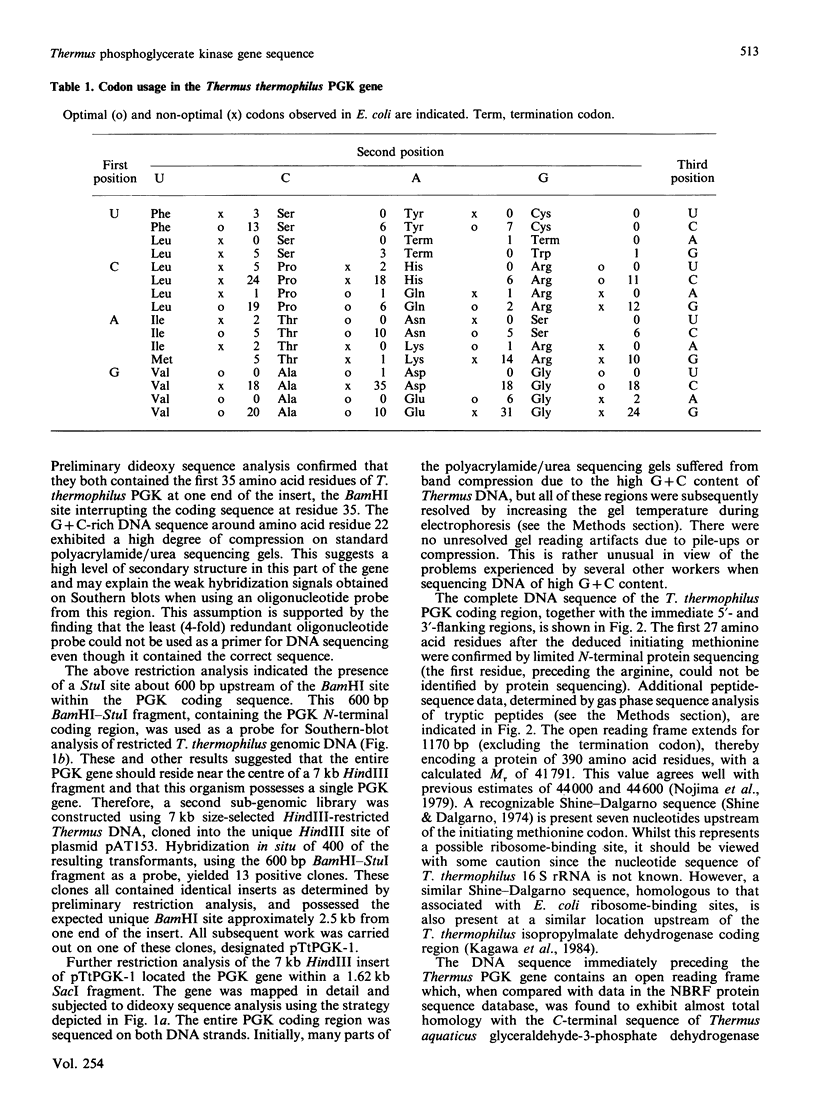
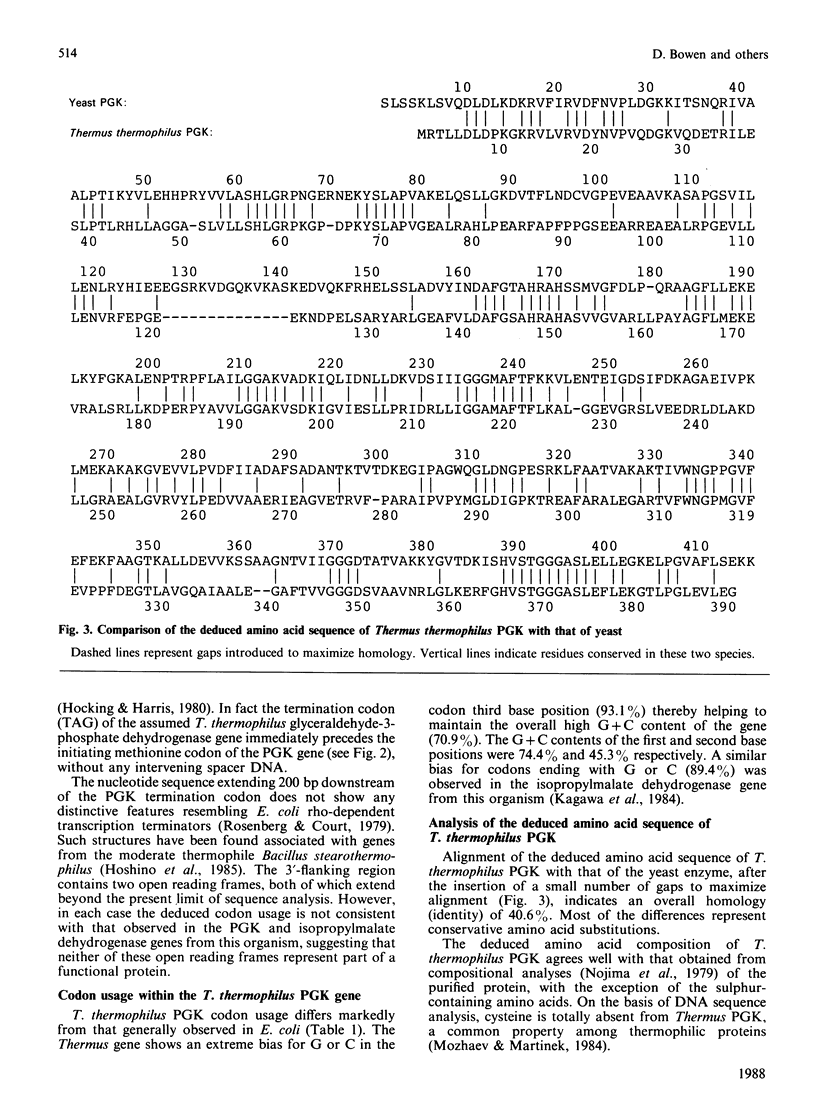
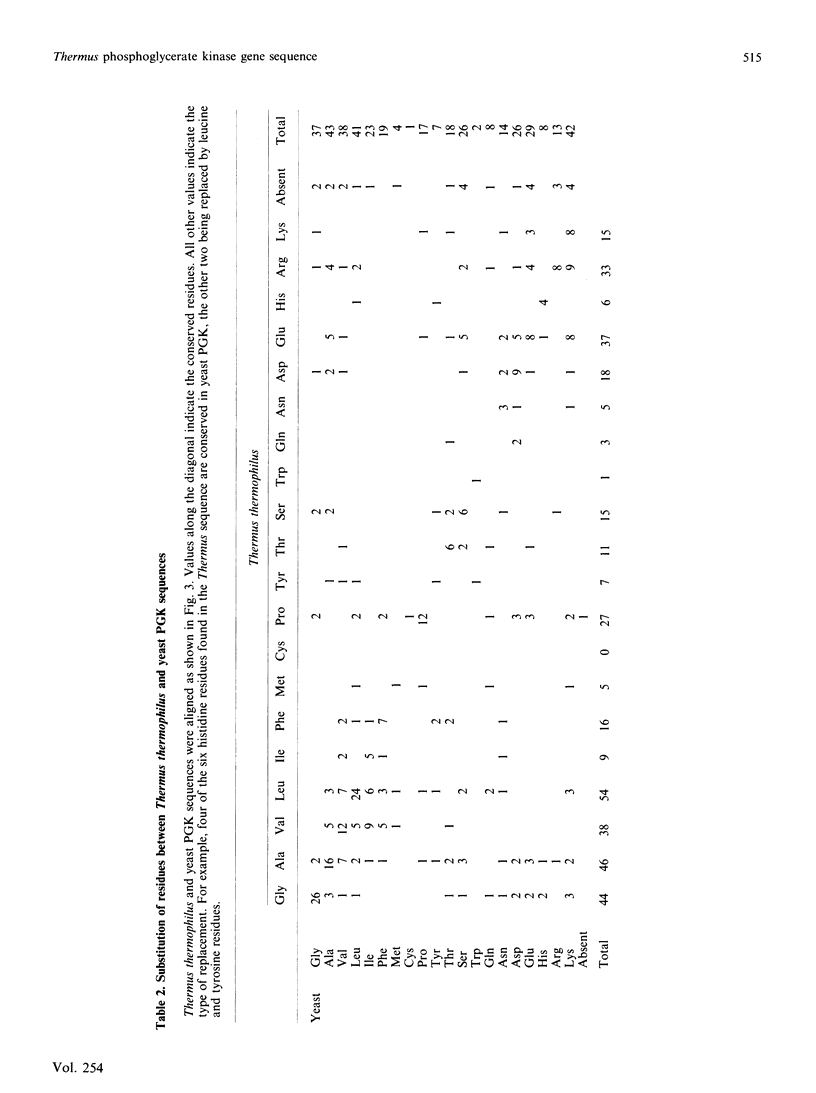
Abstract
Using oligonucleotide probes derived from amino acid sequencing information, the structural gene for phosphoglycerate kinase from the extreme thermophile, Thermus thermophilus, was cloned in Escherichia coli and its complete nucleotide sequence determined. The gene consists of an open reading frame corresponding to a protein of 390 amino acid residues (calculated Mr 41,791) with an extreme bias for G or C (93.1%) in the codon third base position. Comparison of the deduced amino acid sequence with that of the corresponding mesophilic yeast enzyme indicated a number of significant differences. These are discussed in terms of the unusual codon bias and their possible role in enhanced protein thermal stability.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahern T. J., Casal J. I., Petsko G. A., Klibanov A. M. Control of oligomeric enzyme thermostability by protein engineering. Proc Natl Acad Sci U S A. 1987 Feb;84(3):675–679. doi: 10.1073/pnas.84.3.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Clements J. M., Roberts C. F. Transcription and processing signals in the 3-phosphoglycerate kinase (PGK) gene from Aspergillus nidulans. Gene. 1986;44(1):97–105. doi: 10.1016/0378-1119(86)90047-8. [DOI] [PubMed] [Google Scholar]
- Crabb J. W., Murdock A. L., Suzuki T., Hamilton J. W., McLinden J. H., Amelunxen R. E. Sequence homology in the amino-terminal and active-site regions of thermolabile glyceraldehyde-3-phosphate dehydrogenase from a thermophile. J Bacteriol. 1981 Jan;145(1):503–512. doi: 10.1128/jb.145.1.503-512.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita S. C., Oshima T., Imahori K. Purification and properties of D-glyceraldehyde-3-phosphate dehydrogenase from an extreme thermophile, Thermus thermophilus strain HB 8. Eur J Biochem. 1976 Apr 15;64(1):57–68. doi: 10.1111/j.1432-1033.1976.tb10274.x. [DOI] [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy G. W., Darbre A., Merrett M. Primary structure of 3-phosphoglycerate kinase from horse muscle. I. Purification of cyanogen bromide peptides and amino acid sequence of peptide CB5 (104 residues). J Biol Chem. 1981 Oct 25;256(20):10284–10292. [PubMed] [Google Scholar]
- Hitzeman R. A., Hagie F. E., Hayflick J. S., Chen C. Y., Seeburg P. H., Derynck R. The primary structure of the Saccharomyces cerevisiae gene for 3-phosphoglycerate kinase. Nucleic Acids Res. 1982 Dec 11;10(23):7791–7808. doi: 10.1093/nar/10.23.7791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hocking J. D., Harris J. I. D-glyceraldehyde-3-phosphate dehydrogenase. Amino-acid sequence of the enzyme from the extreme thermophile Thermus aquaticus. Eur J Biochem. 1980 Jul;108(2):567–579. doi: 10.1111/j.1432-1033.1980.tb04752.x. [DOI] [PubMed] [Google Scholar]
- Hoshino T., Ikeda T., Tomizuka N., Furukawa K. Nucleotide sequence of the tetracycline resistance gene of pTHT15, a thermophilic Bacillus plasmid: comparison with staphylococcal TcR controls. Gene. 1985;37(1-3):131–138. doi: 10.1016/0378-1119(85)90265-3. [DOI] [PubMed] [Google Scholar]
- Ikai A. Thermostability and aliphatic index of globular proteins. J Biochem. 1980 Dec;88(6):1895–1898. [PubMed] [Google Scholar]
- Kagawa Y., Nojima H., Nukiwa N., Ishizuka M., Nakajima T., Yasuhara T., Tanaka T., Oshima T. High guanine plus cytosine content in the third letter of codons of an extreme thermophile. DNA sequence of the isopropylmalate dehydrogenase of Thermus thermophilus. J Biol Chem. 1984 Mar 10;259(5):2956–2960. [PubMed] [Google Scholar]
- Klibanov A. M. Stabilization of enzymes against thermal inactivation. Adv Appl Microbiol. 1983;29:1–28. doi: 10.1016/s0065-2164(08)70352-6. [DOI] [PubMed] [Google Scholar]
- Mas M. T., Resplandor Z. E., Riggs A. D. Site-directed mutagenesis of glutamate-190 in the hinge region of yeast 3-phosphoglycerate kinase: implications for the mechanism of domain movement. Biochemistry. 1987 Aug 25;26(17):5369–5377. doi: 10.1021/bi00391a023. [DOI] [PubMed] [Google Scholar]
- Merkler D. J., Farrington G. K., Wedler F. C. Protein thermostability. Correlations between calculated macroscopic parameters and growth temperature for closely related thermophilic and mesophilic bacilli. Int J Pept Protein Res. 1981 Nov;18(5):430–442. [PubMed] [Google Scholar]
- Merrett M. Primary structure of 3-phosphoglycerate kinase from horse muscle. II. Amino acid sequence of cyanogen bromide peptides CB1-CB4 and CB6-CB14, sequence of methionine-containing regions, and complete sequence of the enzyme. J Biol Chem. 1981 Oct 25;256(20):10293–10305. [PubMed] [Google Scholar]
- Messing J., Vieira J. A new pair of M13 vectors for selecting either DNA strand of double-digest restriction fragments. Gene. 1982 Oct;19(3):269–276. doi: 10.1016/0378-1119(82)90016-6. [DOI] [PubMed] [Google Scholar]
- Michelson A. M., Markham A. F., Orkin S. H. Isolation and DNA sequence of a full-length cDNA clone for human X chromosome-encoded phosphoglycerate kinase. Proc Natl Acad Sci U S A. 1983 Jan;80(2):472–476. doi: 10.1073/pnas.80.2.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori N., Singer-Sam J., Riggs A. D. Evolutionary conservation of the substrate-binding cleft of phosphoglycerate kinases. FEBS Lett. 1986 Aug 18;204(2):313–317. doi: 10.1016/0014-5793(86)80835-3. [DOI] [PubMed] [Google Scholar]
- Nojima H., Oshima T., Noda H. Purification and properties of phosphoglycerate kinase from Thermus thermophilus strain HB8. J Biochem. 1979 Jun;85(6):1509–1517. doi: 10.1093/oxfordjournals.jbchem.a132480. [DOI] [PubMed] [Google Scholar]
- Osinga K. A., Swinkels B. W., Gibson W. C., Borst P., Veeneman G. H., Van Boom J. H., Michels P. A., Opperdoes F. R. Topogenesis of microbody enzymes: a sequence comparison of the genes for the glycosomal (microbody) and cytosolic phosphoglycerate kinases of Trypanosoma brucei. EMBO J. 1985 Dec 30;4(13B):3811–3817. doi: 10.1002/j.1460-2075.1985.tb04152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins R. E., Conroy S. C., Dunbar B., Fothergill L. A., Tuite M. F., Dobson M. J., Kingsman S. M., Kingsman A. J. The complete amino acid sequence of yeast phosphoglycerate kinase. Biochem J. 1983 Apr 1;211(1):199–218. doi: 10.1042/bj2110199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perutz M. F., Raidt H. Stereochemical basis of heat stability in bacterial ferredoxins and in haemoglobin A2. Nature. 1975 May 15;255(5505):256–259. doi: 10.1038/255256a0. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Rosenberg M., Court D. Regulatory sequences involved in the promotion and termination of RNA transcription. Annu Rev Genet. 1979;13:319–353. doi: 10.1146/annurev.ge.13.120179.001535. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Barrell B. G., Smith A. J., Roe B. A. Cloning in single-stranded bacteriophage as an aid to rapid DNA sequencing. J Mol Biol. 1980 Oct 25;143(2):161–178. doi: 10.1016/0022-2836(80)90196-5. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shine J., Dalgarno L. The 3'-terminal sequence of Escherichia coli 16S ribosomal RNA: complementarity to nonsense triplets and ribosome binding sites. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1342–1346. doi: 10.1073/pnas.71.4.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Twigg A. J., Sherratt D. Trans-complementable copy-number mutants of plasmid ColE1. Nature. 1980 Jan 10;283(5743):216–218. doi: 10.1038/283216a0. [DOI] [PubMed] [Google Scholar]
- Walker J. E., Wonacott A. J., Harris J. I. Heat stability of a tetrameric enzyme, D-glyceraldehyde-3-phosphate dehydrogenase. Eur J Biochem. 1980 Jul;108(2):581–586. doi: 10.1111/j.1432-1033.1980.tb04753.x. [DOI] [PubMed] [Google Scholar]
- Wallace R. B., Shaffer J., Murphy R. F., Bonner J., Hirose T., Itakura K. Hybridization of synthetic oligodeoxyribonucleotides to phi chi 174 DNA: the effect of single base pair mismatch. Nucleic Acids Res. 1979 Aug 10;6(11):3543–3557. doi: 10.1093/nar/6.11.3543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson H. C., Walker N. P., Shaw P. J., Bryant T. N., Wendell P. L., Fothergill L. A., Perkins R. E., Conroy S. C., Dobson M. J., Tuite M. F. Sequence and structure of yeast phosphoglycerate kinase. EMBO J. 1982;1(12):1635–1640. doi: 10.1002/j.1460-2075.1982.tb01366.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson C. A., Hardman N., Fothergill-Gilmore L. A., Gamblin S. J., Watson H. C. Yeast phosphoglycerate kinase: investigation of catalytic function by site-directed mutagenesis. Biochem J. 1987 Jan 15;241(2):609–614. doi: 10.1042/bj2410609. [DOI] [PMC free article] [PubMed] [Google Scholar]



