Abstract
Objective
To assess the impact of an intervention package on the prescription of antibiotic and subsequently the rate of clinical recovery for non-severe acute febrile illnesses at primary health centers.
Methods
Patients over 6 months of age presenting to primary health care centres with fever or history of fever within the past 7 days were randomized to receive either the intervention package constituted of point-of-care tests including COVID-19 antigen tests, a diagnostic algorithm and training and communication packages, or the standard practice. The primary outcomes were antibiotic prescriptions at Day 0 (D0) and the clinical recovery at Day 7 (D7). Secondary outcomes were non-adherence of participants and parents/caregivers to prescriptions, health workers’ non-adherence to the algorithm, and the safety of the intervention.
Results
A total of 1098 patients were enrolled. 551 (50.2%) were randomized to receive the intervention versus 547 (49.8%) received standard care. 1054 (96.0%) completed follow-up and all of them recovered at D7 in both arms. The proportion of patients with antibiotic prescriptions at D0 were 33.2% (183/551) in the intervention arm versus 58.1% (318/547) under standard care, risk difference (RD) -24.9 (95% CI -30.6 to -19.2, p < 0.001), corresponding to one more antibiotic saved every four (95% CI: 3 to 5) consultations. This reduction was also statistically significant in children from 6 to 59 months (RD -34.5; 95% CI -41.7 to -27.3; p < 0.001), patients over 18 years (RD -35.9; 95%CI -58.5 to -13.4; p = 0.002), patients with negative malaria test (RD -46.9; 95% CI -53.9 to -39.8; p < 0.001), those with a respiratory diagnosis (RD -48.9; 95% CI -56.9 to -41.0, p < 0.001) and those not vaccinated against COVID-19 (-24.8% 95%CI -30.7 to -18.9, p-value: <0.001). A significant reduction in non-adherence to prescription by patients was reported (RD -7.1; 95% CI -10.9 to -3.3; p < 0.001).
Conclusion
The intervention was associated with significant reductions of antibiotic prescriptions and non-adherence, chiefly among patients with non-malaria fever, those with respiratory symptoms and children below 5 years of age. The addition of COVID-19 testing did not have a major impact on antibiotic use at primary health centers.
Trial registration
Clinitrial.gov; NCT04081051 registered on 06/09/2019.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12879-024-09787-y.
Keywords: Antibiotic prescription, COVID-19, Point-of-care tests, Respiratory tract infection, Acute fever
Background
In resource-limited settings, most cases of acute febrile illnesses (AFIs) presenting to primary health care are managed empirically according to guidelines based on clinical signs and symptoms and a very limited arsenal of diagnostic tests [1]. The aetiologies of these AFIs vary from viruses, bacteria, fungi or parasites [2–4]. Unfortunately, AFIs commonly present with non-specific signs and symptoms making accurate clinical and etiological diagnosis challenging. This is compounded by the lack of accurate, easy-to-use tools to support clinical diagnosis at primary health care, except malaria for which rapid diagnostic tests (RDT) are widely available [5–7].
With the decrease of malaria burden due to the successful control efforts rolled-out in endemic settings such as sub-Saharan Africa (SSA) countries, the inappropriate prescription of antimalarials pointed out during the pre-RDT era has been replaced by the inappropriate prescription of antibiotics in settings without biomedical laboratory facilities, regardless of malaria RDT results [8, 9]. The efficacy of these antibiotics is limited and their over prescription without biological evidence threaten their effectiveness on the successful treatment of bacterial infections. This situation has exacerbated with the outbreak of COVID-19 in many countries, due to the non-specific signs and symptoms of this disease [10–12]. Unfortunately, antibiotic treatments are known to be ineffective for viral aetiologies, including COVID-19.
In SSA, the overuse (or misuse) of antibiotics had been documented before the COVID-19 pandemic [9, 13]. To achieve the Sustainable Development Goal 3 (SDG3), there has been increasing interest in point-of-care (PoC) tests that can be implemented in low- and middle-income countries (LMICs) during the last decade. Studies conducted in SSA showed that intervention packages including point of care (PoC) tests and/or training of communication component had a positive effect on the management of febrile diseases [14]. In a previous set of studies conducted in SSA under the same overall programme coordinated by FIND [15], a package of interventions including PoC tests, a diagnostic algorithm, and communication showed a significant reduction in antibiotic prescriptions without compromising clinical outcomes. These effects were particularly marked at our study site [16] however, effects were heterogenous across participating countries [17].
To the best of our knowledge, the real burden of COVID-19 in outpatients in rural areas in SSA has not been addressed, leaving a knowledge gap on the impact of the pandemic on standard practice such as antibiotic prescription at primary health centers. The COVID-19 pandemic, added to the burden of other febrile diseases, leaving clinicians and healthcare workers with a daily treatment dilemma of febrile diseases in the field. Building upon the findings of our previous study [16], the present study aimed to assess the impacts of an intervention package of PoC tests including SARS-CoV-2 antigen test and a diagnostic algorithm on the management of non-severe AFIs.
Methods
Study site
This study is part of the second phase of the AMR Diagnostics Use Accelerator project aimed to assess the impact of a package consisting of diagnostic tools, clinical algorithm, and training and communication on antibiotic prescriptions and subsequently clinical outcome, compared to standard care in patients with acute non-severe fever attending the outpatient clinics [18]. The medical centre Saint Louis of Temnaore and the health facility of Pella were included in the study. Malaria transmission is holoendemic and occurs mainly during the raining season from June-July to October-November.
Study design and participants
Study design
This was a prospective, comparative, open label, two-arm, randomized-controlled trial. The study was conducted from February to September 2022. All participants aged over 6 months presenting at the outpatients clinics of the selected recruitment sites with fever (axillary temperature ≥37.5ºC or history of fever within the past 7 days) with no focus or with a suspected respiratory infection according to clinical presentation, were invited to participate in the study after the provision of informed consent [18]. For participants under 18 years, the consent form was signed by the parents/legal guardians. However, an assent was obtained from children over 12 years.
The study was approved by the Burkina Faso national ethical committee for health research (DELIBERATION N°2020-01-010) and the institutional ethical committee for research in health sciences (N/Réf. A09-2029/CEIRES) in Burkina Faso, as well as by the Oxford Tropical Research Ethical Committee (OxTREC Reference: 52 − 19) in the United Kingdom (UK).
Inclusion criteria
The inclusion criteria were defined as follows: outpatients participants of both sexes, aged over 6 months, with fever or history of fever within the past 7 days with no focus or with a suspected respiratory tract infections. Additionally, patients or parents/caregivers who were willing to provide the required biological samples for testing with PoC tests as detailed in the study protocol, adhere to study procedures and come back to the recruitment sites on day 7 (± 2 days) for the follow-up visits were invited to sign the informed consent form.
Randomization and assessment
All the eligible participants consenting to participate to the study were randomized either the intervention arm or the standard care arm. Participants were randomized in the ratio 1:1 in block sizes of 32, 48 or 64 participants. All the participants of both arms enrolled at Day 0 were followed up on Day 7 to assess clinical outcome (clinical recovery) and adherence to the prescription. Adherence to prescriptions at Day 0 was assessed both qualitatively through patient interviews and quantitatively using pill counts. If 90% or more of the expected pills were taken, the patient was considered adherent. During this 7-day period, participants were asked to return to the sites if there was no improvement. The clinical recovery was defined as follows: being alive, with no fever, recovered from day 0 symptoms.
The study hypothesis is that the intervention reduces antibiotics prescription at the time the patients presents to the clinic with fever at Day 0, which subsequently results to better clinical outcome than standard that at Day7 visit. Primary outcomes were le proportion of outpatients receiving antibiotics at Day 0 and the proportion of those recovery at the Day 7 visit. The secondary outcomes were the non-adherence to antibiotics prescriptions at Day 0 and the rate of adverse events and sever adverse events occurring during the follow-up in both arms.
The individual randomization codes were generated by FIND data-management team and sent to CRUN data management team for printing and placing in envelopes. The allocation of randomization codes were made by study team (nurses) in chronological order by allocated each sequence number to the subsequent participant.
Interventions
Intervention package: PoC tests
The descriptions of the intervention packages and the standard practice at the health centers in Burkina Faso have been already described elsewhere [16]. In addition to malaria RDT detecting PfHRP2, the PoC tests implemented in the intervention arm are summarized as follows: pathogen-specific PoC tests such as Influenzae A/B/A (H1N1), Respiratory Syncytial Virus (RSV) antigen test, Group A Streptococcus (GAS), Streptococcus pneumoniae antigen test, dengue NS1/IgG/IgM test, and typhoid IgM test, and non-pathogen specific PoC tests such as such as white blood cell total and differential count (WBC/diff), C-reactive protein (CRP) and urine leukoesterase and urine nitrite test. In addition to these PoC described, a SARS-CoV-2 antigen test was also implemented systematically to all patients in intervention arm. For children under 5 years of age who fulfilled the WHO clinical definition of pneumonia [19], an antibiotic (amoxicillin) was systematically prescribed without further testing.
Intervention: clinical diagnostic algorithm
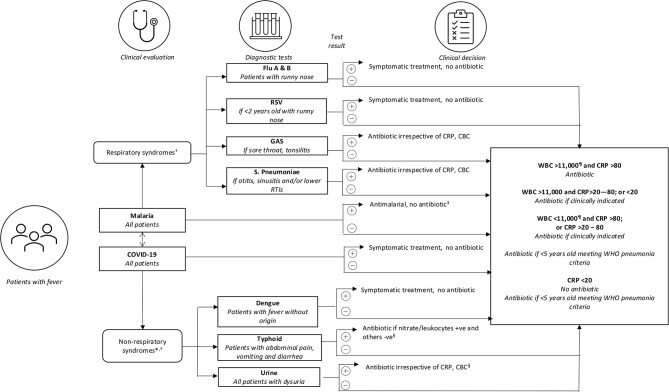
The diagnostic algorithm used in the first phase of the AMR Diagnostic Use Accelerator study [16] was adapted to include results of SARS-CoV-2 testing (see Fig. 1). Briefly, for each patient, the choice of PoC tests were based on the presentation and presumptive diagnosis, whether respiratory or non-respiratory. Patients with respiratory infection symptoms were tested for common respiratory pathogens with GAS, S. pneumoniae, influenza A/B (H1N1) and RSV (if children < 2years) antigenic PoC tests. Dengue NS1/IgM/IgG, typhoid IgG were tested in those with non-respiratory presumptive diagnoses. Malaria and SARS-Cov-2 were tested in all participants in the intervention arm irrespective of presentation, urine leukoesterase and nitrites in all participants with dysuria, and CRP and WBC total and differential counts in all participants who were negative on any of the bacterial PoC antigen tests.
Fig. 1.
Electronic clinical decision aid in intervention arm
Intervention package: training and communication package
Based on the findings on training and communication package implemented during the previous study and reported by Compaore et al., highlighting the factors influencing the adherence to healthcare workers’ prescription [20], study nurses in intervention arm conducted short interviews with participants and parents/legal guardians about their normal experiences of taking antibiotics. This information collected was used by nurses to personalize the prescription adherence message given to the participants and parents/legal guardians at Day 0 at the recruitment health centers. Social scientists followed up with participants and parents/legal guardians to check if they understood the prescription messages.
On the Day 7 visit, participants and parents/legal guardians self-reported their adherence to the prescription during the clinical follow-up and a pill count was taken to assess their adherence. For the sensibility assessment of the adherence to prescription, the patient’s antibiotic intake in accordance with the prescription status and the 90% pill count criteria was used.
Standard care
In the standard practice arm, children under 5 years were managed by the integrated e-diagnostic approach (IeDA) rolled-out by the NGO (non-governmental organization) Terre des Hommes (TdH) [21]. This management approach was based on the IMCI (integrated management of childhood illness) guideline. For patients over 5 years of age, the management was based on diagnostic and treatment guideline based on IMAI (integrated management of adolescent and adult illness) [22]. Malaria RDT remained the only diagnostic tests available to diagnose fever episodes at primary health centers. For the purpose of the study, the COVID-19 test was performed only on the request of the health facilities’ nurses, in case of suspicion of COVID-19.
In both the intervention and standard care arms, adverse events (AEs) or serious adverse events (SAE) reports were based on Division of AIDS (DAIDS) Table for Grading the severity of adult and Paediatric Adverse Events [23]. Adverse event was defined as any undesirable experience associated with the use of the medical product (intervention package) within the 7 days of follow-up. The SAE refers to the AE that results in any of the following outcomes: death, life-threatening adverse event, requires inpatients hospitalization.
Sample size and statistical analysis
The calculation of the sample size was based on the antibiotic prescription rate observed in control arm of the study during the first phase of the AMR Diagnostics Use Accelerator study [16] which is 60% at the time. The study was powered to detect a 30% relative reduction in antibiotic prescriptions by the intervention at 6% precision of the estimation of the measured reduction, a 5% significance level and 80% power. By considering 5% loss of follow-up, 549 participants were recruited per arm, i.e. 1098 in both arms. A statistical analysis plan (SAP) was developed prior to study completion. In the SAP, the estimates of the study outcomes are calculated as proportions based on the definitions of the outcomes to be reported with their 95%-confidence intervals. Analysis was to be performed independently for each country including Burkina Faso. The analysis datasets were defined as follows: Enrolled/Intention-to-Test- all subjects who signed the informed consent form (ICF); Modified Intention to Test- all participants with partial data (but not fully compliant with the protocol; Evaluable/Per-Protocol population- all participants without major protocol deviations (fully complied with the protocol).
The data analysis was performed using SAS software, version 9.4. Analyses were conducted on the per-protocol population (participants who completed the day 7 visit). Sub-group analyses (such as sex, age group, respiratory and malaria diagnostic, and COVID-19 vaccination status) were also performed. Quantitative continuous data are summarised by using means with standard deviations or medians with interquartile ranges. Qualitative data are reported as absolute values and percentages. The primary outcomes were defined as the proportion of patients prescribed antibiotics at day 0 and patients who recovered at day 7. The primary outcomes (clinical outcome after the follow-up of day 7 and antibiotic prescription at day 0) were compared between the two arms and reported in term of relative (risk ratio [RRR]) and absolute (risk difference [RD]) effects, with 95% confidence interval (95% CI); numbers needed to test were calculated as 1/absolute RD, with 95% CI. Asymptotic test for equality was used to compare the proportions. Individual comparison´s significance level has been set to 0.05 (i.e. stat. significance of between-arm difference being concluded in case of individual p-value being ≤ 0.05). No formal adjustment for multiple testing has been done. Individual subgroup specific results (although presented along with p-values) were primarily meant to provide further insight to possible strata-specific prescription patterns. For the assessment of the sensitivity of the adherence to the prescription, a combination of social science interviews and pill count (over 90%) collected at day 7 visit was used.
Results
Participants recruitment and follow-up
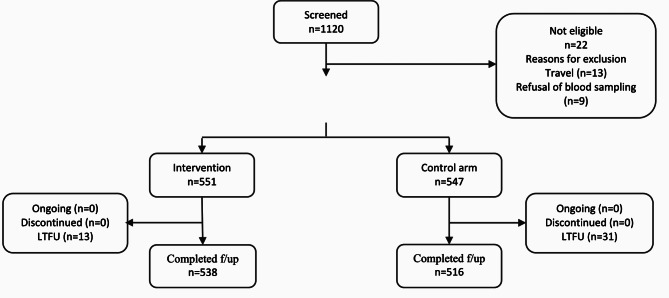
Between 24th February to 9th September 2022, a total of 1120 participants over 6 months of age, attending the recruitment sites with fever or history of fever, were screened and enrolled consecutively, and 1098 (98.0%) were enrolled. Of these participants enrolled, 551(50.2%) were randomized to the intervention arm and 547 (49.8%) to the standard practice arm (control arm). Twenty-two participants were not enrolled for the following reasons: travel (n = 13) and refusal of blood sampling (n = 9). The per-protocol (PP) population consisted of 96.0% (1054/1098) of participants randomized and distributed as follow: 538 (51.0%) participants in the intervention arm and 516 (49.0%) in the control arm. In all, 13 and 31 participants were lost to follow-up for the day 7 follow-up assessment visit, respectively in the intervention arm and the standard practice arm (Fig. 2). The baseline characteristics of the study population did not differ between arms (Table 1).
Fig. 2.
Study flow
Table 1.
Baseline characteristics for the study population in intervention and control arm (standard routine care)
| Characteristic | Total | Study arm | |
|---|---|---|---|
| Intervention | Control | ||
| Demographic | N (%) | n (%) | n (%) |
| Sex | |||
| Male | 543 (49.5) | 287 (52.1) | 256 (46.8) |
| Female | 555 (50.5) | 264 (47.9) | 291 (53.2) |
| Age group | |||
| < 5 years | 650 (59.2) | 327 (59.3) | 323 (59.0) |
| 5 to < 10 years | 269 (24.5) | 136 (24.7) | 133 (24.3) |
| 10 to < 18 years | 113 (10.3) | 53 (9.6) | 60 (11.0) |
| > 18 years | 66 (6.0) | 35 (6.4) | 31 (5.7) |
| Reason of consultation | |||
| Fever | 1098 (100) | 551 (100) | 547 (100) |
| Cough | 364 (33.2) | 168 (30.5) | 196 (35.8) |
| Sneezing and rhinorrhea | 375 (34.2) | 190 (34.5) | 185 (33.8) |
| Headache | 288 (26.2) | 160 (29.0) | 128 (23.4) |
| Abdominal pain | 318 (29.0) | 191 (34.7) | 127 (23.2) |
| Vomiting | 238 (21.7) | 124 (22.5) | 114 (20.8) |
| Sore Throat | 15 (1.4) | 14 (2.5) | 1 (0.2) |
| Ear discharge | 6 (0.5) | 2 (0.4) | 4 (0.7) |
| Diarrhea | 192 (17.5) | 82 (14.9) | 110 (20.1) |
| Dysuria | 4 (0.4) | 1 (0.2) | 3 (0.5) |
| Urinary frequency | 1 (0.1) | 0 (0.0) | 1 (0.2) |
| Loss of sense of smell | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Loss of sense of taste | 8 (0.7) | 8 (1.5) | 0 (0.0) |
| COVID-19 vaccination status | |||
| Fully vaccinated | 53 (4.8) | 27 (4.9) | 26 (4.8) |
| Partially vaccinated | 24 (2.2) | 16 (2.9) | 8 (1.5) |
| Not vaccinated | 1016 (92.5) | 508 (92.2) | 508 (92.9) |
| Unknown | 3 (0.3) | 0 (0.0) | 3 (0.5) |
| Not applicable | 2 (0.2) | 0 (0.0) | 2 (0.4) |
| Respiratory diagnosis | |||
| Yes | 416 (37.9) | 189 (34.3) | 227 (41.5) |
| No | 682 (62.1) | 362 (65.7) | 320 (58.5) |
| Malaria RDT | |||
| Done | 1097 (99.9 | 551 (100) | 546 (99.8) |
| Positive | 571 (52.1%) | 287(52.1) | 284 (52.0%) |
Results of point-of-care (PoC) tests in both arms
In the intervention arm, the PoC test was performed by research nurses. The required times for the management of participants in the intervention and standard practice arms have been already reported [16]. The required time for testing varied between 15 and 20 min in the intervention arm. For the treatment of participants, the required time was 20–30 min in the intervention arm versus 10–15 min in the control arm.
The rates of malaria test positivity, performed in both arms, were similar (51.5% in intervention arm versus 52.0% in standard care arm). Based on bacteria-specific PoC tests in the intervention arm, only 4.0% (22/551) of participants were positive and qualified for antibiotic treatments: 7.0% (20/284) of tested participants were positives to typhoid IgM test, 5.0% (1/20) to Group A Streptococcus antigen test and 10% (1/10) to Streptococcus pneumoniae antigen test. Based on virus-specific PoC tests, 5.4% (30/551) of participants were positive and did not qualify for antibiotic treatments: 4.3% (23/540) of tested participants were positive to SARS-CoV-2 antigen test, 3.4% (5/148) to Influenza A/B/A (H1N1) antigen test, 7.1% (2/28) to Dengue IgM test and 3.7% (1/27) to dengue NS-1Antigen test (Table 2).
Table 2.
Diagnostic test results in the study
| POCT | Intervention (N = 551) |
|
|---|---|---|
| Done, n (%) | Positive, n (%) | |
| Pathogen-specific POCTs | ||
| Malaria Pf/Pan Ag test | 551 (100) | 287 (52.1) |
| SARS-CoV-2 Ag test | 540 (98.0) | 23 (4.3) |
| Typhoid IgM test | 284 (51.5) | 20 (7.0) |
| Group A Strep Ag test | 20 (3.6) | 1 (5.0) |
| Influenza A/B Ag test | 148 (26.9) | 5 (3.4) |
| RSV Ag test | 49 (8.9) | 0 (0.0) |
| S. pneumoniae Ag test | 10 (1.8) | 1 (10.0) |
| Dengue IgM Ag test | 28 (5.1) | 2 (7.1) |
| Dengue NS-1 Ag test | 27 (4.9) | 1 (3.7) # |
| Non-pathogen specific tests | ||
| Done, n (%) | Median (Q1, Q3) | |
| C-Reactive protein [mg/L] | 517 (93.8%) | 28.6 (4.9, 84.0) |
| White Blood Cell counts [x109/L] | 517 (93.8%) | 8.8 (6.5, 11.6) |
| Neutrophil counts [%] | 517 (93.8%) | 55.0 (41.0, 66.0) |
#: The patient tested positive for Dengue NS-1 Ag test also is tested positive for Dengue IgM Ag test
Among participants randomized to the intervention arm, 43.7% (226/517) had a CRP value of < 20 mg/L and did not meet the cuff-off for antibiotic prescriptions, 30.8% (159/517) had a CRP value between 20 and 80 mg/L, and 25.5% (132/517) a CRP value over 80 mg/L. For white blood cell (WBC) counts, 29.6% (153/517) had a WBC count over 11,000 cells/µl and 8.5% (44/517) a neutrophils rate over 75% (Supplementary Table 1). The proportion of participants with a positive malaria test was 31.0% (70/226) for participants with CRP < 20 mg/L, 69.2% (110/159) for those with CRP value between 20 and 80 mg/L, and 77.3% (102/132) for those with CRP value over 80 mg/L.
Clinical outcomes and antibiotics prescription
During the day 7 follow-up visit, no case of unfavourable clinical outcomes was reported in either arm (100% recovered in both arms).
The intervention significantly reduced antibiotic prescription compared to standard practice: in the intervention arm, 33.2% (183/551) of participants were prescribed an antibiotic at day 0, versus 58.1% (318/547) in the standard practice arm: RRR 42.9% (95% CI 34.4 to 50.2), RD -24.9% (95% CI -30.6 to -19.2, p < 0.001), meaning that the intervention globally resulted in 1 fewer antibiotic prescription for every 4 patients tested. In subgroup analyses, antibiotic prescriptions were significantly reduced in patients with respiratory infection [RD -48.9% (95% CI -56.9 to -41.0, p < 0.001); RRR 53.9% (95% CI 45.2 to 61.3)] and in those who tested negative for malaria [RD -46.9% (95% CI -53.9 to -39.8, p < 0.001); RRR 52.7% (95% CI 45.2 to 59.2)]. This translates into 1 fewer antibiotic prescription every 2 consultations for patients with respiratory diagnosis and for those who tested negative for malaria. Regarding the COVID-19 vaccination status, the reduction of antibiotic prescription was very similar for all the vaccination status. Statistically significant difference in reduction is, however, only observed for unvaccinated patients [RD -24.8% (95% CI -30.7 to -18.9; p < 0.001)] due to the small number of patients in the other groups. The reduction of antibiotic prescriptions was also statistically significant in children aged 6–59 months [RRR 50.8% (95% CI 41.7 to 58.6); RD -34.5% (95% CI -41.7 to -27.3, p < 0.001)] and those over 18 years [RRR 55.7% (95% CI 20.5 to 75.3); RD -35.9% (95%CI -58.5 to -13.4, p = 0.002)], meaning that the intervention resulted in 1 fewer antibiotic prescription very 3 consultations in both groups. Unfortunately, this reduction was not statistically significant for those tested positive for malaria [RD -4.5% (95% CI -11.8 to 2.0, p = 0.228)] and those with non-respiratory infections symptoms [RD -6.3% (95% CI -13.3 to 0.7, p = 0.079);]. For the other age groups, the analysis shows reductions of antibiotic prescriptions that are not statistically significant (Tables 3 and 4, and supplementary Table 2).
Table 3.
Antibiotic prescription at Day 0 in intervention and control arms
| Characteristic | Overall | Intervention arm | Control arm | Absolute Risk Difference | p-value | |||
|---|---|---|---|---|---|---|---|---|
| n/N (%) | 95% CI | n/N (%) | 95% CI | n/N (%) | 95% CI | 95% CI | ||
| All | 501/1098 (45.6) | [42.7, 48.6] | 183/551 (33.2) | [29.4, 37.2] | 318/547 (58.1) | [54.0, 62.2] | -24.9% [-30.6, -19.2] | < 0.001 |
| Sex | ||||||||
| Male | 253/543 (46.6) | [42.4, 50.8] | 94/287 (32.8) | [27.6, 38.4] | 159/256 (62.1) | [56.0, 67.8] | -29.4% [-37.4, -21.3] | < 0.001 |
| Female | 248/555 (44.7) | [40.6,48.8] | 89/264 (33.7) | [28.3, 39.6] | 159/291 (54.6%) | [48.9, 60.3] | -20.9% [-29.0, -12.9] | < 0.001 |
| Age group | ||||||||
| < 5 years | 328/650 (50.5) | [46.6, 54.3] | 109/327 (33.3) | [28.4, 38.6] | 219/323 (67.8) | [62.5, 72.7] | -34.5% [-41.7, -27.3] | < 0.001 |
| 5 to < 10 years | 102/269 (37.9) | [32.3, 43.8] | 48/136 (35.3) | [27.8, 43.6] | 54/133 (40.6) | [32.6, 49.1] | -5.3% [-16.9, 6.3] | 0.369 |
| 10 to < 18 years | 41/113 (36.3) | [28.0, 45.5] | 16/53 (30.2) | [19.5, 43.5] | 25/60 (41.7) | [30.1, 54.3] | -11.5% [-29.0, 6.1] | 0.200 |
| > 18 years | 30/66 (45.5) | [34.0, 57.4] | 10/35 (28.6) | [16.3, 45.1] | 20/31 (64.5) | [46.9, 78.9] | -35.9% [-58.5, -13.4] | 0.002 |
| COVID-19 vaccination status | ||||||||
| Fully vaccinated | 30/53 (56.6) | [43.3, 69.0] | 12/27 (44.4) | [27.6, 62.7] | 18/26 (69.2) | [50.0, 83.5] | -24.8% [-50.6, 1.0] | 0.060 |
| Partially vaccinated | 8/24 (33.3) | [18.0, 53.3] | 4/16 (25.0) | [10.2, 49.5] | 4/8 (50.0) | [21.5, 78.5] | -25.0% [-65.6, 15.6] | 0.228 |
| Not vaccinated | 460/1016 (45.3) | [42.2, 48.3] | 167/508 (32.9) | [28.9, 37.1] | 293/508 (57.7) | [53.3, 61.9] | -24.8% [-30.7, -18.9] | < 0.001 |
| Unknown status | 3/5 (60.0) | [23.1, 88.2] | - | - | 3/5 (60.0) | [23.1, 88.2] | - | - |
| Diagnosis | ||||||||
| Respiratory diagnosis | 285/416 (68.5) | [63.9, 72.8] | 79/189 (41.8) | [35.0, 48.9] | 206/227 (90.7) | [86.3, 93.9] | -48.9% [-56.9, -41.0] | < 0.001 |
| Non-respiratory diagnosis | 216/682 (31.7) | [28.3, 35.3] | 104/362 (28.7) | [24.3, 33.6] | 112/320 (35.0) | [30.0, 40.4] | -6.3% [-13.3, 0.7] | 0.079 |
| Malaria test result | ||||||||
| Malaria test negative | 344/526 (65.4) | [61.2, 69.3] | 111/264 (42.0) | [36.2, 48.1] | 233/262 (88.9) | [84.6, 92.2] | -46.9% [-53.9, -39.8] | < 0.001 |
| Malaria test positive | 156/571 (27.3) | [23.8, 31.1] | 72/287 (25.1) | [20.4, 30.4] | 84/284 (29.6) | [24.6, 35.1] | -4.5% [-11.8, 2.8] | 0.228 |
Table 4.
Summary outcomes: relative and absolute effects on antibiotic prescriptions
| ATB prescribed | Effect | ||||
|---|---|---|---|---|---|
| Intervention n/N (%) |
Control n/N (%) |
Relative Risk Reduction [95%CI] |
Absolute (Risk Difference) [95%CI] |
Numbers needed to test to prevent one more antibiotic prescription [95%CI] |
|
| Overall | 183/551 (33.2) | 318/547 (58.1) | 42.9% reduction [from 34.4 to 50.2] | 25 fewer ATB prescriptions per 100 (from 19 to 31) | 1 fewer ATB prescription every 4 patients tested (from 5 to 3) |
| Respiratory | 79/189 (41.8) | 206/227 (90.7) | 53.9% reduction [from 45.2 to 61.3] | 49 fewer ATB prescriptions per 100 (from 41 to 57) | 1 fewer ATB prescription every 2 patients tested (from 2 to 2) |
| Malaria negative | 111/264 (42) | 233/262 (88.9% | 52.7% reduction [from 45.2 to 59.2] | 47 fewer ATB prescriptions per 100 (from 40 to 54) | 1 fewer ATB prescription every 2 patients tested (from 3 to 2) |
| Age under 5 years | 109/327 (33.3) | 219/323 (67.8) | 50.8% reduction [from 41.7 to 58.6] | 34 fewer ATB prescriptions per 100 (from 27 to 42) | 1 fewer ATB prescription every 3 patients tested (from 4 to 2) |
| Age over 18 years | 10/35 (28.6) | 20/31 (64.5) |
55.7% reduction (from 20.5 to 75.3) |
35.9 fewer ATB prescriptions per 100 (from 13.4 to 58.5%) |
1 fewer ATB prescription every 3 patients tested (from 7 to 2)) |
The details of antibiotic prescriptions by CRP and WBC groups and malaria RDT results are reported in Tables 5 and 6. Antibiotic prescription increased with WBC counts (23.4% and 43.1% respectively for counts < 11,000 and ≥ 11,000), neutrophil counts (24.5% and 79.5% for counts < 75% and ≥ 75%), and CRP values (from 20.8 to 31.4 to 40.9% respectively for CRP values < 20, 20–80 and > 80 mg/L) (Table 5). Considering also the malaria test, there was no statistically significant difference in antibiotic prescriptions when CRP was < 20 mg/L, whereas patients with a malaria negative test were prescribed antibiotics more than those with a positive test when CRP was < 20 mg/L (Table 6).
Table 5.
Antibiotics prescriptions by CRP value and white blood cells and neutrophil counts
| Test | n tested/N total (%) | n ATB prescribed /N tested (%) | 95% CI |
|---|---|---|---|
| CRP < 20 | 226/517 (43.7) | 47/226 (20.8) | [16.0, 26.6] |
| CRP 20 to 80 | 159/517 (30.8) | 50/159 (31.4) | [24.7, 39.0] |
| CRP > 80 | 132/517 (25.5) | 54/132 (40.9) | [32.9, 49.4] |
| WBC < 11,000 | 364/517 (70.4) | 85/364 (23.4) | [19.3, 28.0] |
| WBC ≥11,000 | 153/517 (29.6) | 66/153 (43.1) | [35.6, 51.1] |
| Neutrophils < 75% | 473/517 (91.5) | 116/473 (24.5) | [20.9, 28.6] |
| Neutrophils ≥75% | 44/517 (8.5) | 35/44 (79.5) | [65.5, 88.8] |
Table 6.
Antibiotics prescriptions by CRP value and malaria RDT results
| CRP result | Test result n/N (%) |
Overall antibiotic prescriptions n/N (%) |
Malaria RDT-negative | Malaria-RDT positive | Absolute risk reduction | |
|---|---|---|---|---|---|---|
| ATB prescribed n/N [%; 95CI] |
ATB prescribed n/N [%; 95CI] |
95% CI | p-value* | |||
| < 20 | 226/517 (43.7) | 47/226 (20.8) | 37/156 [23.7; 17.7, 31.0] | 10/70 [14.3; 7.9, 24.3] | 9.4 [87.6, 315] | 0.080 |
| 20–80 | 159/517 (30.8) | 50/159 (31.4) | 23/49 [46.9; 33.7, 60.6] | 27/110 [24.5; 17.5, 33.4] | 22.4 [123, 298] | 0.006 |
| > 80 | 132/517 (25,5) | 54/132 (40.9) | 22/30 [73.3; 55.6, 85.8] | 32/102 [31.4; 23.2, 40.9] | 42.0 [163, 335] | < 0.001 |
*: p-values are related to within-CRP group comparison between prescription rates (malaria RDT positive vs. negative cases)
Non-adherence to antibiotic prescription at Day0
Non-adherence to antibiotic prescriptions refers to participants who do not follow the prescription instructions given to them at the Day 0 visit (they do not buy or obtain the prescribed medicine, take it for the prescribed duration, frequency or dose), or, if they are not prescribed an antibiotic, an antibiotic is taken. This has been assessed at day 7 visit. Non-adherence of patients/caregiver to antibiotic prescription is reported in Table 7. In the intervention arm, 7.8% (42/536) did not adhere to the training and communication package versus 14.9% (77/516) in the standard arm: RD: -7.1% (-10.9 to -3.3; p < 0.001). Specifically, non-adherence of patients or parents/caregivers was significantly reduced in intervention arm in patients with respiratory diagnosis [RD -10.8% (95% CI -18.4 to -3.1, p = 0.006)] and in those who tested negative for malaria [RD -12.2% (95% CI -18.4 to -5.7, p < 0.001)]. Regarding the COVID-19 vaccination status, non-adherence was significant reduced in the intervention arm in patients who received the vaccine [RD -28.0% (95% CI -45.6 to -10.4; p = 0.002)] and unvaccinated patients [RD -6.0% (95% CI -10.0 to -2.0; p = 0.003)]. A fewer non-adherence in intervention arm for partially vaccinated subgroup was reported, but non-statistically significant due to sample size.
Table 7.
Non-adherence of patients/care giver to antibiotic prescription at Day 0 in intervention and control arms
| Characteristic | Overall | Intervention arm | Control arm | Risk difference | p-value | |||
|---|---|---|---|---|---|---|---|---|
| n/N (%) | 95% CI | n/N (%) | 95% CI | n/N (%) | 95% CI | % [95% CI] | ||
| All | 119/1052 (11.3) | [9.5, 13.4] | 42/536 (7.8) | [5.8, 10.4] | 77/516 (14.9) | [12.1, 18.3] | -7.1 [-10.9, -3.3] | < 0.001 |
| Age group | ||||||||
| < 5 years | 76/625 (12.2) | [9.8, 15.0] | 26/321 (8.1) | [5.6, 11.6] | 50/304 (16.4) | [12.7, 21.1] | -8.3 [-13.5, -3.2] | 0.001 |
| 5 to < 10 years | 24/260 (9.2) | [6.3, 13.4] | 12/133 (9.0) | [5.2, 15.1] | 12/127 (9.4) | [5.5, 15.8] | -0.4% [-7.5, 6.6] | 0.906 |
| 10 to < 18 years | 10/106 (9.4) | [5.2, 16.5] | 4/51 (7.8) | [3.1, 18.5] | 6/55 (10.9) | [5.1, 21.8] | -3.1 [-14.1, 8.0] | 0.587 |
| > 18 years | 9/61 (14.8) | [8.0, 25.7] | 0/31 (0.0) | [0.0, 11.0] | 9/30 (30.0) | [16.7, 47.9] | -30.0 [-46.4, -13.6] | < 0.001 |
| COVID-19 vaccination status | ||||||||
| Fully vaccinated | 7/50 (14.0) | [7.0, 26.2] | 0/25 (0.0) | [0.0, 13.3] | 7/25 (28.0) | [14.3, 47.6] | -28.0 [-45.6, -10.4] | 0.002 |
| Partially vaccinated | 1/22 (4.5) | [0.8, 21.8] | 0/14 (0.0) | [0.0, 21.5] | 1/8 (12.5) | [2.2, 47.1] | -12.5 [-35.4, 10.4] | 0.285 |
| Not vaccinated | 111/975 (11.4) | [9.5, 13.5] | 42/497 (8.5) | [6.3, 11.2] | 69/478 (14.4) | [11.6, 17.9] | -6.0 [-10.0, -2.0] | 0.003 |
| Unknown status | 0/5 (0.0) | [0.0, 43.4] | - | - | 0/5 (0.0) | [0.0, 43.4] | - | - |
| Diagnosis | ||||||||
| Respiratory diagnosis | 79/397 (19.9) | [16.3, 24.1] | 26/184 (14.1) | [9.8, 19.9] | 53/213 (24.9) | [19.6, 31.1] | -10.8 [-18.4, -3.1] | 0.006 |
| Non-respiratory diagnosis | 40/655 (6.1) | [4.5, 8.2] | 16/352 (4.5) | [12.8, 7.3] | 24/303 (7.9) | [5.4, 11.5] | -3.4 [-7.1, 0.4] | 0.077 |
| Malaria test result | ||||||||
| Malaria test negative | 83/498 (16.7) | [13.7, 20.2] | 27/253 (10.7) | [7.4, 15.1] | 56/245 (22.9) | [10.0, 28.5] | -12.2 [-18.7, -5.7] | < 0.001 |
| Malaria test positive | 36/553 (6.5) | [4.7, 8.9] | 15/283 (5.3) | [3.2, 8.6] | 21/270 (7.8) | [5.1, 11.6] | -2.5 [-6.6, 1.6] | 0.239 |
In the intervention arm, the rate of non-adherence was also significantly reduced in children under 5 years of age [RD -8.3% (95% CI -13.5 to -3.2; p = 0.001)] and patients over 18 years of age [RD -30.0% (95% CI -46.4 to -13.6; p < 0.001)].
Discussion
This study demonstrates the value of the intervention package combining point-of-care diagnostic tests, training and communication, and clinical diagnostic algorithm for the management of uncomplicated acute febrile diseases and SAR-CoV-2 in patients older than 6 months. The intervention resulted in one fewer antibiotic prescription for every four patients tested, with no untoward effect on clinical outcomes, compared to standard practice. Findings are aligned with those of the previous study conducted during the first phase of the study in 2020 to 2021, [16], in which the intervention resulted in one fewer prescription for every six patients. The present study introduced SARS-CoV antigen testing and extended the study population to include adults. Also consistent with the previous study are significant reductions in patients presenting with respiratory symptoms (respiratory diagnosis), those with a negative malaria tests, and children under 5 years of age. This age category accounted for almost 60% of all visits at the recruitment sites for fever or history of fever and represented 69% and 63% of antibiotic prescriptions respectively in standard practice in the phase 2 and 1, despite the implementation of IeDA (integrated e-diagnostic approach) in this age group. This, despite several studies having reported that more than half of febrile children attending the outpatient clinics for healthcare with acute respiratory symptoms such acute bronchitis do not require antibiotics [1, 24, 25]. Although the implementation of IeDA as part of standard care in children under 5 years of age has improved the prescription of antibiotics compared to the pre-IeDA era (antibiotic prescription: 78%) [13], overuse of antibiotics is still common at primary health centres due to the lack of practical tools to identify bacterial infections prior to the prescription of antibiotics [9, 13].
Here we also confirm that this intervention has no effect on antibiotic prescriptions in patients with non-respiratory presentation and those who test positive for malaria. For non-respiratory presentations, this expresses the clinical grey area around fever with no focus in the absence of diagnostic tools to inform case management, and the uncertainties about CRP cut-off values below which bacterial infections are unlikely and antibiotics should not be prescribed. As CRP levels tend to be increased by malaria, CRP also becomes less useful in patients with a positive malaria test.
During the last two decades, the global antibiotics consumption increased by around 70% and should be much higher in 2030 if nothing is done [26–29]. This increase was primarily driven by increased consumption in low and middle-income countries (LMICs) [26, 30], including SSA, due to the burden of infectious diseases in these areas and the lack of laboratory facilities, practical tools or PoC tests for their correct diagnostic, except for malaria [31, 32]. The relationship between overuse of antibiotics and spread of antimicrobial resistance is well documented [33–35].
COVID-19 did not have a major impact on antibiotics use in outpatients at primary health centres, and only one in 25 patients presenting with acute fever had a positive SARS-CoV-2 antigen tests at the time of this study.
The global significant reductions of non-adherence to antibiotic prescription reported in this study due to the implementation of intervention package is an important factor in the roll-out of this intervention (PoC tests and training and communication package) in term of clinical outcome and antibiotic prescriptions at community level. These findings suggested that the intervention package could support adherence of patients to this approach based on clinical and biological evidences.
The significant reductions of non-adherence and antibiotic prescription were both reported in children under 5 years and adult over 18 years of age (even less represented), unvaccinated patients (regarding the COVID-19 vaccination status), patients with respiratory diagnostic and those tested negative for malaria. This observation suggests that participant in intervention arm where antibiotic prescriptions are significantly reduced are more likely to follow healthcare prescription as reported in our previous study [16].
The study has some limitations such the potential contamination of arms. Indeed, this is an individual randomization study and training and communication package was designed for the intervention arm only. The other limitations were the low number of adults enrolled.
Conclusion
The findings of this study confirm the reduction of unnecessary prescription of antibiotics reported during the prior study. The intervention requires only 4 people to be tested for an additional antibiotic prescription to be avoided overall, and 3 for children under 5 and adults even less represented in this study, and 2 for those with a negative malaria test and those with respiratory presentation. The prevalence of COVID-19 was low in the study setting and did not influence the outcomes. In general, the implementation of PoC tests in outpatients has the potential to reduce the inappropriate prescription of antibiotics by guaranteeing a favourable clinical outcome, and securing effectiveness of existing antibiotics for long-time.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank all the participants and their parents or guardians for their participation. We acknowledge the national ethical committee for research in health science and the Institutional Ethical Committee of IRSS. We acknowledge the staff of IRSS-CRUN to provide technical support during the study implementation. We extend our thank to the health district of Nanoro, the staff of the medical center Saint Louis of Temnaore and the health facility of Pella.
Abbreviations
- AFIs
acute febrile illnesses
- AMR
antimicrobial resistance
- CI
confidence interval
- CRP
C-reactive protein
- CRUN
Clinical Research Unit of Nanoro
- FIND
foundation for innovative new diagnostics
- GAS
Group A Streptococcus
- IeDA
integrated e-diagnostic approach
- IMAI
integrated management of adolescent and adult illness
- IMCI
integrated management of childhood illness
- IRSS
Institut de Recherche en Sciences de la Santé
- LMIC
low- and middle-income countries
- NGO
non-governmental organization
- PoC
point-of-care
- RD
risk difference
- RRR
relative risk ration
- RDT
rapid diagnostic tests
- RSV
Respiratory Syncytial Virus
- SAS
Statistical Analysis System
- SDG3
Sustainable Development Goal 3
- SSA
sub-Saharan Africa
- TdH
Terre des Hommes
- UK
United Kingdom
- WBC
white blood cells
- WHO
World Health Organization
Author contributions
SD, JN, HT and FK conceived and designed the study; FK, DV, BK, ANK, TR, AWB, DYS, SS, AMS, AC, JN, PH and HT supervised the implementation of the study in the field and data collection; SW, TK, FK, DV, BK, TR, HT, PH, ABI and JN analyzed and drafted the paper; all the coauthors revised the manuscript; FK, HT, PO and JN supervised the manuscript written.
Funding
This part of the study was funded by the Swiss Agency for Development and Cooperation (SDC),
the German Federal Ministry of Economic Cooperation and Development (BMZ), and the UK Department for International Development (DFID), now Foreign, Commonwealth and Development Office (FCDO).
Data availability
For any data request, please contact the Project Manager at juvenal.nkeramahame@finddx.org) and the local PI Halidou Tinto (by e-mail at the following address halidoutinto@gmail.com).
Declarations
Ethics approval and consent to participate
In Burkina Faso, the study protocol was reviewed and approved by the national ethical committee for health research (DELIBERATION N°2020-01-010) and the institutional ethical committee for research in health sciences (N/Réf. A09-2029/CEIRES). The study was also approved by the Oxford Tropical Research Ethical Committee (OxTREC Reference: 52 − 19). The study was conducted in accordance with the following guidelines: (i) Consensus ethical principles derived from international guidelines including the Declaration of Helsinki, (ii) International Conference on Harmonization Good Clinical Practice guidelines: ICH GCP E6 (R2) and (iii) applicable laws and regulations. Written informed consent was obtained from all participants and/or from parents/guardians before enrollment into the study.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.World Health Organization. WHO Informal Consultation on Fever Management in Peripheral Health Care settings: A Global Review of evidence and practice. WHO. 2013.
- 2.Kiemde F, Tahita MC, Lompo P, Rouamba T, Some AM, Tinto H et al. Treatable causes of fever among children under five years in a seasonal malaria transmission area in Burkina Faso. Infect Dis Poverty. 2018;7(1). [DOI] [PMC free article] [PubMed]
- 3.Osei-Kwakye K, Asante KP, Mahama E, Apanga S, Owusu R, Kwara E et al. The benefits or otherwise of managing Malaria cases with or without laboratory diagnosis: the experience in a District Hospital in Ghana. PLoS ONE. 2013;8(3). [DOI] [PMC free article] [PubMed]
- 4.O’Meara WP, Mangeni JN, Steketee R, Greenwood B. Changes in the burden of malaria in sub-Saharan Africa. Vol. 10, The Lancet Infectious Diseases. 2010. pp. 545–55. [DOI] [PubMed]
- 5.Akpede GO, Akenzua GI. Management of children with prolonged fever of unknown origin and difficulties in the management of fever of unknown origin in children in developing countries. Paediatr Drugs. 2001;3(4):247–62. 10.2165/00128072-200103040-00002 [DOI] [PubMed] [Google Scholar]
- 6.Suttinont C, Losuwanaluk K, Niwatayakul K, Hoontrakul S, Intaranongpai W, Silpasakorn S, et al. Causes of acute, undifferentiated, febrile illness in rural Thailand: results of a prospective observational study. Ann Trop Med Parasitol. 2006;100(4):363–70. 10.1179/136485906X112158 [DOI] [PubMed] [Google Scholar]
- 7.Joshi R, Colford JM, Reingold AL, Kalantri S. Nonmalarial acute undifferentiated fever in a rural hospital in central India: diagnostic uncertainty and overtreatment with antimalarial agents. Am J Trop Med Hyg. 2008;78(3):393–9. 10.4269/ajtmh.2008.78.393 [DOI] [PubMed] [Google Scholar]
- 8.Geneva: World Health Organization. World Malaria Report 2021. Angew Chem Int Ed. 2021;6(11):951–2. [Google Scholar]
- 9.Baiden F, Webster J, Owusu-Agyei S, Chandramohan D. Would rational use of antibiotics be compromised in the era of test-based management of malaria? Trop Med Int Health. 2011;16(2):142–4. 10.1111/j.1365-3156.2010.02692.x [DOI] [PubMed] [Google Scholar]
- 10.Langford BJ, So M, Raybardhan S, Leung V, Soucy J-PR, Westwood D, et al. Antibiotic prescribing in patients with COVID-19: rapid review and meta-analysis. Clin Microbiol Infect. 2021;27(4):520–31. 10.1016/j.cmi.2020.12.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tsay SV, Bartoces M, Gouin K, Kabbani S, Hicks LA. Antibiotic prescriptions Associated with COVID-19 outpatient visits among Medicare Beneficiaries, April 2020 to April 2021. JAMA. 2022;327(20):2018. 10.1001/jama.2022.5471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bednarčuk N, Golić Jelić A, Stoisavljević Šatara S, Stojaković N, Marković Peković V, Stojiljković MP, et al. Antibiotic utilization during COVID-19: are we over-prescribing? Antibiotics. 2023;12(2):308. 10.3390/antibiotics12020308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bonko Mdit, Kiemde A, Tahita F, Lompo MC, Some P, Tinto AM. H, The effect of malaria rapid diagnostic tests results on antimicrobial prescription practices of health care workers in Burkina Faso. Annals Clin Microbiol Antimicrobialsand Antimicrobials. 2019;18(5). [DOI] [PMC free article] [PubMed]
- 14.Crowell V, Yukich JO, Briet OJ, Ross A, Smith TA. A novel approach for measuring the burden of uncomplicated Plasmodium falciparum malaria: application to data from Zambia. PLoS ONE [Electronic Resource] [Internet]. 2013;8:e57297. http://ovidsp.ovid.com/ovidweb.cgi?T=JS&CSC=Y&NEWS=N&PAGE=fulltext&D=medl&AN=23468961. http://digitaal.uba.uva.nl:9003/uva-linker?sid=OVID:medline&id=pmid:23468961&id=doi:10.1371%2Fjournal.pone.0057297&issn=1932-6203&isbn=&volume=8&issue=2&spage=e57297&pag. [DOI] [PMC free article] [PubMed]
- 15.Olliaro P, Nkeramahame J, Salami O, Moore CE, Horgan P, Baiden R, et al. Advancing Access to Diagnostic Tools Essential for Universal Health Coverage and Antimicrobial Resistance Prevention: an overview of trials in Sub-saharan Africa. Clin Infect Dis. 2023;77(Supplement2):S125–33. 10.1093/cid/ciad326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kiemde F, Valia D, Kabore B, Rouamba T, Kone AN, Sawadogo S, et al. A randomized trial to assess the impact of a Package of Diagnostic tools and Diagnostic Algorithm on Antibiotic prescriptions for the management of Febrile Illnesses among Children and Adolescents in Primary Health Facilities in Burkina Faso. Clin Infect Dis. 2023;77(Supplement2):S134–44. 10.1093/cid/ciad331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Olliaro P, Nkeramahame J, Horgan P, Tinto H, Kiemde F, Baiden R, et al. Synthesis and Meta-analysis of 3 Randomized Trials Conducted in Burkina Faso, Ghana, and Uganda Comparing the Effects of Point-of-Care Tests and Diagnostic Algorithms Versus Routine Care on Antibiotic Prescriptions and Clinical Outcomes in Ambulatory Patients <18 Years of Age With Acute Febrile Illness. Clinical Infectious Diseases. 2023;77(Supplement_2):S199–205. [DOI] [PMC free article] [PubMed]
- 18.Salami O, Horgan P, Moore CE, Giri A, Sserwanga A, Pathak A et al. Impact of a package of diagnostic tools, clinical algorithm, and training and communication on outpatient acute fever case management in low- and middle-income countries: protocol for a randomized controlled trial. Trials. 2020;21(1). [DOI] [PMC free article] [PubMed]
- 19.World Health Organization. Pneumonia in children. https://www.who.int/news-room/fact-sheets/detail/pneumonia#:~:text=In%20children%20under%205%20years,the%20chest%20expands%20during%20inhalation). 2022.
- 20.Compaoré A, Nikièma J, Kiemdé F, Tinto H, Salami O, Nkeramahame J, et al. What influences patients’ adherence to Healthcare Worker prescription in Primary Healthcare Facilities in Burkina Faso? A qualitative account of barriers and facilitators. Clin Infect Dis. 2023;77(Supplement2):S171–81. 10.1093/cid/ciad347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sarrassat S, Lewis JJ, Some AS, Somda S, Cousens S, Blanchet K. An Integrated eDiagnosis Approach (IeDA) versus standard IMCI for assessing and managing childhood illness in Burkina Faso: a stepped-wedge cluster randomised trial. BMC Health Serv Res. 2021;21(1):354. 10.1186/s12913-021-06317-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.World Health Organization. Acute Care - Integrated Management of Adolescent and Adult Illness (IMAI) modules. WHO/CDS/IMAI/20041 Rev 2. 2005.
- 23.National Institute of Health. Division of AIDS (DAIDS). Table for Grading the Severity of Adult and Pediatric adverse events. Corrected Version 2.1. [July 2017]. U.S. Department of Health and Human Services, National Institutes of Health, National Institute of Allergy and Infectious Diseases, Division of AIDS; 2017.
- 24.Smith SM, Fahey T, Smucny J, Becker LA. Antibiotics for acute bronchitis. Cochrane Database Syst Rev. 2014;(3):Cd000245. [DOI] [PubMed]
- 25.Albert RH. Diagnosis and treatment of acute bronchitis. Am Fam Physician. 2010;82(11):1345–50. [PubMed] [Google Scholar]
- 26.Sulis G, Adam P, Nafade V, Gore G, Daniels B, Daftary A et al. Antibiotic prescription practices in primary care in low- and middle-income countries: a systematic review and meta-analysis. PLoS Med. 2020;17(6). [DOI] [PMC free article] [PubMed]
- 27.Okoth C, Opanga S, Okalebo F, Oluka M, Baker Kurdi A, Godman B. Point prevalence survey of antibiotic use and resistance at a referral hospital in Kenya: findings and implications. Hosp Pract (1995). 2018;46(3). [DOI] [PubMed]
- 28.Klein EY, Van Boeckel TP, Martinez EM, Pant S, Gandra S, Levin SA, et al. Global increase and geographic convergence in antibiotic consumption between 2000 and 2015. Proc Natl Acad Sci U S A. 2018;115:15. 10.1073/pnas.1717295115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gasson J, Blockman M, Willems B. Antibiotic prescribing practice and adherence to guidelines in primary care in the Cape Town Metro District, South Africa. South Afr Med J. 2018;108(4):304. 10.7196/SAMJ.2018.v108i4.12564 [DOI] [PubMed] [Google Scholar]
- 30.Van Boeckel TP, Gandra S, Ashok A, Caudron Q, Grenfell BT, Levin SA et al. Global antibiotic consumption 2000 to 2010: an analysis of national pharmaceutical sales data. Lancet Infect Dis. 2014;14(8). [DOI] [PubMed]
- 31.The World Bank. The global burden of DIsease: main findings for Sub-saharan Africa. The World Bank; 2013. (87).
- 32.World Health Organization. Guidelines for the treatment of malaria third edition. Trans R Soc Trop Med Hyg. 2015;85(4).
- 33.Van De Sande-Bruinsma N, Grundmann H, Verloo D, Tiemersma E, Monen J, Goossens H et al. Antimicrobial drug use and resistance in Europe. Emerg Infect Dis. 2008;14(11). [DOI] [PMC free article] [PubMed]
- 34.Albrich WC, Monnet DL, Harbarth S. Antibiotic selection pressure and resistance in Streptococcus pneumoniae and Streptococcus pyogenes. Emerg Infect Dis. 2004;10(3). [DOI] [PMC free article] [PubMed]
- 35.Goossens H, Ferech M, Vander Stichele R, Elseviers M. Outpatient antibiotic use in Europe and association with resistance: a cross-national database study. Lancet. 2005;365(9459). [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
For any data request, please contact the Project Manager at juvenal.nkeramahame@finddx.org) and the local PI Halidou Tinto (by e-mail at the following address halidoutinto@gmail.com).