Abstract
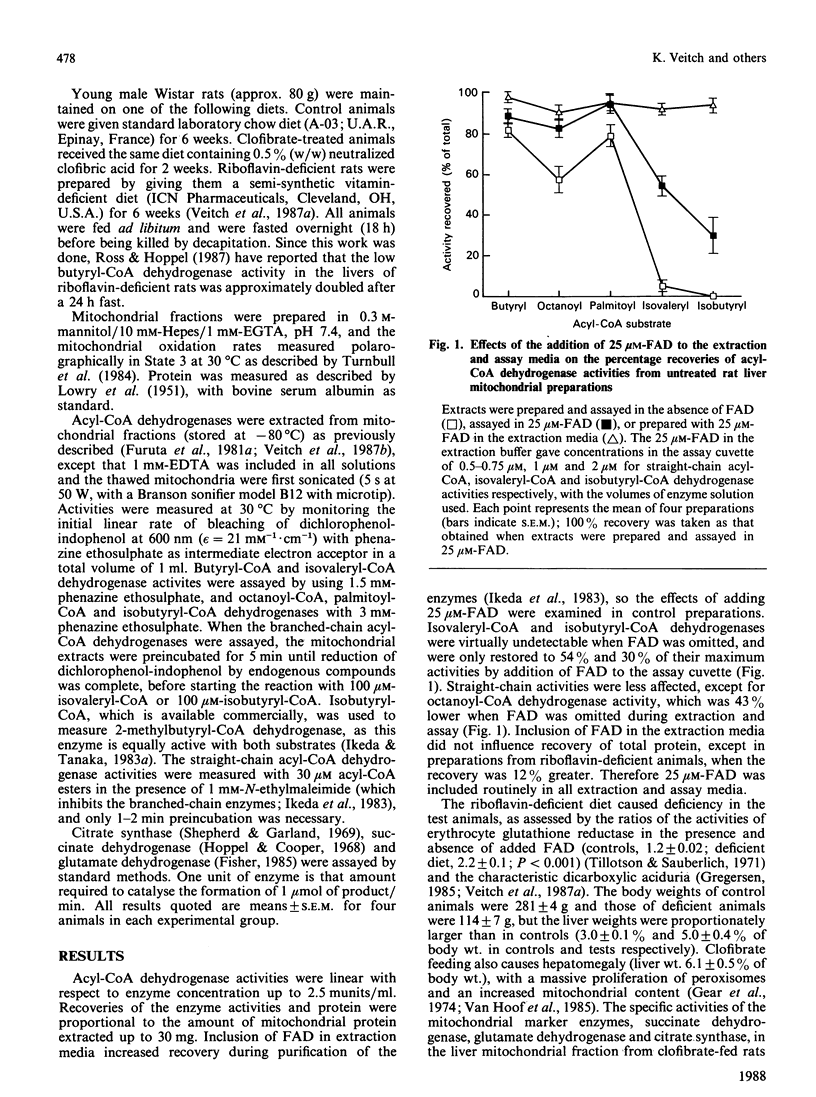
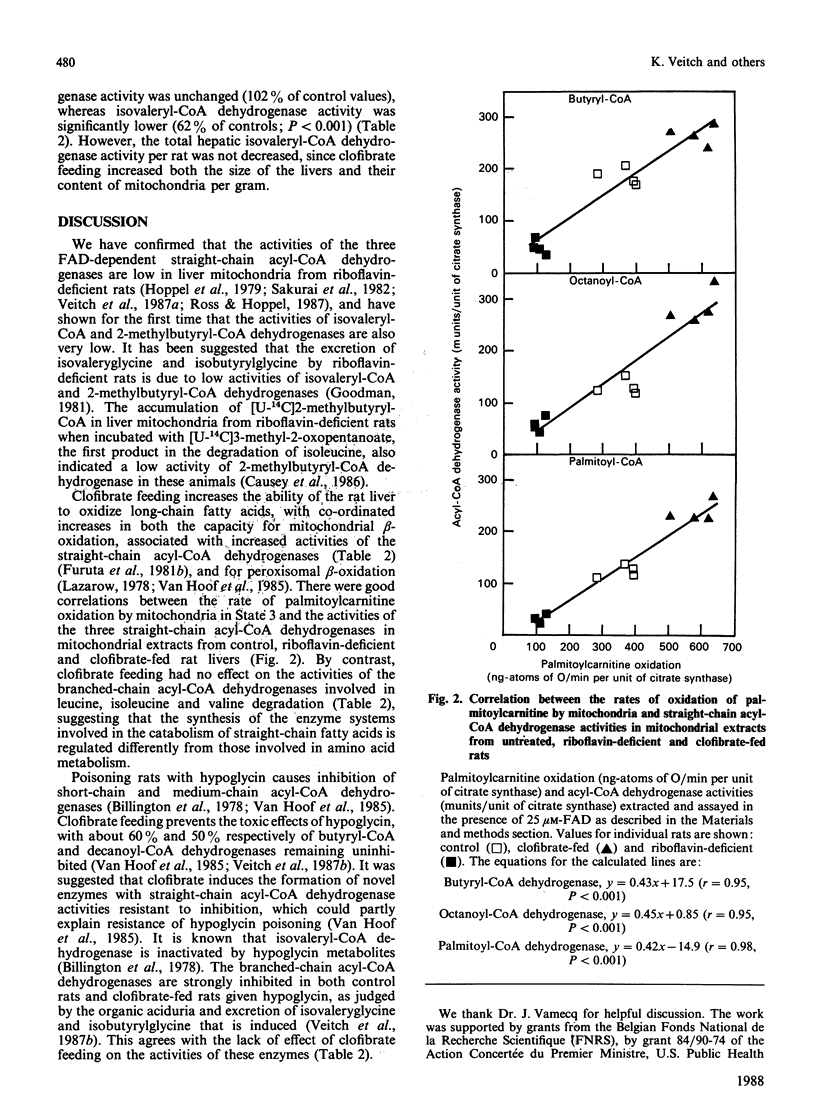
Rats were maintained on a riboflavin-deficient diet or on a diet containing clofibrate (0.5%, w/w). The activities of the mitochondrial FAD-dependent straight-chain acyl-CoA dehydrogenases (butyryl-CoA, octanoyl-CoA and palmitoyl-CoA) and the branched-chain acyl-CoA dehydrogenases (isovaleryl-CoA and isobutyryl-CoA) involved in the degradation of branched-chain acyl-CoA esters derived from branched-chain amino acids were assayed in liver mitochondrial extracts prepared in the absence and presence of exogenous FAD. These activities were low in livers from riboflavin-deficient rats (11, 28, 16, 6 and less than 2% of controls respectively) when prepared in the absence of exogenous FAD, and were not restored to control values when prepared in 25 microM-FAD (29, 47, 28, 7 and 17%). Clofibrate feeding increased the activities of butyryl-CoA, octanoyl-CoA and palmitoyl-CoA dehydrogenases (by 48, 116 and 98% of controls respectively), but not, by contrast, the activities of isovaleryl-CoA and isobutyryl-CoA dehydrogenases (62 and 102% of controls respectively). The mitochondrial fractions from riboflavin-deficient and from clofibrate-fed rats oxidized palmitoylcarnitine in State 3 at rates of 32 and 163% respectively of those from control rats.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Billington D., Osmundsen H., Sherratt H. S. Mechanisms of the metabolic disturbances caused by hypoglycin and by pent-4-enoic acid. In vitro studies. Biochem Pharmacol. 1978;27(24):2879–2890. doi: 10.1016/0006-2952(78)90204-6. [DOI] [PubMed] [Google Scholar]
- Causey A. G., Middleton B., Bartlett K. A study of the metabolism of [U-14C]3-methyl-2-oxopentanoate by rat liver mitochondria using h.p.l.c. with continuous on-line monitoring of radioactive intact acyl-coenzyme A intermediates. Biochem J. 1986 Apr 15;235(2):343–350. doi: 10.1042/bj2350343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher H. F. L-Glutamate dehydrogenase from bovine liver. Methods Enzymol. 1985;113:16–27. doi: 10.1016/s0076-6879(85)13006-5. [DOI] [PubMed] [Google Scholar]
- Furuta S., Miyazawa S., Hashimoto T. Induction of acyl-CoA dehydrogenases and electron transfer flavoprotein and their roles in fatty acid oxidation in rat liver mitochondria. J Biochem. 1981 Dec;90(6):1751–1756. doi: 10.1093/oxfordjournals.jbchem.a133652. [DOI] [PubMed] [Google Scholar]
- Furuta S., Miyazawa S., Hashimoto T. Purification and properties of rat liver acyl-CoA dehydrogenases and electron transfer flavoprotein. J Biochem. 1981 Dec;90(6):1739–1750. doi: 10.1093/oxfordjournals.jbchem.a133651. [DOI] [PubMed] [Google Scholar]
- Gear A. R., Albert A. D., Bednarek J. M. The effect of the hypocholesterolemic drug clofibrate on liver mitochondrial biogenesis. A role for neutral mitochondrial proteases. J Biol Chem. 1974 Oct 25;249(20):6495–6504. [PubMed] [Google Scholar]
- Goodman S. I. Organic aciduria in the riboflavin-deficient rat. Am J Clin Nutr. 1981 Nov;34(11):2434–2437. doi: 10.1093/ajcn/34.11.2434. [DOI] [PubMed] [Google Scholar]
- Hoppel C., Cooper C. The action of digitonin on rat liver mitochondria. The effects on enzyme content. Biochem J. 1968 Apr;107(3):367–375. doi: 10.1042/bj1070367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoppel C., DiMarco J. P., Tandler B. Riboflavin and rat hepatic cell structure and function. Mitochondrial oxidative metabolism in deficiency states. J Biol Chem. 1979 May 25;254(10):4164–4170. [PubMed] [Google Scholar]
- Ikeda Y., Dabrowski C., Tanaka K. Separation and properties of five distinct acyl-CoA dehydrogenases from rat liver mitochondria. Identification of a new 2-methyl branched chain acyl-CoA dehydrogenase. J Biol Chem. 1983 Jan 25;258(2):1066–1076. [PubMed] [Google Scholar]
- Ikeda Y., Tanaka K. Purification and characterization of 2-methyl-branched chain acyl coenzyme A dehydrogenase, an enzyme involved in the isoleucine and valine metabolism, from rat liver mitochondria. J Biol Chem. 1983 Aug 10;258(15):9477–9487. [PubMed] [Google Scholar]
- Ikeda Y., Tanaka K. Purification and characterization of isovaleryl coenzyme A dehydrogenase from rat liver mitochondria. J Biol Chem. 1983 Jan 25;258(2):1077–1085. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lazarow P. B. Rat liver peroxisomes catalyze the beta oxidation of fatty acids. J Biol Chem. 1978 Mar 10;253(5):1522–1528. [PubMed] [Google Scholar]
- Ross N. S., Hoppel C. L. Acyl-CoA dehydrogenase activity in the riboflavin-deficient rat. Effects of starvation. Biochem J. 1987 Jun 1;244(2):387–391. doi: 10.1042/bj2440387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai T., Miyazawa S., Furuta S., Hashimoto T. Riboflavin deficiency and beta-oxidation systems in rat liver. Lipids. 1982 Sep;17(9):598–604. doi: 10.1007/BF02535365. [DOI] [PubMed] [Google Scholar]
- Tanaka K. On the mode of action of hypoglycin A. 3. Isolation and identification of cis-4-decene-1,10-dioic, cis, cis-4,7-decadiene-1,10-dioic, cis-4-octene-1,8-dioic, glutaric, and adipic acids, N-(methylenecyclopropyl)acetylglycine, and N-isovalerylglycine from urine of hypoglycin A-treated rats. J Biol Chem. 1972 Dec 10;247(23):7465–7478. [PubMed] [Google Scholar]
- Tillotson J. A., Sauberlich H. E. Effect of riboflavin depletion and repletion on the erythrocyte glutathione reductase in the rat. J Nutr. 1971 Nov;101(11):1459–1466. doi: 10.1093/jn/101.11.1459. [DOI] [PubMed] [Google Scholar]
- Vamecq J., de Hoffmann E., Van Hoof F. The microsomal dicarboxylyl-CoA synthetase. Biochem J. 1985 Sep 15;230(3):683–693. doi: 10.1042/bj2300683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Hoof F., Hue L., Vamecq J., Sherratt H. S. Protection of rats by clofibrate against the hypoglycaemic and toxic effects of hypoglycin and pent-4-enoate. An ultrastructural and biochemical study. Biochem J. 1985 Jul 15;229(2):387–397. doi: 10.1042/bj2290387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veitch R. K., Sherratt H. S., Bartlett K. Organic aciduria in rats made resistant to hypoglycin toxicity by pretreatment with clofibrate. Biochem J. 1987 Sep 15;246(3):775–778. doi: 10.1042/bj2460775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenz A., Thorpe C., Ghisla S. Inactivation of general acyl-CoA dehydrogenase from pig kidney by a metabolite of hypoglycin A. J Biol Chem. 1981 Oct 10;256(19):9809–9812. [PubMed] [Google Scholar]


