Abstract
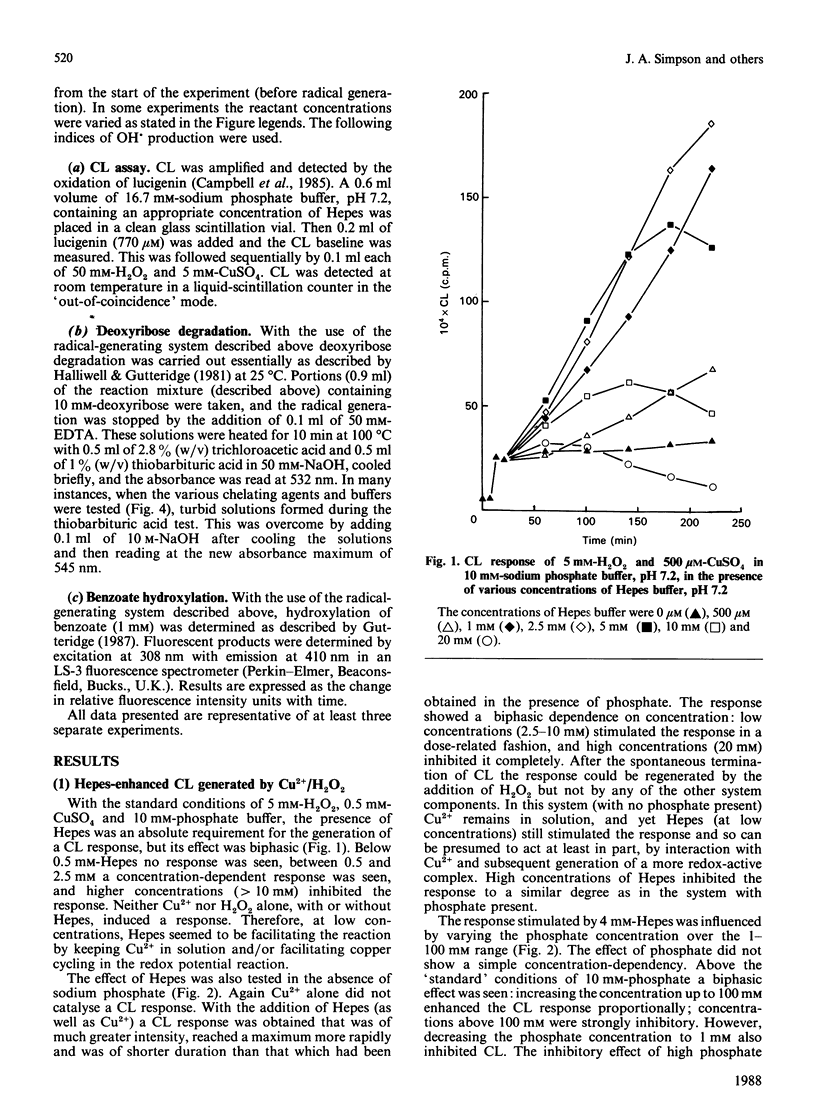
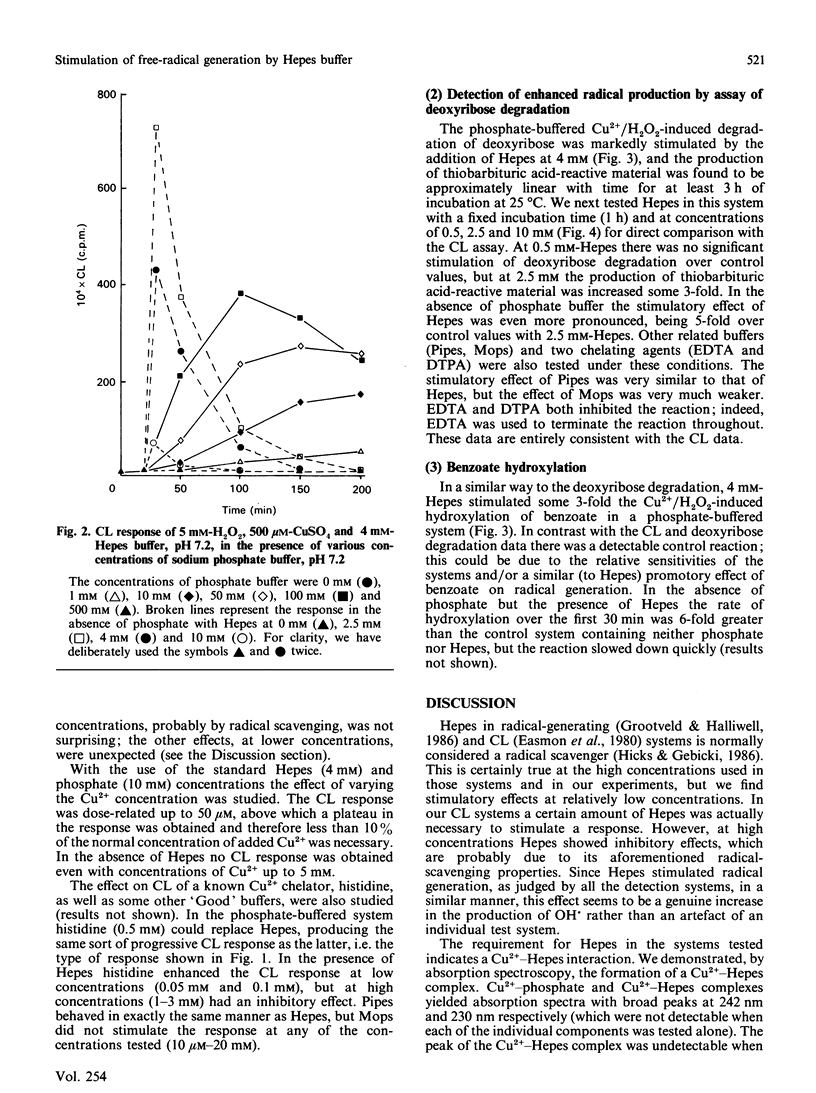
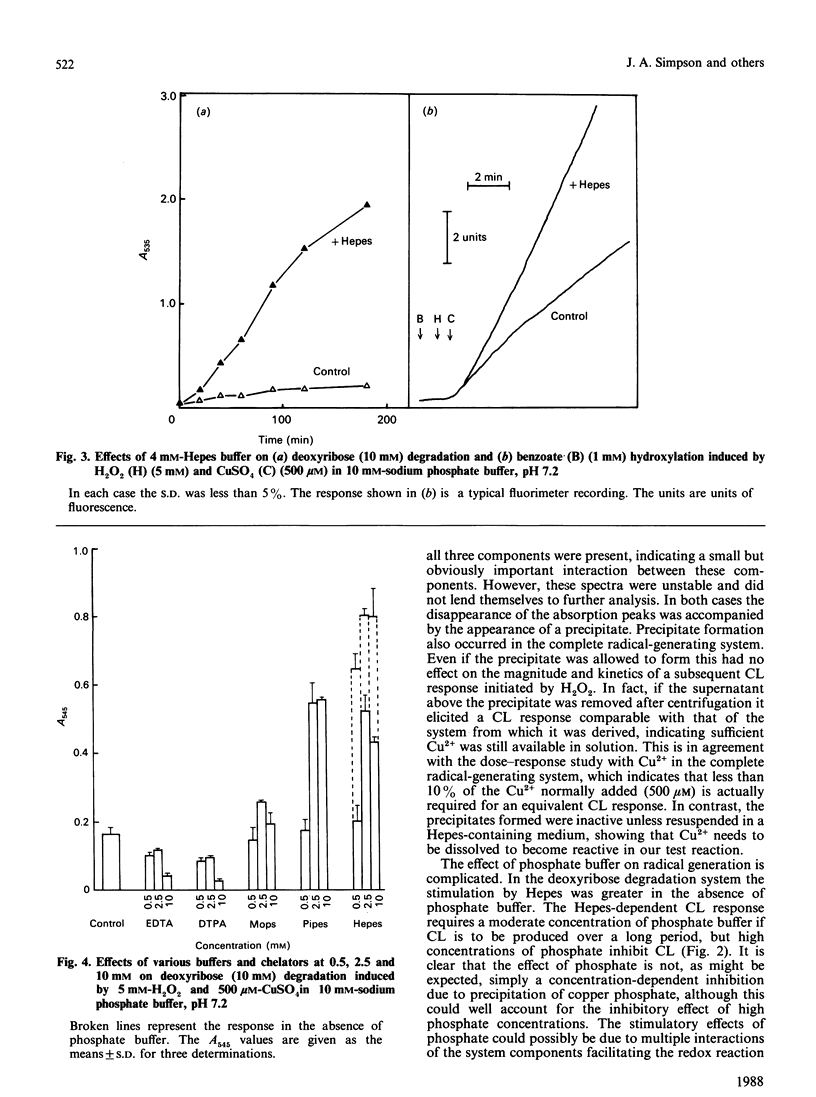
Hydroxyl radicals (OH.), generated by a phosphate-buffered Cu2+/H2O2 system, were detected by lucigenin-amplified chemiluminescence, deoxyribose degradation and benzoate hydroxylation. In each system the buffer, Hepes, was found to stimulate radical generation significantly. There are two main reasons for this effect: Hepes increases Cu2+ solubility in phosphate-buffered systems, and forms a complex with Cu2+ that is effective in generating OH. from H2O2. Pipes, a structurally similar buffer, and histidine, a known Cu2+ chelator, were found to have a similar effect. These data suggest that the crucial factor in such free-radical-generating systems is the availability of Cu2+, and that these actions of Hepes should be considered in the design of studies utilizing such systems.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Campbell A. K., Hallett M. B., Weeks I. Chemiluminescence as an analytical tool in cell biology and medicine. Methods Biochem Anal. 1985;31:317–416. doi: 10.1002/9780470110522.ch7. [DOI] [PubMed] [Google Scholar]
- Clark I. A., Cowden W. B., Hunt N. H. Free radical-induced pathology. Med Res Rev. 1985 Jul-Sep;5(3):297–332. doi: 10.1002/med.2610050303. [DOI] [PubMed] [Google Scholar]
- Dean R. T. A mechanism for accelerated degradation of intracellular proteins after limited damage by free radicals. FEBS Lett. 1987 Aug 17;220(2):278–282. doi: 10.1016/0014-5793(87)80829-3. [DOI] [PubMed] [Google Scholar]
- Easmon C. S., Cole P. J., Williams A. J., Hastings M. The measurement of opsonic and phagocytic function by Luminol-dependent chemiluminescence. Immunology. 1980 Sep;41(1):67–74. [PMC free article] [PubMed] [Google Scholar]
- Good N. E., Winget G. D., Winter W., Connolly T. N., Izawa S., Singh R. M. Hydrogen ion buffers for biological research. Biochemistry. 1966 Feb;5(2):467–477. doi: 10.1021/bi00866a011. [DOI] [PubMed] [Google Scholar]
- Graf E., Mahoney J. R., Bryant R. G., Eaton J. W. Iron-catalyzed hydroxyl radical formation. Stringent requirement for free iron coordination site. J Biol Chem. 1984 Mar 25;259(6):3620–3624. [PubMed] [Google Scholar]
- Grootveld M., Halliwell B. An aromatic hydroxylation assay for hydroxyl radicals utilizing high-performance liquid chromatography (HPLC). Use to investigate the effect of EDTA on the Fenton reaction. Free Radic Res Commun. 1986;1(4):243–250. doi: 10.3109/10715768609051634. [DOI] [PubMed] [Google Scholar]
- Gutteridge J. M. Ferrous-salt-promoted damage to deoxyribose and benzoate. The increased effectiveness of hydroxyl-radical scavengers in the presence of EDTA. Biochem J. 1987 May 1;243(3):709–714. doi: 10.1042/bj2430709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutteridge J. M., Wilkins S. Copper salt-dependent hydroxyl radical formation. Damage to proteins acting as antioxidants. Biochim Biophys Acta. 1983 Aug 23;759(1-2):38–41. doi: 10.1016/0304-4165(83)90186-1. [DOI] [PubMed] [Google Scholar]
- Halliwell B., Butt V. S. Flavin mononucleotide-sensitized photo-oxidation of glyoxylate in Good's buffers. Biochem J. 1972 Oct;129(5):1157–1158. doi: 10.1042/bj1291157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell B., Gutteridge J. M. Formation of thiobarbituric-acid-reactive substance from deoxyribose in the presence of iron salts: the role of superoxide and hydroxyl radicals. FEBS Lett. 1981 Jun 15;128(2):347–352. doi: 10.1016/0014-5793(81)80114-7. [DOI] [PubMed] [Google Scholar]
- Halliwell B., Gutteridge J. M. Oxygen free radicals and iron in relation to biology and medicine: some problems and concepts. Arch Biochem Biophys. 1986 May 1;246(2):501–514. doi: 10.1016/0003-9861(86)90305-x. [DOI] [PubMed] [Google Scholar]
- Halliwell B., Gutteridge J. M. Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem J. 1984 Apr 1;219(1):1–14. doi: 10.1042/bj2190001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt J. V., Simpson J. A., Dean R. T. Hydroperoxide-mediated fragmentation of proteins. Biochem J. 1988 Feb 15;250(1):87–93. doi: 10.1042/bj2500087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller-Peddinghaus R. In vitro determination of phagocyte activity by luminol- and lucigenin-amplified chemiluminescence. Int J Immunopharmacol. 1984;6(5):455–466. doi: 10.1016/0192-0561(84)90084-5. [DOI] [PubMed] [Google Scholar]
- Slater T. F. Free-radical mechanisms in tissue injury. Biochem J. 1984 Aug 15;222(1):1–15. doi: 10.1042/bj2220001. [DOI] [PMC free article] [PubMed] [Google Scholar]


