Abstract
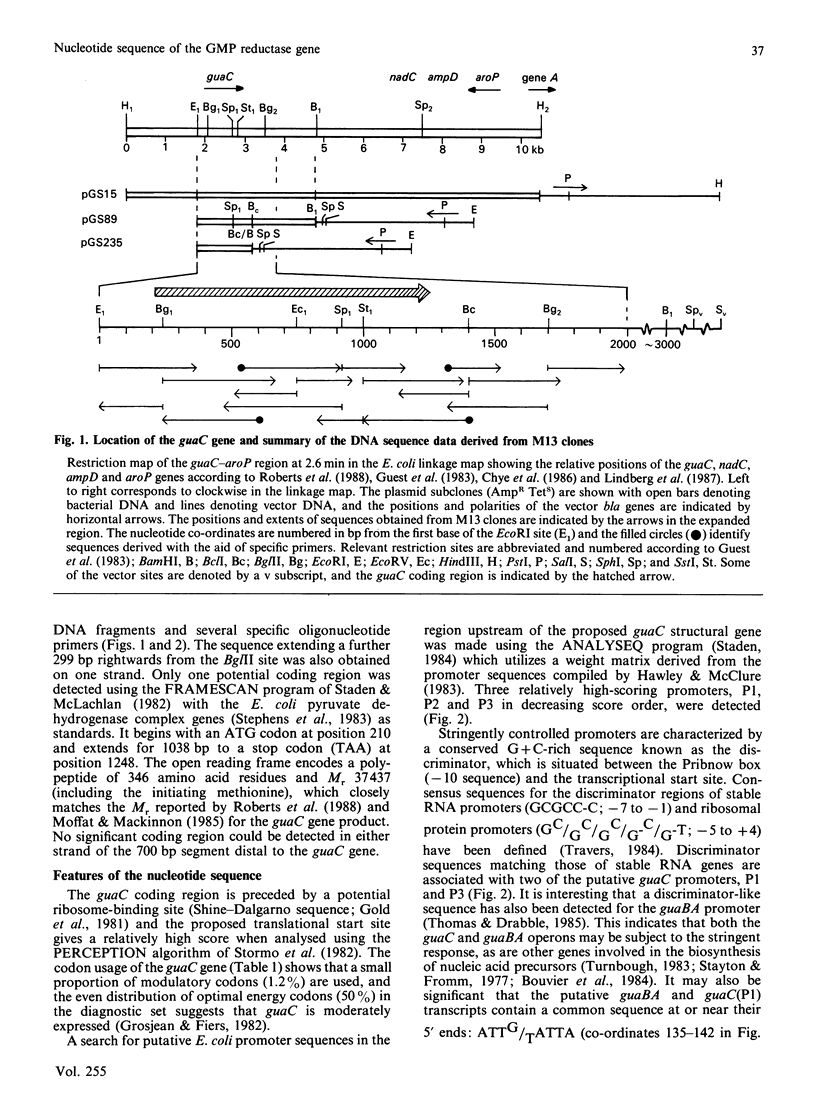
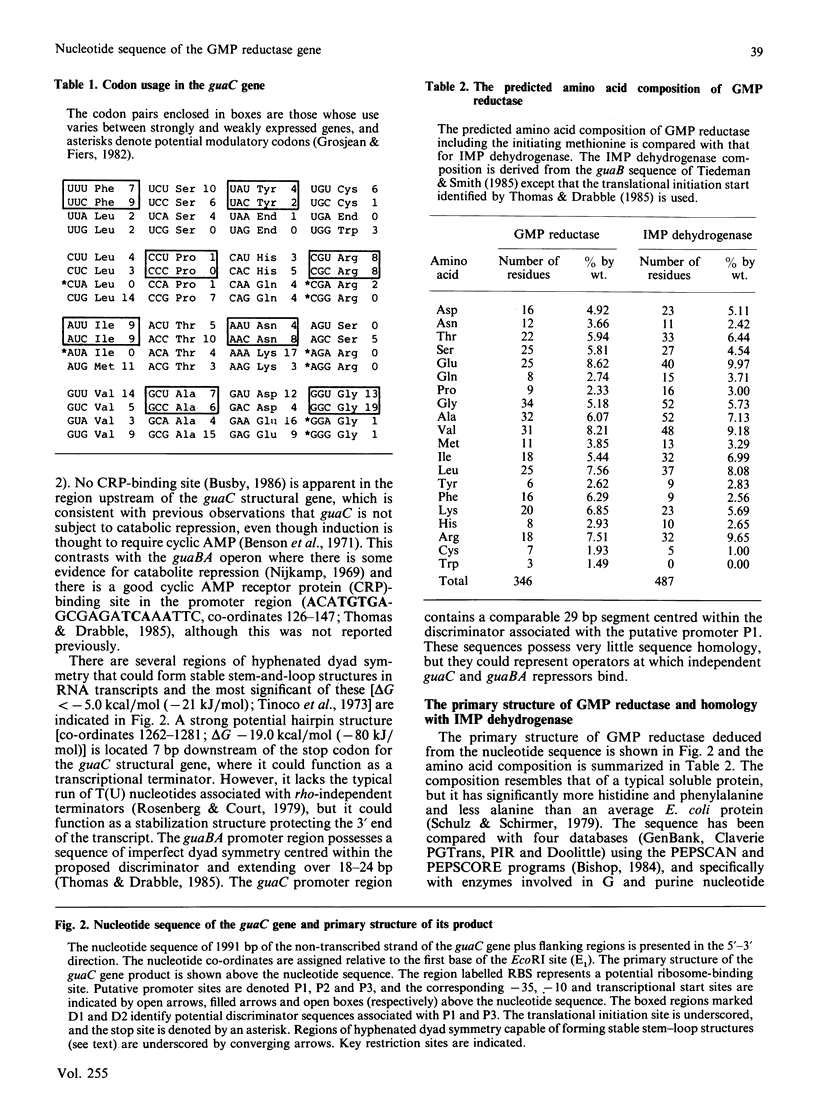
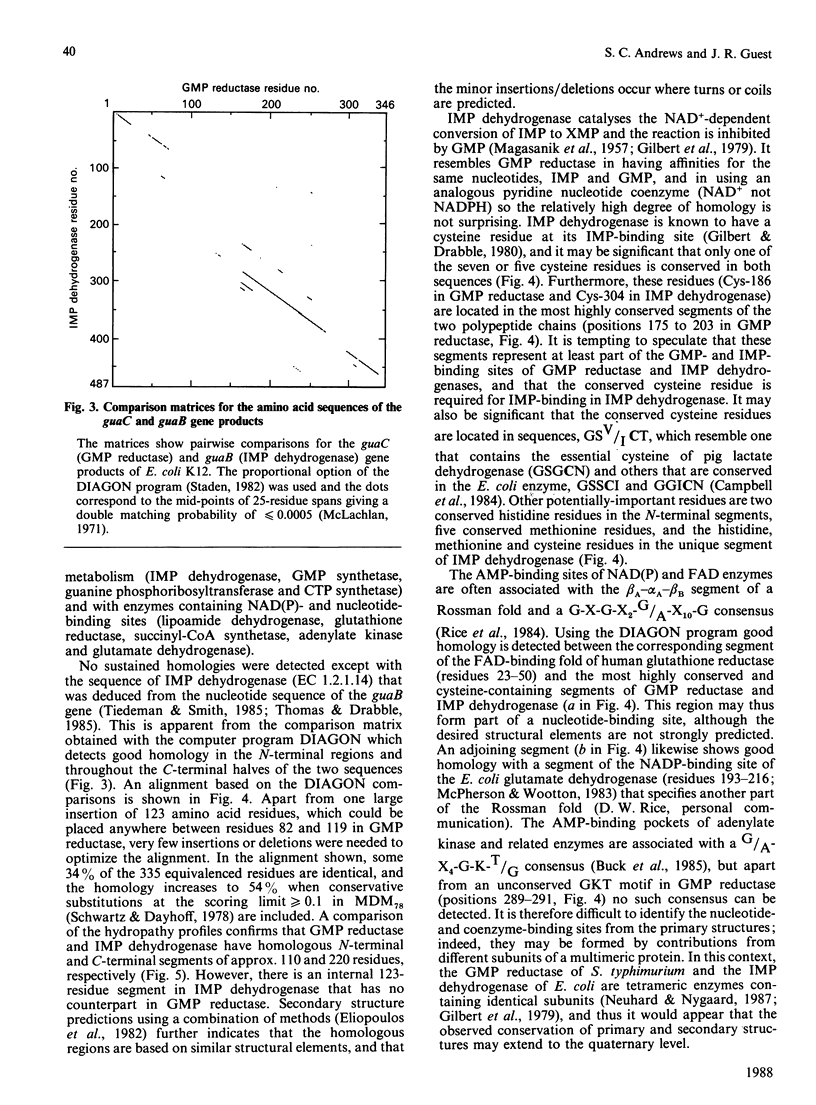
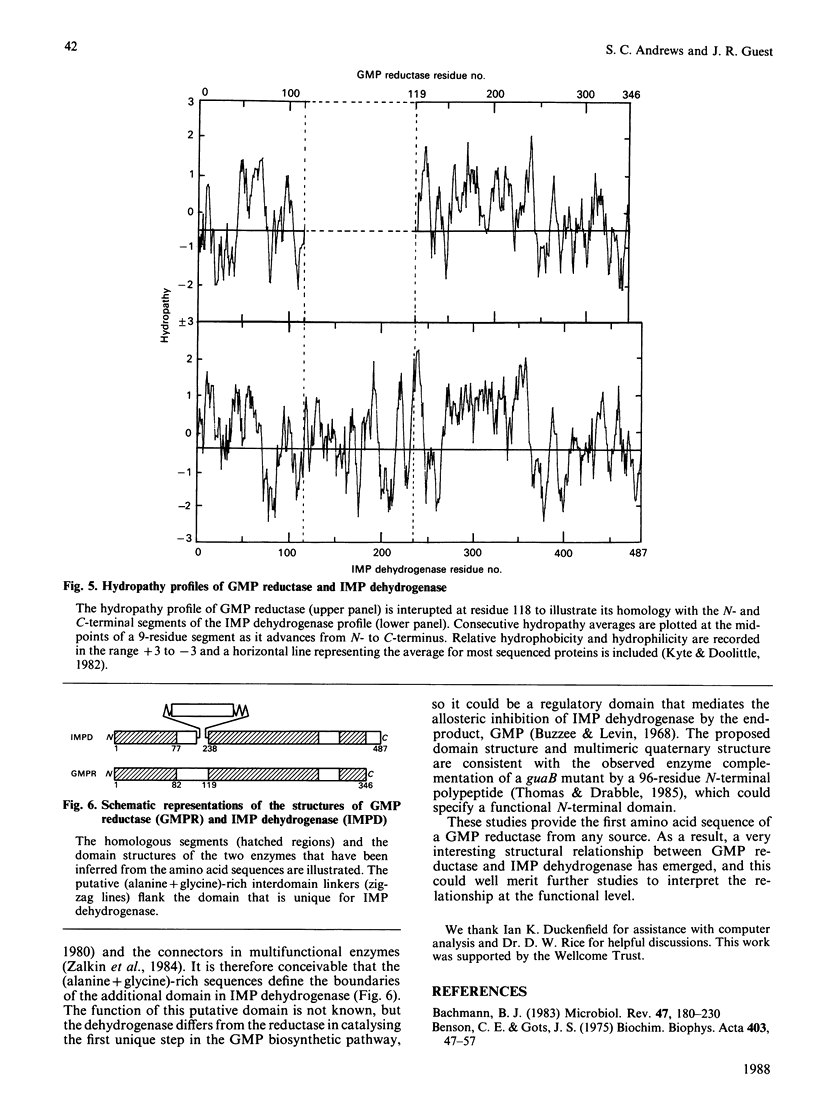
(1) The nucleotide sequence of a 1991 bp segment of DNA that expresses the GMP reductase (guaC) gene of Escherichia coli K12 was determined. (2) This gene comprises 1038 bp, 346 codons (including the initiation codon but excluding the termination codon), and it encodes a polypeptide of Mr 37,437 which is in good agreement with previous maxicell studies. (3) The sequence contains a putative promoter 102 bp upstream of the translational start codon, and this is immediately followed by a (G + C)-rich discriminator sequence suggesting that guaC expression may be under stringent control (4) The GMP reductase exhibits a high degree of sequence identity (34%) with IMP dehydrogenase (the guaB gene product) indicative of a close evolutionary relationship between the salvage pathway and the biosynthetic enzymes, GMP reductase and IMP dehydrogenase, respectively. (5) A single conserved cysteine residue, possibly involved in IMP binding to IMP dehydrogenase, was located within a region that possesses some of the features of a nucleotide binding site. (6) The IMP dehydrogenase polypeptide contains an internal segment of 123 amino acid residues that has no counterpart in GMP reductase and may represent an independent folding domain flanked by (alanine + glycine)-rich interdomain linkers.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B. J. Linkage map of Escherichia coli K-12, edition 7. Microbiol Rev. 1983 Jun;47(2):180–230. doi: 10.1128/mr.47.2.180-230.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson C. E., Brehmeyer B. A., Gots J. S. Requirement of cyclic AMP for induction of GMP reductase in Escherichia coli. Biochem Biophys Res Commun. 1971 Jun 4;43(5):1089–1094. doi: 10.1016/0006-291x(71)90573-0. [DOI] [PubMed] [Google Scholar]
- Benson C. E., Gots J. S. Regulation of GMP reductase in Salmonella typhimurium. Biochim Biophys Acta. 1975 Sep 22;403(1):47–57. doi: 10.1016/0005-2744(75)90007-8. [DOI] [PubMed] [Google Scholar]
- Biggin M. D., Gibson T. J., Hong G. F. Buffer gradient gels and 35S label as an aid to rapid DNA sequence determination. Proc Natl Acad Sci U S A. 1983 Jul;80(13):3963–3965. doi: 10.1073/pnas.80.13.3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouvier J., Patte J. C., Stragier P. Multiple regulatory signals in the control region of the Escherichia coli carAB operon. Proc Natl Acad Sci U S A. 1984 Jul;81(13):4139–4143. doi: 10.1073/pnas.81.13.4139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck D., Spencer M. E., Guest J. R. Primary structure of the succinyl-CoA synthetase of Escherichia coli. Biochemistry. 1985 Oct 22;24(22):6245–6252. doi: 10.1021/bi00343a031. [DOI] [PubMed] [Google Scholar]
- Buzzee D. H., Levin A. P. Demonstration of an effector site for the enzyme inosine 5'-phosphate dehydrogenase. Biochem Biophys Res Commun. 1968 Mar 27;30(6):673–677. doi: 10.1016/0006-291x(68)90565-2. [DOI] [PubMed] [Google Scholar]
- Campbell H. D., Rogers B. L., Young I. G. Nucleotide sequence of the respiratory D-lactate dehydrogenase gene of Escherichia coli. Eur J Biochem. 1984 Oct 15;144(2):367–373. doi: 10.1111/j.1432-1033.1984.tb08473.x. [DOI] [PubMed] [Google Scholar]
- Chye M. L., Guest J. R., Pittard J. Cloning of the aroP gene and identification of its product in Escherichia coli K-12. J Bacteriol. 1986 Aug;167(2):749–753. doi: 10.1128/jb.167.2.749-753.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford I. P., Nichols B. P., Yanofsky C. Nucleotide sequence of the trpB gene in Escherichia coli and Salmonella typhimurium. J Mol Biol. 1980 Oct 5;142(4):489–502. doi: 10.1016/0022-2836(80)90259-4. [DOI] [PubMed] [Google Scholar]
- Gilbert H. J., Drabble W. T. Active-site modification of native and mutant forms of inosine 5'-monophosphate dehydrogenase from Escherichia coli K12. Biochem J. 1980 Nov 1;191(2):533–541. doi: 10.1042/bj1910533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert H. J., Lowe C. R., Drabble W. T. Inosine 5'-monophosphate dehydrogenase of Escherichia coli. Purification by affinity chromatography, subunit structure and inhibition by guanosine 5'-monophosphate. Biochem J. 1979 Dec 1;183(3):481–494. doi: 10.1042/bj1830481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold L., Pribnow D., Schneider T., Shinedling S., Singer B. S., Stormo G. Translational initiation in prokaryotes. Annu Rev Microbiol. 1981;35:365–403. doi: 10.1146/annurev.mi.35.100181.002053. [DOI] [PubMed] [Google Scholar]
- Gots J. S., Benson C. E., Jochimsen B., Koduri K. R. Microbial models and regulatory elements in the control of purine metabolism. Ciba Found Symp. 1977;(48):23–41. doi: 10.1002/9780470720301.ch3. [DOI] [PubMed] [Google Scholar]
- Grosjean H., Fiers W. Preferential codon usage in prokaryotic genes: the optimal codon-anticodon interaction energy and the selective codon usage in efficiently expressed genes. Gene. 1982 Jun;18(3):199–209. doi: 10.1016/0378-1119(82)90157-3. [DOI] [PubMed] [Google Scholar]
- Guest J. R., Roberts R. E., Stephens P. E. Hybrid plasmids containing the pyruvate dehydrogenase complex genes and gene-DNA relationships in the 2 to 3 minute region of the Escherichia coli chromosome. J Gen Microbiol. 1983 Mar;129(3):671–680. doi: 10.1099/00221287-129-3-671. [DOI] [PubMed] [Google Scholar]
- Guest J. R., Stephens P. E. Molecular cloning of the pyruvate dehydrogenase complex genes of Escherichia coli. J Gen Microbiol. 1980 Dec;121(2):277–292. doi: 10.1099/00221287-121-2-277. [DOI] [PubMed] [Google Scholar]
- Hawley D. K., McClure W. R. Compilation and analysis of Escherichia coli promoter DNA sequences. Nucleic Acids Res. 1983 Apr 25;11(8):2237–2255. doi: 10.1093/nar/11.8.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler A. I., Gots J. S. Regulation of guaC expression in Escherichia coli. J Bacteriol. 1985 Dec;164(3):1288–1293. doi: 10.1128/jb.164.3.1288-1293.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Lindberg F., Lindquist S., Normark S. Inactivation of the ampD gene causes semiconstitutive overproduction of the inducible Citrobacter freundii beta-lactamase. J Bacteriol. 1987 May;169(5):1923–1928. doi: 10.1128/jb.169.5.1923-1928.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAGASANIK B., MOYED H. S., GEHRING L. B. Enzymes essential for the biosynthesis of nucleic acid guanine; inosine 5'-phosphate dehydrogenase of Aerobacter aerogenes. J Biol Chem. 1957 May;226(1):339–350. [PubMed] [Google Scholar]
- MAGER J., MAGASANIK B. Guanosine 5'-phosphate reductase and its role in the interconversion of purine nucleotides. J Biol Chem. 1960 May;235:1474–1478. [PubMed] [Google Scholar]
- Marinus M. G., Morris N. R. Isolation of deoxyribonucleic acid methylase mutants of Escherichia coli K-12. J Bacteriol. 1973 Jun;114(3):1143–1150. doi: 10.1128/jb.114.3.1143-1150.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLachlan A. D. Tests for comparing related amino-acid sequences. Cytochrome c and cytochrome c 551 . J Mol Biol. 1971 Oct 28;61(2):409–424. doi: 10.1016/0022-2836(71)90390-1. [DOI] [PubMed] [Google Scholar]
- McPherson M. J., Wootton J. C. Complete nucleotide sequence of the Escherichia coli gdhA gene. Nucleic Acids Res. 1983 Aug 11;11(15):5257–5266. doi: 10.1093/nar/11.15.5257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehra R. K., Drabble W. T. Dual control of the gua operon of Escherichia coli K12 by adenine and guanine nucleotides. J Gen Microbiol. 1981 Mar;123(1):27–37. doi: 10.1099/00221287-123-1-27. [DOI] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Moffat K. G., Mackinnon G. Cloning of the Escherichia coli K-12 guaC gene following its transposition into the RP4::Mu cointegrate. Gene. 1985;40(1):141–143. doi: 10.1016/0378-1119(85)90034-4. [DOI] [PubMed] [Google Scholar]
- Nijkamp H. J., De Haan P. G. Genetic and biochemical studies of the guanosine 5'-monophosphate pathway in Escherichia coli. Biochim Biophys Acta. 1967 Aug 22;145(1):31–40. doi: 10.1016/0005-2787(67)90651-x. [DOI] [PubMed] [Google Scholar]
- Nijkamp H. J. Regulatory role of adenine nucleotides in the biosynthesis of guanosine 5'-monophosphate. J Bacteriol. 1969 Nov;100(2):585–593. doi: 10.1128/jb.100.2.585-593.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radford S. E., Laue E. D., Perham R. N., Miles J. S., Guest J. R. Segmental structure and protein domains in the pyruvate dehydrogenase multienzyme complex of Escherichia coli. Genetic reconstruction in vitro and 1H-n.m.r. spectroscopy. Biochem J. 1987 Nov 1;247(3):641–649. doi: 10.1042/bj2470641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renart M. F., Sillero A. GMP reductase in Artemia salina. Biochim Biophys Acta. 1974 Mar 21;341(1):178–186. doi: 10.1016/0005-2744(74)90078-3. [DOI] [PubMed] [Google Scholar]
- Rice D. W., Schulz G. E., Guest J. R. Structural relationship between glutathione reductase and lipoamide dehydrogenase. J Mol Biol. 1984 Apr 15;174(3):483–496. doi: 10.1016/0022-2836(84)90332-2. [DOI] [PubMed] [Google Scholar]
- Roberts R. E., Lienhard C. I., Gaines C. G., Smith J. M., Guest J. R. Genetic and molecular characterization of the guaC-nadC-aroP region of Escherichia coli K-12. J Bacteriol. 1988 Jan;170(1):463–467. doi: 10.1128/jb.170.1.463-467.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg M., Court D. Regulatory sequences involved in the promotion and termination of RNA transcription. Annu Rev Genet. 1979;13:319–353. doi: 10.1146/annurev.ge.13.120179.001535. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Barrell B. G., Smith A. J., Roe B. A. Cloning in single-stranded bacteriophage as an aid to rapid DNA sequencing. J Mol Biol. 1980 Oct 25;143(2):161–178. doi: 10.1016/0022-2836(80)90196-5. [DOI] [PubMed] [Google Scholar]
- Spector T., Jones T. E. Guanosine 5'-monophosphate reductase from Leishmania donovani. A possible chemotherapeutic target. Biochem Pharmacol. 1982 Dec 1;31(23):3891–3897. doi: 10.1016/0006-2952(82)90307-0. [DOI] [PubMed] [Google Scholar]
- Spector T., Jones T. E., Miller R. L. Reaction mechanism and specificity of human GMP reductase. Substrates, inhibitors, activators, and inactivators. J Biol Chem. 1979 Apr 10;254(7):2308–2315. [PubMed] [Google Scholar]
- Staden R. A new computer method for the storage and manipulation of DNA gel reading data. Nucleic Acids Res. 1980 Aug 25;8(16):3673–3694. doi: 10.1093/nar/8.16.3673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staden R. A strategy of DNA sequencing employing computer programs. Nucleic Acids Res. 1979 Jun 11;6(7):2601–2610. doi: 10.1093/nar/6.7.2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staden R. An interactive graphics program for comparing and aligning nucleic acid and amino acid sequences. Nucleic Acids Res. 1982 May 11;10(9):2951–2961. doi: 10.1093/nar/10.9.2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staden R. Computer methods to locate signals in nucleic acid sequences. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 2):505–519. doi: 10.1093/nar/12.1part2.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staden R., McLachlan A. D. Codon preference and its use in identifying protein coding regions in long DNA sequences. Nucleic Acids Res. 1982 Jan 11;10(1):141–156. doi: 10.1093/nar/10.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stayton M. M., Fromm H. J. Guanosine 5'-diphosphate-3'-diphosphate inhibition of adenylosuccinate synthetase. J Biol Chem. 1979 Apr 25;254(8):2579–2581. [PubMed] [Google Scholar]
- Stephens P. E., Lewis H. M., Darlison M. G., Guest J. R. Nucleotide sequence of the lipoamide dehydrogenase gene of Escherichia coli K12. Eur J Biochem. 1983 Oct 3;135(3):519–527. doi: 10.1111/j.1432-1033.1983.tb07683.x. [DOI] [PubMed] [Google Scholar]
- Stephens R. W., Whittaker V. K. Calf thymus GMP reductase: control by XMP. Biochem Biophys Res Commun. 1973 Aug 6;53(3):975–981. doi: 10.1016/0006-291x(73)90187-3. [DOI] [PubMed] [Google Scholar]
- Stormo G. D., Schneider T. D., Gold L., Ehrenfeucht A. Use of the 'Perceptron' algorithm to distinguish translational initiation sites in E. coli. Nucleic Acids Res. 1982 May 11;10(9):2997–3011. doi: 10.1093/nar/10.9.2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas M. S., Drabble W. T. Nucleotide sequence and organisation of the gua promoter region of Escherichia coli. Gene. 1985;36(1-2):45–53. doi: 10.1016/0378-1119(85)90068-x. [DOI] [PubMed] [Google Scholar]
- Tiedeman A. A., Smith J. M. Nucleotide sequence of the guaB locus encoding IMP dehydrogenase of Escherichia coli K12. Nucleic Acids Res. 1985 Feb 25;13(4):1303–1316. doi: 10.1093/nar/13.4.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinoco I., Jr, Borer P. N., Dengler B., Levin M. D., Uhlenbeck O. C., Crothers D. M., Bralla J. Improved estimation of secondary structure in ribonucleic acids. Nat New Biol. 1973 Nov 14;246(150):40–41. doi: 10.1038/newbio246040a0. [DOI] [PubMed] [Google Scholar]
- Travers A. A. Conserved features of coordinately regulated E. coli promoters. Nucleic Acids Res. 1984 Mar 26;12(6):2605–2618. doi: 10.1093/nar/12.6.2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbough C. L., Jr Regulation of Escherichia coli aspartate transcarbamylase synthesis by guanosine tetraphosphate and pyrimidine ribonucleoside triphosphates. J Bacteriol. 1983 Feb;153(2):998–1007. doi: 10.1128/jb.153.2.998-1007.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Zalkin H., Paluh J. L., van Cleemput M., Moye W. S., Yanofsky C. Nucleotide sequence of Saccharomyces cerevisiae genes TRP2 and TRP3 encoding bifunctional anthranilate synthase: indole-3-glycerol phosphate synthase. J Biol Chem. 1984 Mar 25;259(6):3985–3992. [PubMed] [Google Scholar]


