 Jul 15;146(1):147–153. doi: 10.1016/0006-291x(87)90703-0. [DOI] [PubMed] [Google Scholar]
- Egerer P., Simon H. Isotopic and kinetic studies and influence of dicoumarol on the soluble hydrogenase from Alcaligenes eutrophus H16. Biochim Biophys Acta. 1982 May 3;703(2):158–170. doi: 10.1016/0167-4838(82)90044-9. [DOI] [PubMed] [Google Scholar]
- Gruzinskii I. V., Gogotov I. N., Bechina E. M., Semenov Ia V. Gidrogenaznaia aktivnost' vodorodokisliaiushchikh bakterii Alcaligenes eutrophus. Mikrobiologiia. 1977 Jul-Aug;46(4):625–631. [PubMed] [Google Scholar]
- Hornhardt S., Schneider K., Schlegel H. G. Characterization of a native subunit of the NAD-linked hydrogenase isolated from a mutant of Alcaligenes eutrophus H16. Biochimie. 1986 Jan;68(1):15–24. doi: 10.1016/s0300-9084(86)81063-x. [DOI] [PubMed] [Google Scholar]
- Hyman M. R., Fox C. A., Arp D. J. Role of hydrogen in the activation and regulation of hydrogen oxidation by the soluble hydrogenase from Alcaligenes eutrophus H16. Biochem J. 1988 Sep 1;254(2):463–468. doi: 10.1042/bj2540463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasna A. I., Rittenberg D. THE INHIBITION OF HYDROGENASE BY NITRIC OXIDE. Proc Natl Acad Sci U S A. 1954 Apr;40(4):225–227. doi: 10.1073/pnas.40.4.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer J. Comparison of carbon monoxide, nitric oxide, and nitrite as inhibitors of the nitrogenase from Clostridium pasteurianum. Arch Biochem Biophys. 1981 Aug;210(1):246–256. doi: 10.1016/0003-9861(81)90186-7. [DOI] [PubMed] [Google Scholar]
- Pinchukova E. E., Varfolomeev S. D., Kondrat'eva E. N. Vydelenie, ochistka i issledovanie stabil'nosti rastvorimoi gidrogenazy Alcaligenes eutrophus Z-1. Biokhimiia. 1979 Apr;44(4):605–615. [PubMed] [Google Scholar]
- Popov V. O., Ovchinnikov A. N., Egorov A. M., Berezin I. V. NAD+-dependent hydrogenase from the hydrogen oxidizing bacterium Alcaligenes eutrophus Z1. Stabilization against temperature and urea induced inactivation. Biochimie. 1986 Jan;68(1):63–68. doi: 10.1016/s0300-9084(86)81069-0. [DOI] [PubMed] [Google Scholar]
- Reddy D., Lancaster J. R., Jr, Cornforth D. P. Nitrite inhibition of Clostridium botulinum: electron spin resonance detection of iron-nitric oxide complexes. Science. 1983 Aug 19;221(4612):769–770. doi: 10.1126/science.6308761. [DOI] [PubMed] [Google Scholar]
- Schneider K., Cammack R., Schlegel H. G., Hall D. O. The iron-sulphur centres of soluble hydrogenase from Alcaligenes eutrophus. Biochim Biophys Acta. 1979 Jun 19;578(2):445–461. doi: 10.1016/0005-2795(79)90175-2. [DOI] [PubMed] [Google Scholar]
- Schneider K., Piechulla B. Isolation and immunological characterization of the four non-identical subunits of the soluble NAD-linked hydrogenase from Alcaligenes eutrophus H16. Biochimie. 1986 Jan;68(1):5–13. doi: 10.1016/s0300-9084(86)81062-8. [DOI] [PubMed] [Google Scholar]
- Schneider K., Schlegel H. G., Jochim K. Effect of nickel on activity and subunit composition of purified hydrogenase from Nocardia opaca 1 b. Eur J Biochem. 1984 Feb 1;138(3):533–541. doi: 10.1111/j.1432-1033.1984.tb07948.x. [DOI] [PubMed] [Google Scholar]
- Schneider K., Schlegel H. G. Production of superoxide radicals by soluble hydrogenase from Alcaligenes eutrophus H16. Biochem J. 1981 Jan 1;193(1):99–107. doi: 10.1042/bj1930099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider K., Schlegel H. G. Purification and properties of soluble hydrogenase from Alcaligenes eutrophus H 16. Biochim Biophys Acta. 1976 Nov 8;452(1):66–80. doi: 10.1016/0005-2744(76)90058-9. [DOI] [PubMed] [Google Scholar]
- Tibelius K. H., Knowles R. Hydrogenase activity in Azospirillum brasilense is inhibited by nitrite, nitric oxide, carbon monoxide, and acetylene. J Bacteriol. 1984 Oct;160(1):103–106. doi: 10.1128/jb.160.1.103-106.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]


